Rare among Rare: Phenotypes of Uncommon CMT Genotypes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
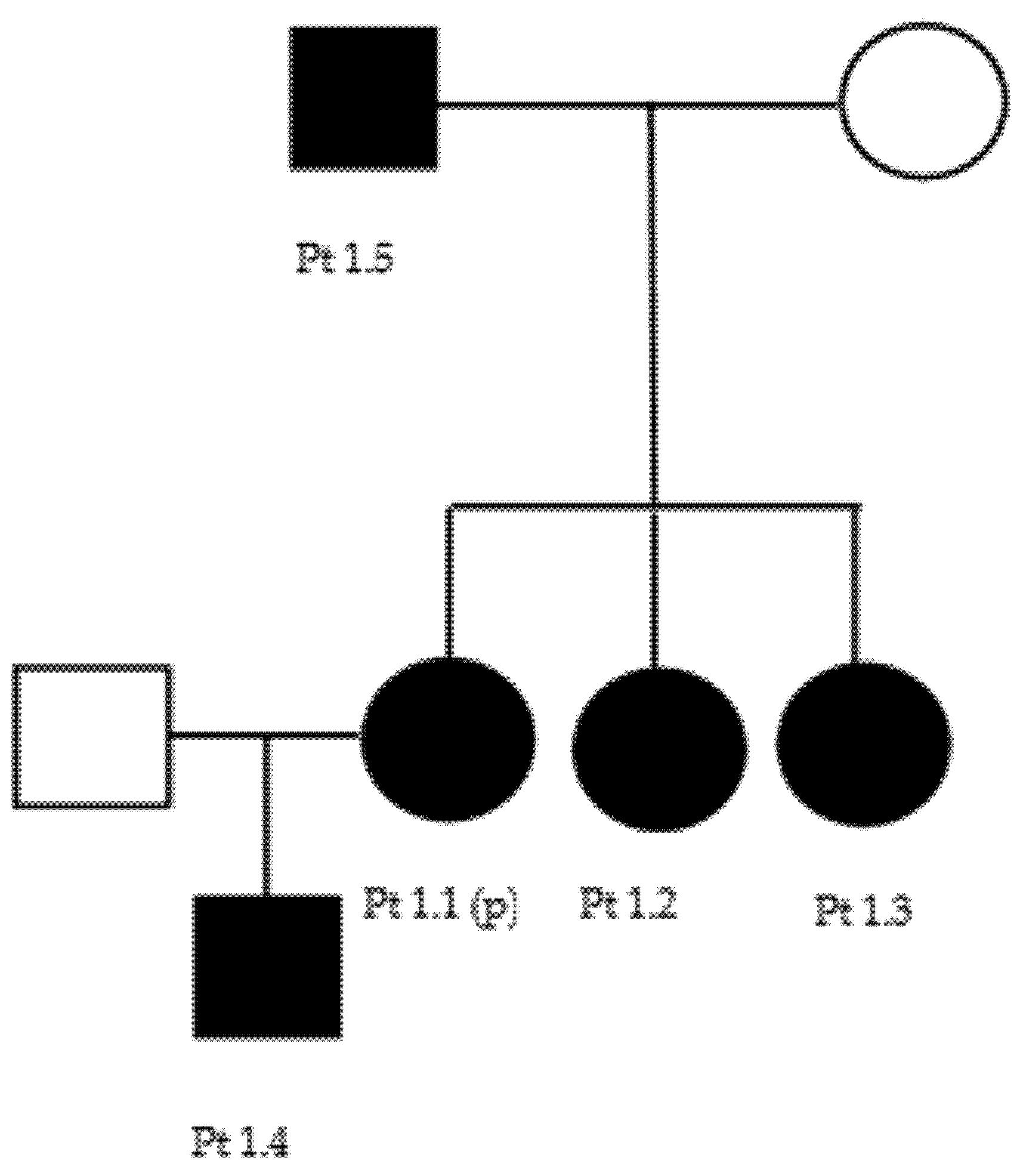
3.1. BSCL2 (CMT2) (OMIM 606158)
3.2. MORC2 (CMT2) (OMIM 616661)
3.3. HINT1 (CMT2) (OMIM 601314)
3.4. LITAF (CMT1) (OMIM 603795)
3.5. GARS1 (CMT2) (OMIM 600287)
3.6. GDAP1 (CMT1/CMT2) (OMIM 606598)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Pareyson, D.; Saveri, P.; Pisciotta, C. New Developments in Charcot–Marie–Tooth Neuropathy and Related Diseases. Curr. Opin. Neurol. 2017, 30, 471–480. [Google Scholar] [CrossRef]
- Bienfait, H.M.E.; Baas, F.; Koelman, J.H.T.M.; de Haan, R.J.; van Engelen, B.G.M.; Gabreels-Festen, A.A.W.M.; Ongerboer de Visser, B.W.; Meggouh, F.; Weterman, M.A.J.; De Jonghe, P.; et al. Phenotype of Charcot-Marie-Tooth Disease Type 2. Neurology 2007, 68, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Cardellini, D.; Zanette, G.; Taioli, F.; Bertolasi, L.; Ferrari, S.; Cavallaro, T.; Fabrizi, G.M. CIDP, CMT1B, or CMT1B plus CIDP? Neurol. Sci. 2021, 42, 1127–1130. [Google Scholar] [CrossRef]
- Mazzeo, A.; Stancanelli, C.; Russo, M.; Granata, F.; Gentile, L.; Di Leo, R.; Vita, G.; Nobile-Orazio, E.; Toscano, A. Subacute Inflammatory Demyelinating Polyneuropathy Disclosed by Massive Nerve Root Enhancement in CMT1A: Letter to the Editor. Muscle Nerve 2012, 45, 451–452. [Google Scholar] [CrossRef]
- Laurá, M.; Pipis, M.; Rossor, A.M.; Reilly, M.M. Charcot–Marie–Tooth Disease and Related Disorders: An Evolving Landscape. Curr. Opin. Neurol. 2019, 32, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.M.; Laura, M.; Fawcett, K.; Pandraud, A.; Liu, Y.-T.; Davidson, G.L.; Rossor, A.M.; Polke, J.M.; Castleman, V.; Manji, H.; et al. Charcot–Marie–Tooth Disease: Frequency of Genetic Subtypes and Guidelines for Genetic Testing. J. Neurol. Neurosurg. Psychiatry 2012, 83, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Saporta, A.S.D.; Sottile, S.L.; Miller, L.J.; Feely, S.M.E.; Siskind, C.E.; Shy, M.E. Charcot-Marie-Tooth Disease Subtypes and Genetic Testing Strategies. Ann. Neurol. 2011, 69, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Gess, B.; Schirmacher, A.; Boentert, M.; Young, P. Charcot-Marie-Tooth Disease: Frequency of Genetic Subtypes in a German Neuromuscular Center Population. Neuromuscul. Disord. 2013, 23, 647–651. [Google Scholar] [CrossRef]
- Stancanelli, C.; Taioli, F.; Testi, S.; Fabrizi, G.M.; Arena, M.G.; Granata, F.; Russo, M.; Gentile, L.; Vita, G.; Mazzeo, A. Unusual Features of Central Nervous System Involvement in CMTX Associated with a Novel Mutation of GJB1 Gene. J. Peripher. Nerv. Syst. 2012, 17, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Sivera, R.; Sevilla, T.; Vilchez, J.J.; Martinez-Rubio, D.; Chumillas, M.J.; Vazquez, J.F.; Muelas, N.; Bataller, L.; Millan, J.M.; Palau, F.; et al. Charcot-Marie-Tooth Disease: Genetic and Clinical Spectrum in a Spanish Clinical Series. Neurology 2013, 81, 1617–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manganelli, F.; Tozza, S.; Pisciotta, C.; Bellone, E.; Iodice, R.; Nolano, M.; Geroldi, A.; Capponi, S.; Mandich, P.; Santoro, L. Charcot-Marie-Tooth Disease: Frequency of Genetic Subtypes in a Southern Italy Population: Manganelli et Al. J. Peripher. Nerv. Syst. 2014, 19, 292–298. [Google Scholar] [CrossRef]
- Lorefice, L.; Murru, M.R.; Coghe, G.; Fenu, G.; Corongiu, D.; Frau, J.; Tranquilli, S.; Tacconi, P.; Vannelli, A.; Marrosu, G.; et al. Charcot–Marie–Tooth Disease: Genetic Subtypes in the Sardinian Population. Neurol. Sci. 2017, 38, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.; Russo, M.; Fabrizi, G.M.; Taioli, F.; Ferrarini, M.; Testi, S.; Alfonzo, A.; Aguennouz, M.; Toscano, A.; Vita, G.; et al. Charcot-Marie-Tooth Disease: Experience from a Large Italian Tertiary Neuromuscular Center. Neurol. Sci. 2020, 41, 1239–1243. [Google Scholar] [CrossRef]
- Stancanelli, C.; Fabrizi, G.M.; Ferrarini, M.; Cavallaro, T.; Taioli, F.; Di Leo, R.; Russo, M.; Gentile, L.; Toscano, A.; Vita, G.; et al. Charcot–Marie–Tooth 2F: Phenotypic Presentation of the Arg136Leu HSP27 Mutation in a Multigenerational Family. Neurol. Sci. 2015, 36, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Ramchandren, S. Charcot-Marie-Tooth Disease and Other Genetic Polyneuropathies. Contin. Lifelong Learn. Neurol. 2017, 23, 1360–1377. [Google Scholar] [CrossRef] [PubMed]
- Rohkamm, B.; Reilly, M.M.; Lochmüller, H.; Schlotter-Weigel, B.; Barisic, N.; Schöls, L.; Nicholson, G.; Pareyson, D.; Laurà, M.; Janecke, A.R.; et al. Further Evidence for Genetic Heterogeneity of Distal HMN Type V, CMT2 with Predominant Hand Involvement and Silver Syndrome. J. Neurol. Sci. 2007, 263, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Eulate, G.; Fernández-Torrón, R.; Guisasola, A.; Gaspar, M.T.I.; Diaz-Manera, J.; Maneiro, M.; Zulaica, M.; Olasagasti, V.; Formica, A.F.; Espinal, J.B.; et al. Phenotypic Correlations in a Large Single-center Cohort of Patients with BSCL2 Nerve Disorders: A Clinical, Neurophysiological and Muscle Magnetic Resonance Imaging Study. Eur. J. Neurol. 2020, 27, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Okamoto, Y.; Tanabe, H.; Yoshimura, A.; Higuchi, Y.; Yuan, J.; Hashiguchi, A.; Ishiura, H.; Mitsui, J.; Suwazono, S.; et al. Clinical Features of Inherited Neuropathy with BSCL2 Mutations in Japan. J. Peripher. Nerv. Syst. 2020, 25, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Windpassinger, C.; Auer-Grumbach, M.; Irobi, J.; Patel, H.; Petek, E.; Hörl, G.; Malli, R.; Reed, J.A.; Dierick, I.; Verpoorten, N.; et al. Heterozygous Missense Mutations in BSCL2 Are Associated with Distal Hereditary Motor Neuropathy and Silver Syndrome. Nat. Genet. 2004, 36, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zheng, R.; Luan, X.; Zhang, W.; Wang, Z.; Yuan, Y. Clincial and Pathological Study of Distal Motor Neuropathy with N88S Mutation in BSCL2. Neuropathology 2009, 29, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, Y.; Magot, A.; Latour, P.; Perrier, J.; Mercier, S.; Maisonobe, T.; Péréon, Y. Clinical and Electrophysiological Features in a French Family Presenting with Seipinopathy. Neuromuscul. Disord. 2015, 25, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Cafforio, G.; Calabrese, R.; Morelli, N.; Mancuso, M.; Piazza, S.; Martinuzzi, A.; Bassi, M.T.; Crippa, F.; Siciliano, G. The First Italian Family with Evidence of Pyramidal Impairment as Phenotypic Manifestation of Silver Syndrome BSCL2 Gene Mutation. Neurol. Sci. 2008, 29, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-O.; Park, M.-H.; Chung, K.W.; Woo, H.-M.; Koo, H.; Chung, H.-K.; Choi, K.-G.; Park, K.D.; Lee, H.J.; Hyun, Y.S.; et al. Clinical and Histopathological Study of Charcot-Marie-Tooth Neuropathy with a Novel S90W Mutation in BSCL2. Neurogenetics 2013, 14, 35–42. [Google Scholar] [CrossRef]
- Monteiro, A.; Real, R.; Nadais, G.; Silveira, F.; Leão, M. BSCL2 N88S Mutation in A Portuguese Patient with the Silver Syndrome. Muscle Nerve 2015, 51, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Rakočević-Stojanović, V.; Milić-Rašić, V.; Perić, S.; Baets, J.; Timmerman, V.; Dierick, I.; Pavlović, S.; De Jonghe, P. N88S Mutation in the BSCL2 Gene in a Serbian Family with Distal Hereditary Motor Neuropathy Type V or Silver Syndrome. J. Neurol. Sci. 2010, 296, 107–109. [Google Scholar] [CrossRef]
- Sevilla, T.; Lupo, V.; Martínez-Rubio, D.; Sancho, P.; Sivera, R.; Chumillas, M.J.; García-Romero, M.; Pascual-Pascual, S.I.; Muelas, N.; Dopazo, J.; et al. Mutations in the MORC2 Gene Cause Axonal Charcot–Marie–Tooth Disease. Brain 2016, 139, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Sancho, P.; Bartesaghi, L.; Miossec, O.; García-García, F.; Ramírez-Jiménez, L.; Siddell, A.; Åkesson, E.; Hedlund, E.; Laššuthová, P.; Pascual-Pascual, S.I.; et al. Characterization of Molecular Mechanisms Underlying the Axonal Charcot–Marie–Tooth Neuropathy Caused by MORC2 Mutations. Hum. Mol. Genet. 2019, 28, 1629–1644. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Okamoto, Y.; Yoshimura, A.; Yuan, J.-H.; Hiramatsu, Y.; Higuchi, Y.; Hashiguchi, A.; Mitsui, J.; Ishiura, H.; Fukumura, S.; et al. Clinical and Mutational Spectrum of Charcot–Marie–Tooth Disease Type 2Z Caused by MORC2 Variants in Japan. Eur. J. Neurol. 2017, 24, 1274–1282. [Google Scholar] [CrossRef]
- Peeters, K.; Chamova, T.; Tournev, I.; Jordanova, A. Axonal Neuropathy with Neuromyotonia: There Is a HINT. Brain 2016, 140, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Shchagina, O.A.; Milovidova, T.B.; Murtazina, A.F.; Rudenskaya, G.E.; Nikitin, S.S.; Dadali, E.L.; Polyakov, A.V. HINT1 Gene Pathogenic Variants: The Most Common Cause of Recessive Hereditary Motor and Sensory Neuropathies in Russian Patients. Mol. Biol. Rep. 2020, 47, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Amor-Barris, S.; Høyer, H.; Brauteset, L.V.; De Vriendt, E.; Strand, L.; Jordanova, A.; Braathen, G.J.; Peeters, K. HINT1 Neuropathy in Norway: Clinical, Genetic and Functional Profiling. Orphanet J. Rare Dis. 2021, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Fu, J.; Lv, H.; Zhang, W.; Wang, Z.; Yuan, Y. Novel Mutations in HINT1 Gene Cause Autosomal Recessive Axonal Neuropathy with Neuromyotonia in Two Cases of Sensorimotor Neuropathy and One Case of Motor Neuropathy. Neuromuscul. Disord. 2018, 28, 646–651. [Google Scholar] [CrossRef]
- Scarpini, G.; Spagnoli, C.; Salerno, G.G.; Rizzi, S.; Frattini, D.; Fusco, C. Autosomal Recessive Axonal Neuropathy Caused by HINT1 Mutation: New Association of a Psychiatric Disorder to the Neurologic Phenotype. Neuromuscul. Disord. 2019, 29, 979. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.R.; Ho, A.K.; Laurá, M.; Horvath, R.; Reilly, M.M.; Luzio, J.P.; Roberts, R.C. A Dysfunctional Endolysosomal Pathway Common to Two Sub-Types of Demyelinating Charcot–Marie–Tooth Disease. Acta Neuropathol. Commun. 2020, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, P.; Luigetti, M.; Geroldi, A.; Capponi, S.; Pezzini, I.; Gulli, R.; Pazzaglia, C.; Padua, L.; Massa, R.; Mandich, P.; et al. A Novel LITAF/SIMPLE Mutation within a Family with a Demyelinating Form of Charcot–Marie–Tooth Disease. J. Neurol. Sci. 2014, 343, 183–186. [Google Scholar] [CrossRef]
- Jerath, N.U.; Shy, M.E. Charcot-Marie-Tooth Disease Type 1C: Clinical and Electrophysiological Findings for the c.334G > A (p.Gly112Ser) Litaf/Simple Mutation: EDx, Clinical Findings in CMT1C. Muscle Nerve 2017, 56, 1092–1095. [Google Scholar] [CrossRef]
- Guimarães-Costa, R.; Iancu Ferfoglia, R.; Leonard-Louis, S.; Ziegler, F.; Magy, L.; Fournier, E.; Dubourg, O.; Bouche, P.; Maisonobe, T.; Lacour, A.; et al. Phenotypic Spectrum of Charcot−Marie−Tooth Disease Due to LITAF/SIMPLE Mutations: A Study of 18 Patients. Eur. J. Neurol. 2017, 24, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, E.S.; Nikonov, O.S.; Nikonova, E.Y. Associations between Neurological Diseases and Mutations in the Human Glycyl-TRNA Synthetase. Biochem. Mosc. 2021, 86, S12–S23. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, R.; Locatelli, F.; Corti, S.; Scarlato, M.; Ghezzi, S.; Prelle, A.; Fagiolari, G.; Moggio, M.; Carpo, M.; Bresolin, N.; et al. Coexistence of CMT-2D and Distal SMA-V Phenotypes in an Italian Family with a GARS Gene Mutation. Neurology 2006, 66, 752–754. [Google Scholar] [CrossRef]
- Cantarero, L.; Juárez-Escoto, E.; Civera-Tregón, A.; Rodríguez-Sanz, M.; Roldán, M.; Benítez, R.; Hoenicka, J.; Palau, F. Mitochondria–Lysosome Membrane Contacts Are Defective in GDAP1-Related Charcot–Marie–Tooth Disease. Hum. Mol. Genet. 2021, 29, 3589–3605. [Google Scholar] [CrossRef]
- Pezzini, I.; Geroldi, A.; Capponi, S.; Gulli, R.; Schenone, A.; Grandis, M.; Doria-Lamba, L.; La Piana, C.; Cremonte, M.; Pisciotta, C.; et al. GDAP1 Mutations in Italian Axonal Charcot–Marie–Tooth Patients: Phenotypic Features and Clinical Course. Neuromuscul. Disord. 2016, 26, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sivera, R.; Frasquet, M.; Lupo, V.; García-Sobrino, T.; Blanco-Arias, P.; Pardo, J.; Fernández-Torrón, R.; de Munain, A.L.; Márquez-Infante, C.; Villarreal, L.; et al. Distribution and Genotype-Phenotype Correlation of GDAP1 Mutations in Spain. Sci. Rep. 2017, 7, 6677. [Google Scholar] [CrossRef] [Green Version]
- Pakhrin, P.S.; Xie, Y.; Hu, Z.; Li, X.; Liu, L.; Huang, S.; Wang, B.; Yang, Z.; Zhang, J.; Liu, X.; et al. Genotype–Phenotype Correlation and Frequency of Distribution in a Cohort of Chinese Charcot–Marie–Tooth Patients Associated with GDAP1 Mutations. J. Neurol. 2018, 265, 637–646. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Zimoń, M.; Baets, J.; Almeida-Souza, L.; De Vriendt, E.; Nikodinovic, J.; Parman, Y.; Battaloǧlu, E.; Matur, Z.; Guergueltcheva, V.; Tournev, I.; et al. Loss-of-Function Mutations in HINT1 Cause Axonal Neuropathy with Neuromyotonia. Nat. Genet. 2012, 44, 1080–1083. [Google Scholar] [CrossRef]
- Lerat, J.; Cintas, P.; Beauvais-Dzugan, H.; Magdelaine, C.; Sturtz, F.; Lia, A.-S. A Complex Homozygous Mutation in ABHD12 Responsible for PHARC Syndrome Discovered with NGS and Review of the Literature: A Complex Homozygous Mutation in ABHD12 Responsible. J. Peripher. Nerv. Syst. 2017, 22, 77–84. [Google Scholar] [CrossRef]
- Kabala, A.M.; Lasserre, J.-P.; Ackerman, S.H.; di Rago, J.-P.; Kucharczyk, R. Defining the Impact on Yeast ATP Synthase of Two Pathogenic Human Mitochondrial DNA Mutations, T9185C and T9191C. Biochimie 2014, 100, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães-Costa, R.; Villar-Quiles, R.-N.; Latour, P.; Sole, G.; Husson, I.; Lacour, A.; Leonard-Louis, S.; Stojkovic, T. Confounding Clinical Presentation and Different Disease Progression in CMT4B1. Neuromuscul. Disord. 2020, 30, 576–582. [Google Scholar] [CrossRef]
- Miller-Vedam, L.E.; Bräuning, B.; Popova, K.D.; Schirle Oakdale, N.T.; Bonnar, J.L.; Prabu, J.R.; Boydston, E.A.; Sevillano, N.; Shurtleff, M.J.; Stroud, R.M.; et al. Structural and Mechanistic Basis of the EMC-Dependent Biogenesis of Distinct Transmembrane Clients. ELife 2020, 9, e62611. [Google Scholar] [CrossRef] [PubMed]
- Vita, G.; Vita, G.L.; Stancanelli, C.; Gentile, L.; Russo, M.; Mazzeo, A. Genetic Neuromuscular Disorders: Living the Era of a Therapeutic Revolution. Part 1: Peripheral Neuropathies. Neurol. Sci. 2019, 40, 661–669. [Google Scholar] [CrossRef]
| Gene | Phenotypes | Atypical Clinical Features |
|---|---|---|
| BSCL2 | AD-CMT2 | pyramidal signs [18,22,24,25] respiratory dysfunction [18] sensory hearing loss [22] |
| MORC2 | AD-CMT2 | asymmetric proximal weakness [28] pyramidal signs [28] cerebellar atrophy [28] diaphragmatic paralysis [28] tongue atrophy [28] mental retardation [28] spinal cord atrophy [28] |
| HINT1 | AD-CMT2 | Neuromyotonia [31,32] neuro-psychiatric symptoms [32,33] |
| LITAF | AD-CMT1 | young age of onset [36] foot deformities [35,37] plantar ulcers [35,37] scoliosis [35,37] |
| GARS1 | AD-CMT2 | prominent distal upper limb involvement [16] |
| GDAP1 | AR-CMT1/AD-CMT2 | early and rapidly progressive phenotype (AR-CMT1) [41] |
| Family | Gene Reference Transcript | Variant (cDNA Protein) | Allele ID | Genotype | Classification (ACMG 2015 Guidelines) | ACMG/AMP Criteria Codes | Variant First Reported by: |
|---|---|---|---|---|---|---|---|
| 1, 2 | BSCL2 NM_001130702 | c.263A > G p.Asn88Ser | 931315 | htz | pathogenic | PS3, PM2, PP3, PP5 | [19] |
| 3 | MORC2 NM_014941.3 | c.1503A > T p.Gln501His | n.a. | htz | Uncertain significance | PM2 | Present report |
| 4 | HINT1 NM_005340.6 | c.110G > C p.Arg37Pro | 45887 | hmz | pathogenic | PVS1, PS3, PM1, PM2, PP2, PP3, PP5 | [45] |
| 5 | LITAF NM_001136472.1 | c.404C > G p.Pro135Arg | 204476 | htz | Uncertain significance/likely pathogenic | PM2, PP3 | [35] |
| 6 | GARS1 NM_002047 | c.1660G > A p.Asp554Asn | 24247 | htz | Uncertain significance/likely pathogenic | PS3 | [39] |
| 7, 8 | GDAP1 NM_018972 | c.374G > A p.Arg125Gln | 315143 | htz | Likely pathogenic | PM1, PM2, PP2, PP3 | Present report |
| Family | Gene | Variant | Patient | Age (yrs) | Weakness | Sensory Deficit | Reflexes | Unusual Features | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UL | LL | UL | LL | UL | LL | ||||||
| 1 | BSCL2 | c.263A > G p.Asn88Ser | 1.1 (p) | 39 | ++ | / | / | / | +++ | ++ | Pyramidal signs |
| 1.2 | 43 | / | + | / | ++ | ++ | ++ | Pyramidal signs | |||
| 1.3 | 48 | + | + | / | / | / | +++ | pigmentosus retinitis, bilateral cataract, pyramidal signs | |||
| 1.4 | 16 | / | / | / | / | / | / | bilateral sensory hypoacusia | |||
| 1.5 | 74 | / | / | / | / | / | / | / | |||
| 2 | BSCL2 | c.263A > G p.Asn88Ser | 2.1 (p) | 16 | +++ | + | / | / | / | ++ | Proximal UL weakness, pyramidal signs |
| 2.2 | 43 | / | + | / | / | / | + | N.A. | |||
| 2.3 | 72 | / | / | / | / | / | / | N.A. | |||
| 3 | MORC2 | c.1503A > T p.Gln501His | 3.1 | 54 | / | / | ++ | ++ | -- | --- | High CSF protein |
| 4 | HINT1 | c.110G > C p.Arg37Pro | 4.1 | 15 | ++ | +++ | / | / | --- | --- | Distal UL postural tremor |
| 5 | LITAF | c.404C > G p.Pro135Arg | 5.1 (p) | 41 | + | ++ | / | ++ | --- | --- | Distal UL postural tremor |
| 5.2 | 6 | / | + | / | / | / | -- | N.A. | |||
| 6 | GARS1 | c.1660G > A p.Asp554Asn | 6.1 (p) | 53 | / | + | / | / | -- | / | N.A. |
| 6.2 | 28 | / | / | / | / | / | / | N.A. | |||
| 6.3 | 14 | / | / | / | / | / | / | N.A. | |||
| 7 | GDAP1 | c.374G > A p.Arg125Gln | 7.1 | 52 | / | +++ | / | / | +++ | ++ | Proximal LL weakness, pyramidal signs |
| 8 | GDAP1 | c.374G > A p.Arg125Gln | 8.1 | 50 | / | ++ | / | ++ | +++ | / | Asymmetric LL weakness, optic nerve subatrophy, neurosensorial hypoacusia, pyramidal signs |
| Family | Gene | Variant | Pt. | Neurophysiologic Phenotype | Ulnar Motor | Ulnar Sensitive | Peroneal | Sural | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amplitude | Velocity | Onset Latency | Amplitude | Velocity | Onset Latency | Amplitude | Velocity | Onset Latency | Amplitude | Velocity | |||||
| 1 | BSCL2 | c.263A > G p.Asn88Ser | 1.1 (p) | Axonal | 10 | 57 | 2.4 | 48 | 61 | 2 | 0 | 0 | 0 | 8 | 61 |
| 1.2 | Axonal | 12 | 69 | 2.3 | 79 | 71 | 2.1 | 2 | 41 | 6 | 28 | 46 | |||
| 1.3 | Axonal | 14 | 57 | 2.2 | 59 | 86 | 2 | 3 | 46 | 6.2 | 6 | 40 | |||
| 1.4 | Axonal | 14 | 55 | 2.8 | 44 | 60 | 5.1 | 1 | 39 | 5.2 | 27 | 59 | |||
| 1.5 | / | ||||||||||||||
| 2 | BSCL2 | c.263A > G p.Asn88Ser | 2.1 (p) | Axonal | 18 | 58 | 2.6 | 93 | 63 | 2.2 | 1 | 40 | 4 | 12 | 45 |
| 2.2 | Axonal | 6 | 51 | 3.4 | 20 | 50 | 3 | 0 | 0 | 0 | 11 | 44 | |||
| 2.3 | / | ||||||||||||||
| 3 | MORC2 | c.1503A > T p.Gln501His | 3.1 | Intermediate | 13 | 33 | 5.3 | 10 | 36 | 5.5 | 3 | 22 | 9.7 | 0 | 0 |
| 4 | HINT1 | c.110G > C p.Arg37Pro | 4.1 | Intermediate | 10 | 45 | 4.1 | 35 | 55 | 2.1 | 1 | 34 | 7.7 | 5 | 33 |
| 5 | LITAF | c.404C > G p.Pro135Arg | 5.1 (p) | Demyelinating | 9 | 31 | 6.8 | 12 | 37 | 5.3 | 6 | 22 | 8.6 | 0 | 0 |
| 5.2 | / | ||||||||||||||
| 6 | GARS1 | c.1660G > A p.Asp554Asn | 6.1 (p) | Axonal | 5 | 52 | 4.9 | 24 | 65 | 3.5 | 0 | 0 | 0 | 12 | 45 |
| 6.2 | / | ||||||||||||||
| 6.3 | / | ||||||||||||||
| 7 | GDAP1 | c.374G > A p.Arg125Gln | 7.1 | Axonal | 3 | 39 | 4.8 | 48 | 61 | 2 | 0 | 0 | 0 | 8 | 61 |
| 8 | GDAP1 | c.374G > A p.Arg125Gln | 8.1 | Axonal | 9 | 48 | 4.1 | 44 | 60 | 5.1 | 0 | 0 | 0 | 27 | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, L.; Russo, M.; Taioli, F.; Ferrarini, M.; Aguennouz, M.; Rodolico, C.; Toscano, A.; Fabrizi, G.M.; Mazzeo, A. Rare among Rare: Phenotypes of Uncommon CMT Genotypes. Brain Sci. 2021, 11, 1616. https://doi.org/10.3390/brainsci11121616
Gentile L, Russo M, Taioli F, Ferrarini M, Aguennouz M, Rodolico C, Toscano A, Fabrizi GM, Mazzeo A. Rare among Rare: Phenotypes of Uncommon CMT Genotypes. Brain Sciences. 2021; 11(12):1616. https://doi.org/10.3390/brainsci11121616
Chicago/Turabian StyleGentile, Luca, Massimo Russo, Federica Taioli, Moreno Ferrarini, M’Hammed Aguennouz, Carmelo Rodolico, Antonio Toscano, Gian Maria Fabrizi, and Anna Mazzeo. 2021. "Rare among Rare: Phenotypes of Uncommon CMT Genotypes" Brain Sciences 11, no. 12: 1616. https://doi.org/10.3390/brainsci11121616







