The Relationship between Body Composition, Fatty Acid Metabolism and Diet in Spinal Muscular Atrophy
Abstract
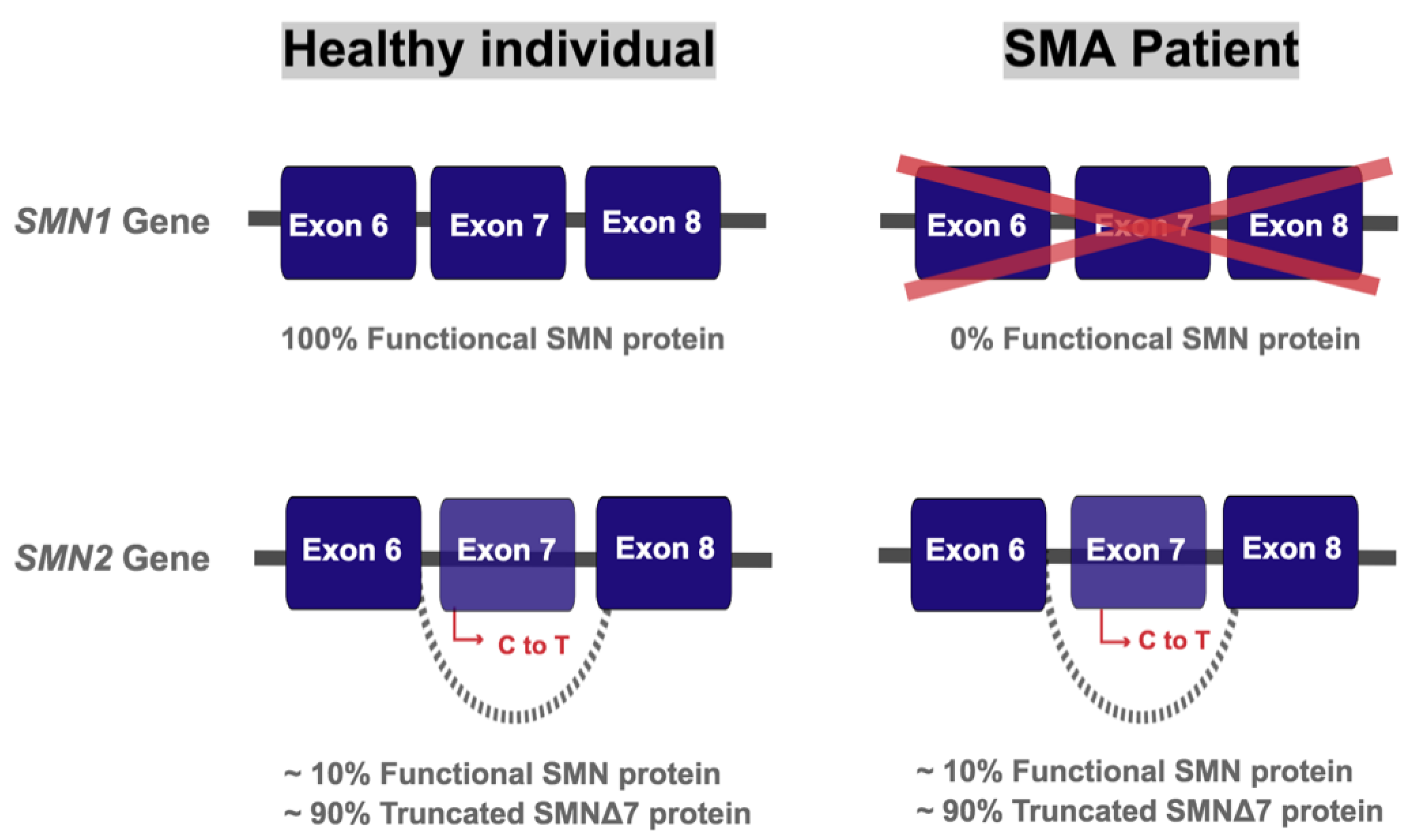
:1. Introduction
2. Body Composition of SMA Patients
3. Fatty Acid Oxidation and Metabolism
3.1. Basics of Fatty Acid Oxidation and Metabolism
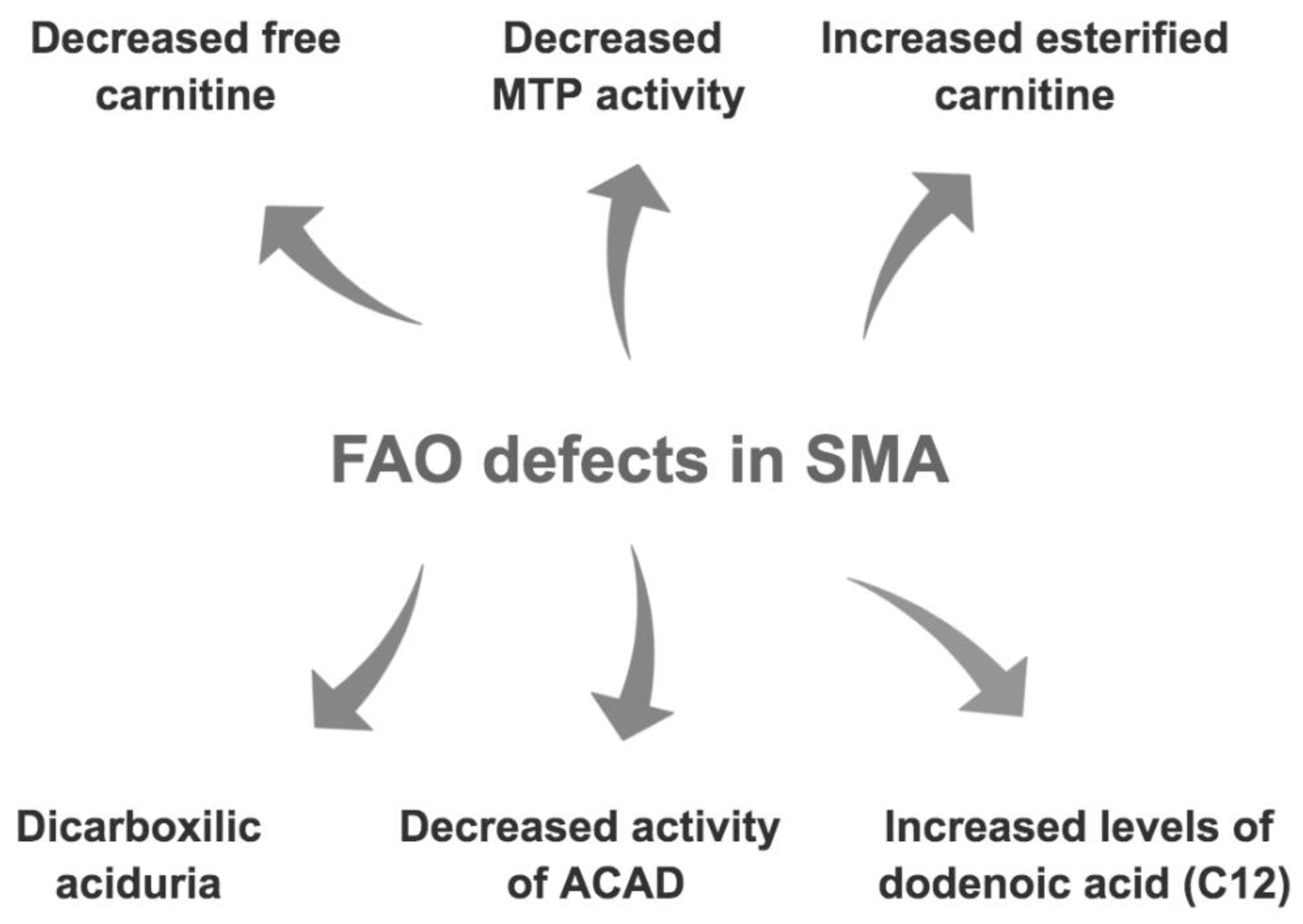
3.2. Fatty Acid Oxidation and Metabolism Defects in SMA
4. Nutrition and Fatty Acid Metabolism in SMA
4.1. Dietary Approaches Evaluated in SMA Mouse Models
4.2. Dietary Interventions in SMA Patients
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugarman, E.A.; Nagan, N.; Zhu, H.; Akmaev, V.R.; Zhou, Z.; Rohlfs, E.M.; Flynn, K.; Hendrickson, B.C.; Scholl, T.; Sirko-Osadsa, D.A.; et al. Pan-Ethnic Carrier Screening and Prenatal Diagnosis for Spinal Muscular Atrophy: Clinical Laboratory Analysis of >72,400 Specimens. Eur. J. Hum. Genet. EJHG 2012, 20, 27–32. [Google Scholar] [CrossRef]
- D’Amico, A.; Mercuri, E.; Tiziano, F.D.; Bertini, E. Spinal Muscular Atrophy. Orphanet J. Rare Dis. 2011, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Burghes, A.H.M.; Beattie, C.E. Spinal Muscular Atrophy: Why Do Low Levels of SMN Make Motor Neurons Sick? Nat. Rev. Neurosci. 2009, 10, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and Characterization of a Spinal Muscular Atrophy-Determining Gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Nurputra, D.K.; Lai, P.S.; Harahap, N.I.F.; Morikawa, S.; Yamamoto, T.; Nishimura, N.; Kubo, Y.; Takeuchi, A.; Saito, T.; Takeshima, Y.; et al. Spinal Muscular Atrophy: From Gene Discovery to Clinical Trials. Ann. Hum. Genet. 2013, 77, 435–463. [Google Scholar] [CrossRef]
- Feldkötter, M.; Schwarzer, V.; Wirth, R.; Wienker, T.F.; Wirth, B. Quantitative Analyses of SMN1 and SMN2 Based on Real-Time LightCycler PCR: Fast and Highly Reliable Carrier Testing and Prediction of Severity of Spinal Muscular Atrophy. Am. J. Hum. Genet. 2002, 70, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A Single Nucleotide in the SMN Gene Regulates Splicing and Is Responsible for Spinal Muscular Atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef] [Green Version]
- Cartegni, L.; Hastings, M.L.; Calarco, J.A.; de Stanchina, E.; Krainer, A.R. Determinants of Exon 7 Splicing in the Spinal Muscular Atrophy Genes, SMN1 and SMN2. Am. J. Hum. Genet. 2006, 78, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Lorson, M.A.; Lorson, C.L. SMN-Inducing Compounds for the Treatment of Spinal Muscular Atrophy. Future Med. Chem. 2012, 4, 2067–2084. [Google Scholar] [CrossRef] [Green Version]
- Prior, T.W.; Swoboda, K.J.; Scott, H.D.; Hejmanowski, A.Q. Homozygous SMN1 Deletions in Unaffected Family Members and Modification of the Phenotype by SMN2. Am. J. Med. Genet. A. 2004, 130A, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Butchbach, M.E.R. Copy Number Variations in the Survival Motor Neuron Genes: Implications for Spinal Muscular Atrophy and Other Neurodegenerative Diseases. Front. Mol. Biosci. 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russman, B.S. Spinal Muscular Atrophy: Clinical Classification and Disease Heterogeneity. J. Child Neurol. 2007, 22, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Lunn, M.R.; Wang, C.H. Spinal Muscular Atrophy. Lancet 2008, 371, 2120–2133. [Google Scholar] [CrossRef]
- Kostova, F.V.; Williams, V.C.; Heemskerk, J.; Iannaccone, S.; Didonato, C.; Swoboda, K.; Maria, B.L. Spinal Muscular Atrophy: Classification, Diagnosis, Management, Pathogenesis, and Future Research Directions. J. Child Neurol. 2007, 22, 926–945. [Google Scholar] [CrossRef]
- Qian, Y.; McGraw, S.; Henne, J.; Jarecki, J.; Hobby, K.; Yeh, W.-S. Understanding the Experiences and Needs of Individuals with Spinal Muscular Atrophy and Their Parents: A Qualitative Study. BMC Neurol. 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Sproule, D.M.; Kaufmann, P. Therapeutic Developments in Spinal Muscular Atrophy. Ther. Adv. Neurol. Disord. 2010, 3, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Arnold, W.D.; Kassar, D.; Kissel, J.T. Spinal Muscular Atrophy: Diagnosis and Management in a New Therapeutic Era. Muscle Nerve 2015, 51, 157–167. [Google Scholar] [CrossRef]
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef] [Green Version]
- Wirth, B. Spinal Muscular Atrophy: In the Challenge Lies a Solution. Trends Neurosci. 2021. [Google Scholar] [CrossRef]
- Monani, U.R.; Sendtner, M.; Coovert, D.D.; Parsons, D.W.; Andreassi, C.; Le, T.T.; Jablonka, S.; Schrank, B.; Rossoll, W.; Rossol, W.; et al. The Human Centromeric Survival Motor Neuron Gene (SMN2) Rescues Embryonic Lethality in Smn(-/-) Mice and Results in a Mouse with Spinal Muscular Atrophy. Hum. Mol. Genet. 2000, 9, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, T.W. The Spliceosome: The Most Complex Macromolecular Machine in the Cell? BioEssays News Rev. Mol. Cell. Dev. Biol. 2003, 25, 1147–1149. [Google Scholar] [CrossRef]
- Shababi, M.; Lorson, C.L.; Rudnik-Schöneborn, S.S. Spinal Muscular Atrophy: A Motor Neuron Disorder or a Multi-Organ Disease? J. Anat. 2014, 224, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, K.; Tiuca, I.-D. Importance of Fatty Acids in Physiopathology of Human Body. Fat. Acids 2017. [Google Scholar] [CrossRef] [Green Version]
- Bertoli, S.; De Amicis, R.; Mastella, C.; Pieri, G.; Giaquinto, E.; Battezzati, A.; Leone, A.; Baranello, G. Spinal Muscular Atrophy, Types I and II: What Are the Differences in Body Composition and Resting Energy Expenditure? Clin. Nutr. Edinb. Scotl. 2017, 36, 1674–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poruk, K.E.; Davis, R.H.; Smart, A.L.; Chisum, B.S.; Lasalle, B.A.; Chan, G.M.; Gill, G.; Reyna, S.P.; Swoboda, K.J. Observational Study of Caloric and Nutrient Intake, Bone Density, and Body Composition in Infants and Children with Spinal Muscular Atrophy Type I. Neuromuscul. Disord. NMD 2012, 22, 966–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproule, D.M.; Montes, J.; Montgomery, M.; Battista, V.; Koenigsberger, D.; Shen, W.; Punyanitya, M.; De Vivo, D.C.; Kaufmann, P. Increased Fat Mass and High Incidence of Overweight despite Low Body Mass Index in Patients with Spinal Muscular Atrophy. Neuromuscul. Disord. NMD 2009, 19, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, N.M.; Newman, H.; Tarrant, S.; Graham, R.J. Nutritional Status and Nutrient Intake Challenges in Children with Spinal Muscular Atrophy. Pediatr. Neurol. 2016, 57, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Cutillo, L.; Pizziconi, C.; Tozzi, A.E.; Verrillo, E.; Testa, M.B.C.; Cutrera, R. Predicted and Measured Resting Energy Expenditure in Children with Spinal Muscular Atrophy 2. J. Pediatr. 2014, 164, 1228–1230. [Google Scholar] [CrossRef]
- Maretina, M.A.; Zheleznyakova, G.Y.; Lanko, K.M.; Egorova, A.A.; Baranov, V.S.; Kiselev, A.V. Molecular Factors Involved in Spinal Muscular Atrophy Pathways as Possible Disease-Modifying Candidates. Curr. Genom. 2018, 19, 339–355. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Triacylglycerols Are Highly Concentrated Energy Stores. 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK22369/ (accessed on 27 November 2020).
- Jo, Y.; Okazaki, H.; Moon, Y.-A.; Zhao, T. Regulation of Lipid Metabolism and Beyond. Int. J. Endocrinol. 2016, 2016. [Google Scholar] [CrossRef]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J.A. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hames, D.; Hooper, N. Nige BIOS Instant Notes in Biochemistry; Taylor & Francis: Abingdon, UK, 2011. [Google Scholar]
- Cooper, G.M.; Cooper, G.M. The Cell, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000; ISBN 978-0-87893-106-4. [Google Scholar]
- Casteels, M.; Foulon, V.; Mannaerts, G.P.; Van Veldhoven, P.P. Alpha-Oxidation of 3-Methyl-Substituted Fatty Acids and Its Thiamine Dependence. Eur. J. Biochem. 2003, 270, 1619–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanders, R.J.A.; Waterham, H.R.; Ferdinandusse, S. Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum. Front. Cell Dev. Biol. 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, R.-J.; Ofman, R.; Duran, M.; Kemp, S.; Wanders, R.J.A. Omega-Oxidation of Very Long-Chain Fatty Acids in Human Liver Microsomes. Implications for X-Linked Adrenoleukodystrophy. J. Biol. Chem. 2006, 281, 13180–13187. [Google Scholar] [CrossRef] [PubMed]
- Kompare, M.; Rizzo, W.B. Mitochondrial Fatty-Acid Oxidation Disorders. Semin. Pediatr. Neurol. 2008, 15, 140–149. [Google Scholar] [CrossRef]
- Kelley, R.I.; Sladky, J.T. Dicarboxylic Aciduria in an Infant with Spinal Muscular Atrophy. Ann. Neurol. 1986, 20, 734–736. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Harpey, J.P.; Charpentier, C.; Paturneau-Jouas, M.; Renault, F.; Romero, N.; Fardeau, M. Secondary Metabolic Defects in Spinal Muscular Atrophy Type II. Lancet 1990, 336, 629–630. [Google Scholar] [CrossRef]
- Tein, I.; Sloane, A.E.; Donner, E.J.; Lehotay, D.C.; Millington, D.S.; Kelley, R.I. Fatty Acid Oxidation Abnormalities in Childhood-Onset Spinal Muscular Atrophy: Primary or Secondary Defect(s)? Pediatr. Neurol. 1995, 12, 21–30. [Google Scholar] [CrossRef]
- Tein, I. Disorders of Fatty Acid Oxidation. Handb. Clin. Neurol. 2013, 113, 1675–1688. [Google Scholar] [CrossRef]
- Crawford, T.O.; Sladky, J.T.; Hurko, O.; Besner-Johnston, A.; Kelley, R.I. Abnormal Fatty Acid Metabolism in Childhood Spinal Muscular Atrophy. Ann. Neurol. 1999, 45, 337–343. [Google Scholar] [CrossRef]
- Costa, C.C.; de Almeida, I.T.; Jakobs, C.; Poll-The, B.T.; Duran, M. Dynamic Changes of Plasma Acylcarnitine Levels Induced by Fasting and Sunflower Oil Challenge Test in Children. Pediatr. Res. 1999, 46, 440–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, R.R. Metabolic Interactions between Glucose and Fatty Acids in Humans. Am. J. Clin. Nutr. 1998, 67, 519S–526S. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of Carnitine in Disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bebee, T.W.; Dominguez, C.E.; Chandler, D.S. Mouse Models of SMA: Tools for Disease Characterization and Therapeutic Development. Hum. Genet. 2012, 131, 1277–1293. [Google Scholar] [CrossRef]
- Deguise, M.-O.; Baranello, G.; Mastella, C.; Beauvais, A.; Michaud, J.; Leone, A.; De Amicis, R.; Battezzati, A.; Dunham, C.; Selby, K.; et al. Abnormal Fatty Acid Metabolism Is a Core Component of Spinal Muscular Atrophy. Ann. Clin. Transl. Neurol. 2019, 6, 1519–1532. [Google Scholar] [CrossRef] [Green Version]
- Butchbach, M.E.R.; Rose, F.F.; Rhoades, S.; Marston, J.; McCrone, J.T.; Sinnott, R.; Lorson, C.L. Effect of Diet on the Survival and Phenotype of a Mouse Model for Spinal Muscular Atrophy. Biochem. Biophys. Res. Commun. 2010, 391, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Kelishadi, R.; Hadi, B.; Iranpour, R.; Khosravi-Darani, K.; Mirmoghtadaee, P.; Farajian, S.; Poursafa, P. A Study on Lipid Content and Fatty Acid of Breast Milk and Its Association with Mother’s Diet Composition. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012, 17, 824–827. [Google Scholar]
- Scopesi, F.; Ciangherotti, S.; Lantieri, P.B.; Risso, D.; Bertini, I.; Campone, F.; Pedrotti, A.; Bonacci, W.; Serra, G. Maternal Dietary PUFAs Intake and Human Milk Content Relationships during the First Month of Lactation. Clin. Nutr. Edinb. Scotl. 2001, 20, 393–397. [Google Scholar] [CrossRef]
- Deguise, M.-O.; Chehade, L.; Tierney, A.; Beauvais, A.; Kothary, R. Low Fat Diets Increase Survival of a Mouse Model of Spinal Muscular Atrophy. Ann. Clin. Transl. Neurol. 2019, 6, 2340–2346. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The Mechanisms of Pharmacokinetic Food-Drug Interactions—A Perspective from the UNGAP Group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Narver, H.L.; Kong, L.; Burnett, B.G.; Choe, D.W.; Bosch-Marcé, M.; Taye, A.A.; Eckhaus, M.A.; Sumner, C.J. Sustained Improvement of Spinal Muscular Atrophy Mice Treated with Trichostatin A plus Nutrition. Ann. Neurol. 2008, 64, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Sumner, C.J.; Paushkin, S.; Ko, C.-P. Spinal Muscular Atrophy: Disease Mechanisms and Therapy; Academic Press: Cambridge, MA, USA, 2016; ISBN 978-0-12-803686-0. [Google Scholar]
- Moore, G.E.; Lindenmayer, A.W.; McConchie, G.A.; Ryan, M.M.; Davidson, Z.E. Describing Nutrition in Spinal Muscular Atrophy: A Systematic Review. Neuromuscul. Disord. NMD 2016, 26, 395–404. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and Management of Spinal Muscular Atrophy: Part 1: Recommendations for Diagnosis, Rehabilitation, Orthopedic and Nutritional Care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Russell, R.I.; Hall, M.J. Elemental Diet Therapy in the Management of Complicated Crohn’s Disease. Scott. Med. J. 1979, 24, 291–295. [Google Scholar] [CrossRef]
- Horiuchi, A.; Nakayama, Y.; Sakai, R.; Suzuki, M.; Kajiyama, M.; Tanaka, N. Elemental Diets May Reduce the Risk of Aspiration Pneumonia in Bedridden Gastrostomy-Fed Patients. Am. J. Gastroenterol. 2013, 108, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.H.; Godshall, B.J.; Seffrood, E.; Marcus, M.; LaSalle, B.A.; Wong, B.; Schroth, M.K.; Swoboda, K.J. Nutritional Practices at a Glance: Spinal Muscular Atrophy Type I Nutrition Survey Findings. J. Child Neurol. 2014, 29, 1467–1472. [Google Scholar] [CrossRef] [Green Version]
- Farthing, M.J.; Jarrett, E.B.; Williams, G.; Crawford, M.A. Essential Fatty Acid Deficiency after Prolonged Treatment with Elemental Diet. Lancet 1980, 2, 1088–1089. [Google Scholar] [CrossRef]
- Boyer, J.G.; Murray, L.M.; Scott, K.; De Repentigny, Y.; Renaud, J.-M.; Kothary, R. Early Onset Muscle Weakness and Disruption of Muscle Proteins in Mouse Models of Spinal Muscular Atrophy. Skelet. Muscle 2013, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Walter, L.M.; Koch, C.E.; Betts, C.A.; Ahlskog, N.; Meijboom, K.E.; van Westering, T.L.E.; Hazell, G.; Bhomra, A.; Claus, P.; Oster, H.; et al. Light Modulation Ameliorates Expression of Circadian Genes and Disease Progression in Spinal Muscular Atrophy Mice. Hum. Mol. Genet. 2018. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.N.; Howell, M.D.; Ottesen, E.W.; Singh, N.N. Diverse Role of Survival Motor Neuron Protein. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 299–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowerman, M.; Shafey, D.; Kothary, R. Smn Depletion Alters Profilin II Expression and Leads to Upregulation of the RhoA/ROCK Pathway and Defects in Neuronal Integrity. J. Mol. Neurosci. MN 2007, 32, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Oprea, G.E.; Kröber, S.; McWhorter, M.L.; Rossoll, W.; Müller, S.; Krawczak, M.; Bassell, G.J.; Beattie, C.E.; Wirth, B. Plastin 3 Is a Protective Modifier of Autosomal Recessive Spinal Muscular Atrophy. Science 2008, 320, 524–527. [Google Scholar] [CrossRef] [Green Version]
- Janzen, E.; Mendoza-Ferreira, N.; Hosseinibarkooie, S.; Schneider, S.; Hupperich, K.; Tschanz, T.; Grysko, V.; Riessland, M.; Hammerschmidt, M.; Rigo, F.; et al. CHP1 Reduction Ameliorates Spinal Muscular Atrophy Pathology by Restoring Calcineurin Activity and Endocytosis. Brain J. Neurol. 2018, 141, 2343–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, L.M.; Deguise, M.-O.; Meijboom, K.E.; Betts, C.A.; Ahlskog, N.; van Westering, T.L.E.; Hazell, G.; McFall, E.; Kordala, A.; Hammond, S.M.; et al. Interventions Targeting Glucocorticoid-Krüppel-like Factor 15-Branched-Chain Amino Acid Signaling Improve Disease Phenotypes in Spinal Muscular Atrophy Mice. EBioMedicine 2018, 31, 226–242. [Google Scholar] [CrossRef]
- Klingenspor, M.; Xu, P.; Cohen, R.D.; Welch, C.; Reue, K. Altered Gene Expression Pattern in the Fatty Liver Dystrophy Mouse Reveals Impaired Insulin-Mediated Cytoskeleton Dynamics. J. Biol. Chem. 1999, 274, 23078–23084. [Google Scholar] [CrossRef] [Green Version]
- Bertolio, R.; Napoletano, F.; Mano, M.; Maurer-Stroh, S.; Fantuz, M.; Zannini, A.; Bicciato, S.; Sorrentino, G.; Del Sal, G. Sterol Regulatory Element Binding Protein 1 Couples Mechanical Cues and Lipid Metabolism. Nat. Commun. 2019, 10, 1326. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Zheng, Y.; Cho, S.; Jang, C.; England, C.; Dempsey, J.M.; Yu, Y.; Liu, X.; He, L.; Cavaliere, P.M.; et al. Post-Transcriptional Regulation of De Novo Lipogenesis by MTORC1-S6K1-SRPK2 Signaling. Cell 2017, 171, 1545.e18–1558.e18. [Google Scholar] [CrossRef] [Green Version]
- Prosdocimo, D.A.; John, J.E.; Zhang, L.; Efraim, E.S.; Zhang, R.; Liao, X.; Jain, M.K. KLF15 and PPARα Cooperate to Regulate Cardiomyocyte Lipid Gene Expression and Oxidation. PPAR Res. 2015, 2015, 201625. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, D.P.; Forbes, S.; Walker, B.R. Glucocorticoids and Fatty Acid Metabolism in Humans: Fuelling Fat Redistribution in the Metabolic Syndrome. J. Endocrinol. 2008, 197, 189–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.G.; Nicholson Puthenveedu, S.; Shen, Y.; La, K.; Ozlu, C.; Wang, T.; Klompstra, D.; Gultekin, Y.; Chi, J.; Fidelin, J.; et al. CHP1 Regulates Compartmentalized Glycerolipid Synthesis by Activating GPAT4. Mol. Cell 2019, 74, 45.e7–58.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.H.; Miller, E.A.; Zhang, R.Z.; Swoboda, K.J. Responses to Fasting and Glucose Loading in a Cohort of Well Children with Spinal Muscular Atrophy Type II. J. Pediatr. 2015, 167, 1362.e1–1368.e1. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, M.; Swoboda, K.J.; Michalski, J.-P.; Wang, G.-S.; Reeks, C.; Beauvais, A.; Murphy, K.; Woulfe, J.; Screaton, R.A.; Scott, F.W.; et al. Glucose Metabolism and Pancreatic Defects in Spinal Muscular Atrophy. Ann. Neurol. 2012, 72, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Bowerman, M.; Becker, C.G.; Yáñez-Muñoz, R.J.; Ning, K.; Wood, M.J.A.; Gillingwater, T.H.; Talbot, K. Therapeutic Strategies for Spinal Muscular Atrophy: SMN and Beyond. Dis. Model. Mech. 2017, 10, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Tefera, T.W.; Borges, K. Metabolic Dysfunctions in Amyotrophic Lateral Sclerosis Pathogenesis and Potential Metabolic Treatments. Front. Neurosci. 2016, 10, 611. [Google Scholar] [CrossRef]
- Bogie, J.F.J.; Haidar, M.; Kooij, G.; Hendriks, J.J.A. Fatty Acid Metabolism in the Progression and Resolution of CNS Disorders. Adv. Drug Deliv. Rev. 2020, 159, 198–213. [Google Scholar] [CrossRef]
- Bowerman, M.; Murray, L.M.; Scamps, F.; Schneider, B.L.; Kothary, R.; Raoul, C. Pathogenic Commonalities between Spinal Muscular Atrophy and Amyotrophic Lateral Sclerosis: Converging Roads to Therapeutic Development. Eur. J. Med. Genet. 2018, 61, 685–698. [Google Scholar] [CrossRef]
- Steyn, F.J.; Li, R.; Kirk, S.E.; Tefera, T.W.; Xie, T.Y.; Tracey, T.J.; Kelk, D.; Wimberger, E.; Garton, F.C.; Roberts, L.; et al. Altered Skeletal Muscle Glucose-Fatty Acid Flux in Amyotrophic Lateral Sclerosis. Brain Commun. 2020, 2, fcaa154. [Google Scholar] [CrossRef]
- González De Aguilar, J.-L. Lipid Biomarkers for Amyotrophic Lateral Sclerosis. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Chaves-Filho, A.B.; Pinto, I.F.D.; Dantas, L.S.; Xavier, A.M.; Inague, A.; Faria, R.L.; Medeiros, M.H.G.; Glezer, I.; Yoshinaga, M.Y.; Miyamoto, S. Alterations in Lipid Metabolism of Spinal Cord Linked to Amyotrophic Lateral Sclerosis. Sci. Rep. 2019, 9, 11642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szelechowski, M.; Amoedo, N.; Obre, E.; Léger, C.; Allard, L.; Bonneu, M.; Claverol, S.; Lacombe, D.; Oliet, S.; Chevallier, S.; et al. Metabolic Reprogramming in Amyotrophic Lateral Sclerosis. Sci. Rep. 2018, 8, 3953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, J.M.; Frahm, J.L.; Li, L.O.; Coleman, R.A. Acyl-Coenzyme A Synthetases in Metabolic Control. Curr. Opin. Lipidol. 2010, 21, 212–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashek, D.G.; Li, L.O.; Coleman, R.A. Long-Chain Acyl-CoA Synthetases and Fatty Acid Channeling. Future Lipidol. 2007, 2, 465–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacoangeli, A.; Lin, T.; Khleifat, A.A.; Jones, A.R.; Opie-Martin, S.; Coleman, J.R.I.; Shatunov, A.; Sproviero, W.; Williams, K.L.; Garton, F.; et al. Genome-Wide Meta-Analysis Finds the ACSL5-ZDHHC6 Locus Is Associated with ALS and Links Weight Loss to the Disease Genetics. Cell Rep. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Wills, A.-M.; Hubbard, J.; Macklin, E.A.; Glass, J.; Tandan, R.; Simpson, E.P.; Brooks, B.; Gelinas, D.; Mitsumoto, H.; Mozaffar, T.; et al. Hypercaloric Enteral Nutrition in Patients with Amyotrophic Lateral Sclerosis: A Randomised, Double-Blind, Placebo-Controlled Phase 2 Trial. Lancet 2014, 383, 2065–2072. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, L.; Oudart, H.; René, F.; Gonzalez de Aguilar, J.-L.; Loeffler, J.-P. Evidence for Defective Energy Homeostasis in Amyotrophic Lateral Sclerosis: Benefit of a High-Energy Diet in a Transgenic Mouse Model. Proc. Natl. Acad. Sci. USA 2004, 101, 11159–11164. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Lange, D.J.; Voustianiouk, A.; MacGrogan, D.; Ho, L.; Suh, J.; Humala, N.; Thiyagarajan, M.; Wang, J.; Pasinetti, G.M. A Ketogenic Diet as a Potential Novel Therapeutic Intervention in Amyotrophic Lateral Sclerosis. BMC Neurosci. 2006, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Beghi, E.; Pupillo, E.; Bonito, V.; Buzzi, P.; Caponnetto, C.; Chiò, A.; Corbo, M.; Giannini, F.; Inghilleri, M.; Bella, V.L.; et al. Randomized Double-Blind Placebo-Controlled Trial of Acetyl-L-Carnitine for ALS. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 397–405. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, K.S.; Boukhloufi, I.; Bowerman, M.; Parson, S.H. The Relationship between Body Composition, Fatty Acid Metabolism and Diet in Spinal Muscular Atrophy. Brain Sci. 2021, 11, 131. https://doi.org/10.3390/brainsci11020131
Watson KS, Boukhloufi I, Bowerman M, Parson SH. The Relationship between Body Composition, Fatty Acid Metabolism and Diet in Spinal Muscular Atrophy. Brain Sciences. 2021; 11(2):131. https://doi.org/10.3390/brainsci11020131
Chicago/Turabian StyleWatson, Katherine S., Imane Boukhloufi, Melissa Bowerman, and Simon H. Parson. 2021. "The Relationship between Body Composition, Fatty Acid Metabolism and Diet in Spinal Muscular Atrophy" Brain Sciences 11, no. 2: 131. https://doi.org/10.3390/brainsci11020131
APA StyleWatson, K. S., Boukhloufi, I., Bowerman, M., & Parson, S. H. (2021). The Relationship between Body Composition, Fatty Acid Metabolism and Diet in Spinal Muscular Atrophy. Brain Sciences, 11(2), 131. https://doi.org/10.3390/brainsci11020131





