Cerebrolysin Prevents Brain Injury in a Mouse Model of Liver Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis Induction
2.3. Clinical Evaluation and Behavior Testing
2.4. Abdominal Ultrasonography
2.5. Histopathology and Immunohistochemistry
2.6. Statistical Analysis
3. Results
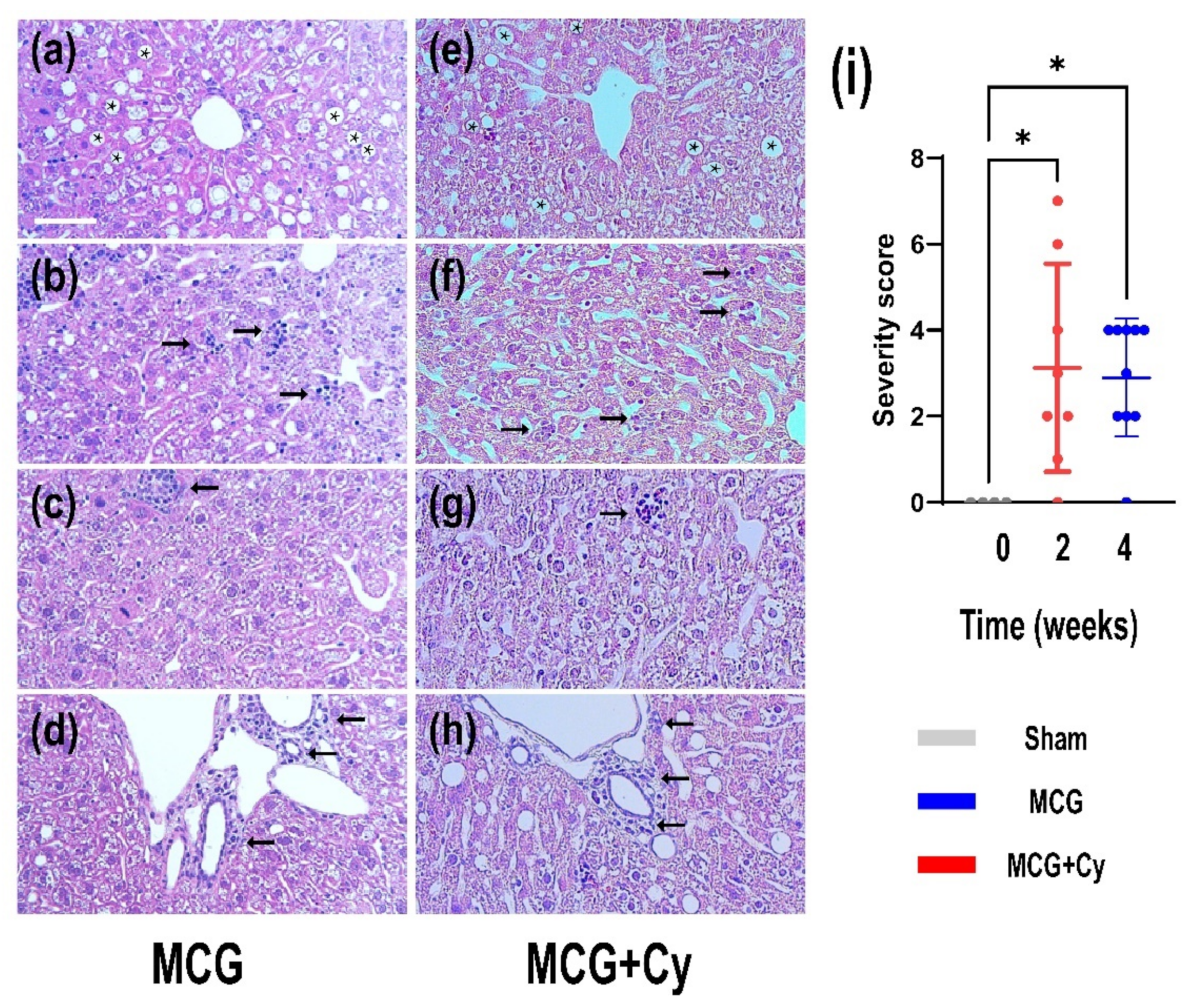
3.1. Cerebrolysyne Does Not Worsened Hepatic Damage Induced by MCD Food
3.2. Cerebrolysine Does Not Improve Bevaivior in Animals Feed MCD Food
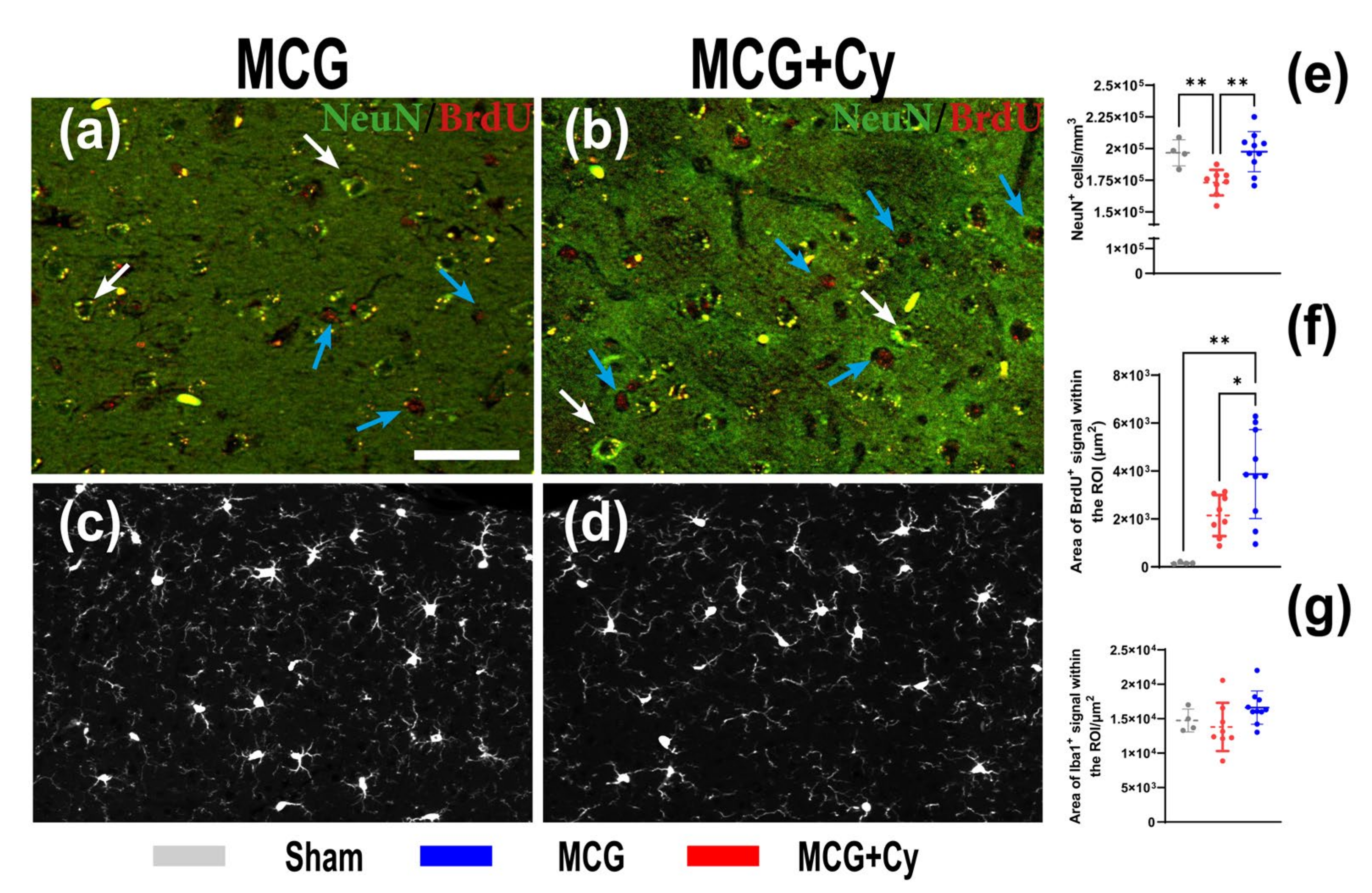
3.3. Cerebrolysin Improves Neuronal Survivability but Has No Impact on Neuroinflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Rose, C.F.; Amodio, P.; Bajaj, J.S.; Dhiman, R.K.; Montagnese, S.; Taylor-Robinson, S.D.; Vilstrup, H.; Jalan, R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J. Hepatol. 2020, 73, 1526–1547. [Google Scholar] [CrossRef]
- Sureka, B.; Bansal, K.; Patidar, Y.; Rajesh, S.; Mukund, A.; Arora, A. Neurologic Manifestations of Chronic Liver Disease and Liver Cirrhosis. Curr. Probl. Diagn. Radiol. 2015, 44, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; O’Leary, J.G.; Tandon, P.; Wong, F.; Garcia-Tsao, G.; Kamath, P.S.; Maliakkal, B.; Biggins, S.W.; Thuluvath, P.J.; Fallon, M.B.; et al. Hepatic Encephalopathy Is Associated With Mortality in Patients With Cirrhosis Independent of Other Extrahepatic Organ Failures. Clin. Gastroenterol. Hepatol. 2017, 15, 565–574.e4. [Google Scholar] [CrossRef]
- Weinstein, G.; Zelber-Sagi, S.; Preis, S.R.; Beiser, A.; DeCarli, C.; Speliotes, E.K.; Satizabal, C.L.; Vasan, R.S.; Seshadri, S. Association of Nonalcoholic Fatty Liver Disease With Lower Brain Volume in Healthy Middle-aged Adults in the Framingham Study. JAMA Neurol. 2018, 75, 97–104. [Google Scholar] [CrossRef]
- Sivell, C. Nonalcoholic Fatty Liver Disease: A Silent Epidemic. Gastroenterol. Nurs. 2019, 42, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among a Largely Middle-Aged Population Utilizing Ultrasound and Liver Biopsy: A Prospective Study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef]
- Lacaille, F.; Zylberberg, H.; Hagège, H.; Roualdès, B.; Meyrignac, C.; Chousterman, M.; Girot, R. Hepatitis C associated with Guillain-Barré syndrome. Liver Int. 2008, 18, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Chlilek, A.; Roger, C.; Muller, L.; Carles, M.-J.; Stephan, R.; Laureillard, D.; Lavigne, J.-P.; Lefrant, J.-Y.; Sotto, A. Severe Guillain-Barré syndrome associated with chronic active hepatitis C and mixed cryoglobulinemia: A case report. BMC Infect. Dis. 2019, 19, 636. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Seshadri, S.; Beiser, A.; Au, R.; Himali, J.J.; Palumbo, C.; Wolf, P.A.; DeCarli, C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011, 77, 461–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorelick, P.B.; Scuteri, A.; Black, S.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular Contributions to Cognitive Impairment and Dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.H. Blood–brain barrier in acute liver failure. Neurochem. Int. 2012, 60, 676–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero, F.; Fernandez, A.; Matías, N.; Martínez, L.; Fucho, R.; Elena, M.; Caballeria, J.; Morales, A.; Fernández-Checa, J.C.; García-Ruiz, C. Specific Contribution of Methionine and Choline in Nutritional Nonalcoholic Steatohepatitis: Impact on mitochondrial s-adenosyl-l-methionine and glutathione. J. Biol. Chem. 2010, 285, 18528–18536. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, Y.; Ozdogan, O. Liver disease as a risk factor for cognitive decline and dementia: An Under-recognized issue. Hepatology 2009, 49, 698. [Google Scholar] [CrossRef]
- Long, M.T.; Wang, N.; Larson, M.G.; Mitchell, G.F.; Palmisano, J.; Vasan, R.S.; Hoffmann, U.; Speliotes, E.K.; Vita, J.A.; Benjamin, E.J.; et al. Nonalcoholic Fatty Liver Disease and Vascular Function: Cross-sectional analysis in the Framingham heart study. Arter. Thromb. Vasc. Biol. 2015, 35, 1284–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, N.; Häring, H.-U. The role of hepatokines in metabolism. Nat. Rev. Endocrinol. 2013, 9, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Howdle, P. The neurology of liver failure. QJM Int. J. Med. 2003, 96, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Ardizzone, G.; Arrigo, A.; Schellino, M.; Stratta, C.; Valzan, S.; Skurzak, S.; Andruetto, P.; Panio, A.; Ballaris, M.; Lavezzo, B.; et al. Neurological Complications of Liver Cirrhosis and Orthotopic Liver Transplant. Transplant. Proc. 2006, 38, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.J.; Fishman, J.A. Central Nervous System Syndromes in Solid Organ Transplant Recipients. Clin. Infect. Dis. 2014, 59, 1001–1011. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Kang, J.K.; Lee, S.A.; Kim, K.H.; Lee, S.G.; Andermann, F. New-Onset Seizures after Liver Transplantation: Clinical Implications and Prognosis in Survivors. Eur. Neurol. 2004, 52, 230–236. [Google Scholar] [CrossRef]
- Dhar, R.; Young, G.B.; Marotta, P. Perioperative Neurological Complications After Liver Transplantation are Best Predicted by Pre-transplant Hepatic Encephalopathy. Neurocritical Care 2008, 8, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Buis, C.I.; Wiesner, R.H.; Krom, R.A.; Kremers, W.K.; Wijdicks, E.F. Acute confusional state following liver transplantation for alcoholic liver disease. Neurology 2002, 59, 601–605. [Google Scholar] [CrossRef]
- Filipović, B.; Marković, O.; Đurić, V.; Filipović, B. Cognitive Changes and Brain Volume Reduction in Patients with Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9638797. [Google Scholar] [CrossRef]
- Catalin, B.; Rogoveanu, O.-C.; Pirici, I.; Balseanu, T.A.; Stan, A.; Tudorica, V.; Balea, M.; Mindrila, I.; Albu, C.V.; Mohamed, G.; et al. Cerebrolysin and Aquaporin 4 Inhibition Improve Pathological and Motor Recovery after Ischemic Stroke. CNS Neurol. Disord.-Drug Targets 2018, 17, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Fiani, B.; Chacon, D.; Jarrah, R.; Barthelmass, M.; Covarrubias, C. Neuroprotective strategies of cerebrolysin for the treatment of infants with neonatal hypoxic–ischemic encephalopathy. Acta Neurol. Belg. 2021, 121, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Toader, L.E.; Roşu, G.C.; Catalin, B.; Pirici, I.; Gîlceavă, I.C.; Albu, V.C.; Istrate-Ofiţeru, A.M.; Mureşanu, D.F.; Pirici, D. Cerebrolysin increases motor recovery and decreases inflammation in a mouse model of autoimmune encephalitis. Rom. J. Morphol. Embryol. 2018, 59, 755–762. [Google Scholar]
- Ziganshina, L.E.; Abakumova, T.; Vernay, L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst. Rev. 2017, 4, CD007026. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.J.; Choi, H.S.; Park, S.Y.; Kim, D.Y. Effects of Cerebrolysin® in Patients With Minimally Conscious State After Stroke: An Observational Retrospective Clinical Study. Front. Neurol. 2019, 10, 803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chopp, M.; Cui, Y.; Wang, L.; Zhang, R.; Zhang, L.; Lu, M.; Szalad, A.; Doppler, E.; Hitzl, M.; et al. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J. Neurosci. Res. 2010, 88, 3275–3281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Tung, Y.-C.; Li, B.; Iqbal, K.; Grundke-Iqbal, I. Trophic factors counteract elevated FGF-2-induced inhibition of adult neurogenesis. Neurobiol. Aging 2007, 28, 1148–1162. [Google Scholar] [CrossRef]
- Hartbauer, M.; Hutter-Paier, B.; Skofitsch, G.; Windisch, M. Antiapoptotic effects of the peptidergic drug Cerebrolysin on primary cultures of embryonic chick cortical neurons. J. Neural Transm. 2001, 108, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chopp, M.; Meier, D.H.; Winter, S.; Wang, L.; Szalad, A.; Lu, M.; Wei, M.; Cui, Y.; Zhang, Z.G. Sonic Hedgehog Signaling Pathway Mediates Cerebrolysin-Improved Neurological Function After Stroke. Stroke 2013, 44, 1965–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, Y.; Kondo, T.; Kanazawa, A.; Itou, T.; Mizuno, Y. Protective effect of FPF 1070 (cerebrolysin) on delayed neuronal death in the gerbil--detection of hydroxyl radicals with salicylic acid. No Shinkei 1993, 45, 325–331. [Google Scholar]
- Itagaki, H.; Shimizu, K.; Morikawa, S.; Ogawa, K.; Ezaki, T. Morphological and functional characterization of non-alcoholic fatty liver disease induced by a methionine-choline-deficient diet in C57BL/6 mice. Int. J. Clin. Exp. Pathol. 2013, 6, 2683–2696. [Google Scholar] [PubMed]
- Rizki, G.; Arnaboldi, L.; Gabrielli, B.; Yan, J.; Lee, G.S.; Ng, R.K.; Turner, S.M.; Badger, T.M.; Pitas, R.E.; Maher, J.J. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J. Lipid Res. 2006, 47, 2280–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyman, R.L.; Giotas, C.; Medwadowski, B.; Miljanich, P. Effect of low methionine, choline deficient diets upon major unsaturated phosphatidyl choline fractions of rat liver and plasma. Lipids 1975, 10, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Cătălin, B.; Stopper, L.; Balseanu, T.-A.; Scheller, A. The in situ morphology of microglia is highly sensitive to the mode of tissue fixation. J. Chem. Neuroanat. 2017, 86, 59–66. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Surugiu, R.; Catalin, B.; Dumbrava, D.; Gresita, A.; Olaru, D.G.; Hermann, D.M.; Popa-Wagner, A. Intracortical Administration of the Complement C3 Receptor Antagonist Trifluoroacetate Modulates Microglia Reaction after Brain Injury. Neural Plast. 2019, 2019, 1071036. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zignego, A.L.; Craxì, A. Extrahepatic Manifestations of Hepatitis C Virus Infection. Clin. Liver Dis. 2008, 12, 611–636. [Google Scholar] [CrossRef] [PubMed]
- Felipo, V.; Montoliu, C.; Llansola, M. Neuroinflammation and neurological alterations in chronic liver diseases. Neuroimmunol. Neuroinflamm. 2015, 2, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Feltracco, P.; Cagnin, A.; Carollo, C.; Barbieri, S.; Ori, C. Neurological disorders in liver transplant candidates: Pathophysiology and clinical assessment. Transplant. Rev. 2017, 31, 193–206. [Google Scholar] [CrossRef]
- Kappus, M.R.; Sterling, R.K. Extrahepatic Manifestations of Acute Hepatitis B Virus Infection. Gastroenterol. Hepatol. 2013, 9, 123–126. [Google Scholar]
- Elwir, S.; Rahimi, R.S. Hepatic Encephalopathy: An Update on the Pathophysiology and Therapeutic Options. J. Clin. Transl. Hepatol. 2017, 5, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, B.C.; Sharma, P.; Lunia, M.K.; Srivastava, S.; Goyal, R.; Sarin, S.K. A Randomized, Double-Blind, Controlled Trial Comparing Rifaximin Plus Lactulose With Lactulose Alone in Treatment of Overt Hepatic Encephalopathy. Am. J. Gastroenterol. 2013, 108, 1458–1463. [Google Scholar] [CrossRef]
- Patel, N.; Yopp, A.C.; Singal, A.G. Diagnostic Delays Are Common Among Patients With Hepatocellular Carcinoma. J. Natl. Compr. Cancer Netw. 2015, 13, 543–549. [Google Scholar] [CrossRef]
- Bustamante, J.; Rimola, A.; Ventura, P.-J.; Navasa, M.; Cirera, I.; Reggiardo, V.; Rodés, J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J. Hepatol. 1999, 30, 890–895. [Google Scholar] [CrossRef]
- Chanubol, R.; Lertbutsayanukul, P. Role of Cerebrolysin® in Rehabilitation in Ischemic Stroke: A Case Report. Am. J. Case Rep. 2021, 22, e932365. [Google Scholar] [CrossRef]
- Álvarez, X.A.; Lombardi, V.R.M.; Fernández-Novoa, L.; García, M.; Sampedro, C.; Cagiao, A.; Cacabelos, R.; Windisch, M. Cerebrolysin® reduces microglial activation in vivo and in vitro: A potential mechanism of neuroprotection. Adv. Dement. Res. 2000, 59, 281–292. [Google Scholar] [CrossRef]
- Zamin, I., Jr.; Mattos, A.A.; Mattos, A.Z.; Migon, E.; Soares, E.; Perry, M.L. Modelo experimental de esteatohepatite não-alcoólica com dieta deficiente em metionina e colina. Arq. Gastroenterol. 2009, 46, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Satoh, A.; Tezuka, H.; Han, S.-I.; Takei, K.; Iwasaki, H.; Yatoh, S.; Yahagi, N.; Suzuki, H.; Iwasaki, Y.; et al. CREB3L3 controls fatty acid oxidation and ketogenesis in synergy with PPARα. Sci. Rep. 2016, 6, 39182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.-J.; Wang, Y.-H.; Wu, C.-Y.; Hsu, F.-Y.; Chien, C.-Y.; Lee, Y.-C. Ketogenic Diet Enhances the Cholesterol Accumulation in Liver and Augments the Severity of CCl4 and TAA-Induced Liver Fibrosis in Mice. Int. J. Mol. Sci. 2021, 22, 2934. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Ueno, T.; Sasaki, N.; Kuhara, K.; Yoshioka, S.; Tateishi, Y.; Nagata, E.; Kage, M.; Sata, M. Comparison of Liver Histology Between Patients with Non-Alcoholic Steatohepatitis and Patients with Alcoholic Steatohepatitis in Japan. Alcohol. Clin. Exp. Res. 2005, 29, 277S–281S. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bodenheimer, H.C., Jr.; Clain, D.J.; Min, A.D.; Theise, N.D. Hepatic lipogranulomas in patients with chronic liver disease: Association with hepatitis C and fatty liver disease. World J. Gastroenterol. 2010, 16, 5065–5069. [Google Scholar] [CrossRef]
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: Observational study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morega, S.; Cătălin, B.; Simionescu, C.E.; Sapalidis, K.; Rogoveanu, I. Cerebrolysin Prevents Brain Injury in a Mouse Model of Liver Damage. Brain Sci. 2021, 11, 1622. https://doi.org/10.3390/brainsci11121622
Morega S, Cătălin B, Simionescu CE, Sapalidis K, Rogoveanu I. Cerebrolysin Prevents Brain Injury in a Mouse Model of Liver Damage. Brain Sciences. 2021; 11(12):1622. https://doi.org/10.3390/brainsci11121622
Chicago/Turabian StyleMorega, Shandiz, Bogdan Cătălin, Cristiana Eugenia Simionescu, Konstantinos Sapalidis, and Ion Rogoveanu. 2021. "Cerebrolysin Prevents Brain Injury in a Mouse Model of Liver Damage" Brain Sciences 11, no. 12: 1622. https://doi.org/10.3390/brainsci11121622





