Elevated Circulating Microparticle Subpopulations in Incidental Cerebral White Matter Hyperintensities: A Multimodal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval, Sample Size Estimation, and Subject Recruitment
2.2. Neurocognitive Assessment
2.3. MPs Enumeration
2.4. Diffusion MRI Protocols
2.5. Image Analysis
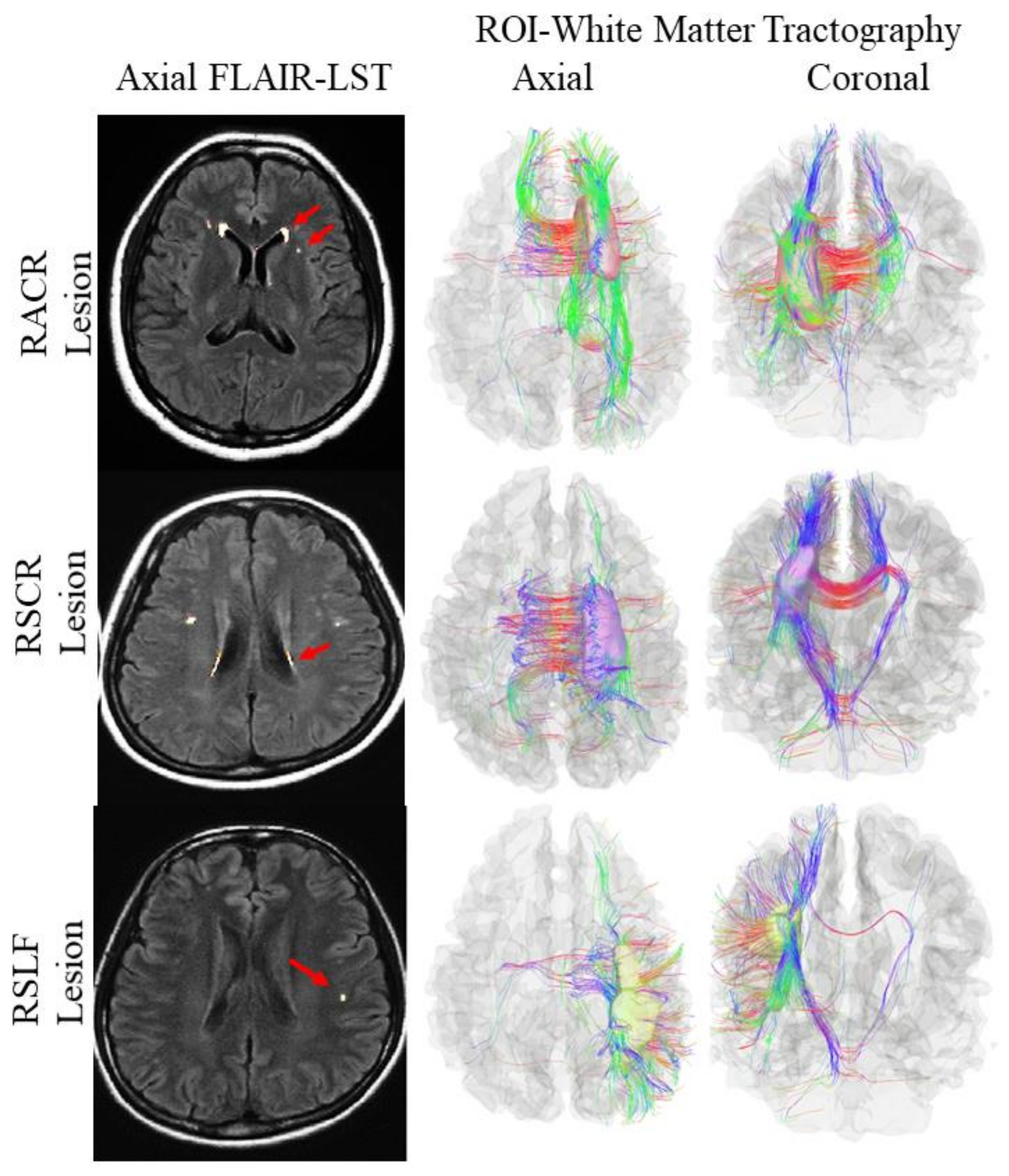
2.6. Region of Interest Analysis and Tractography
2.7. Statistical Data Analysis
3. Results
3.1. Demographics and Cardiocerebrovascular Risk Prediction
3.2. Age vs. the Proportion of WMHs among the Study Subjects
3.3. QRISK2 vs. the Proportion of WMHs among the Study Subjects
3.4. Neurocognitive Profiles vs. the Proportion of WMHs among the Study Subjects
3.5. MPs vs. the Proportion of WMHs among the Study Subjects
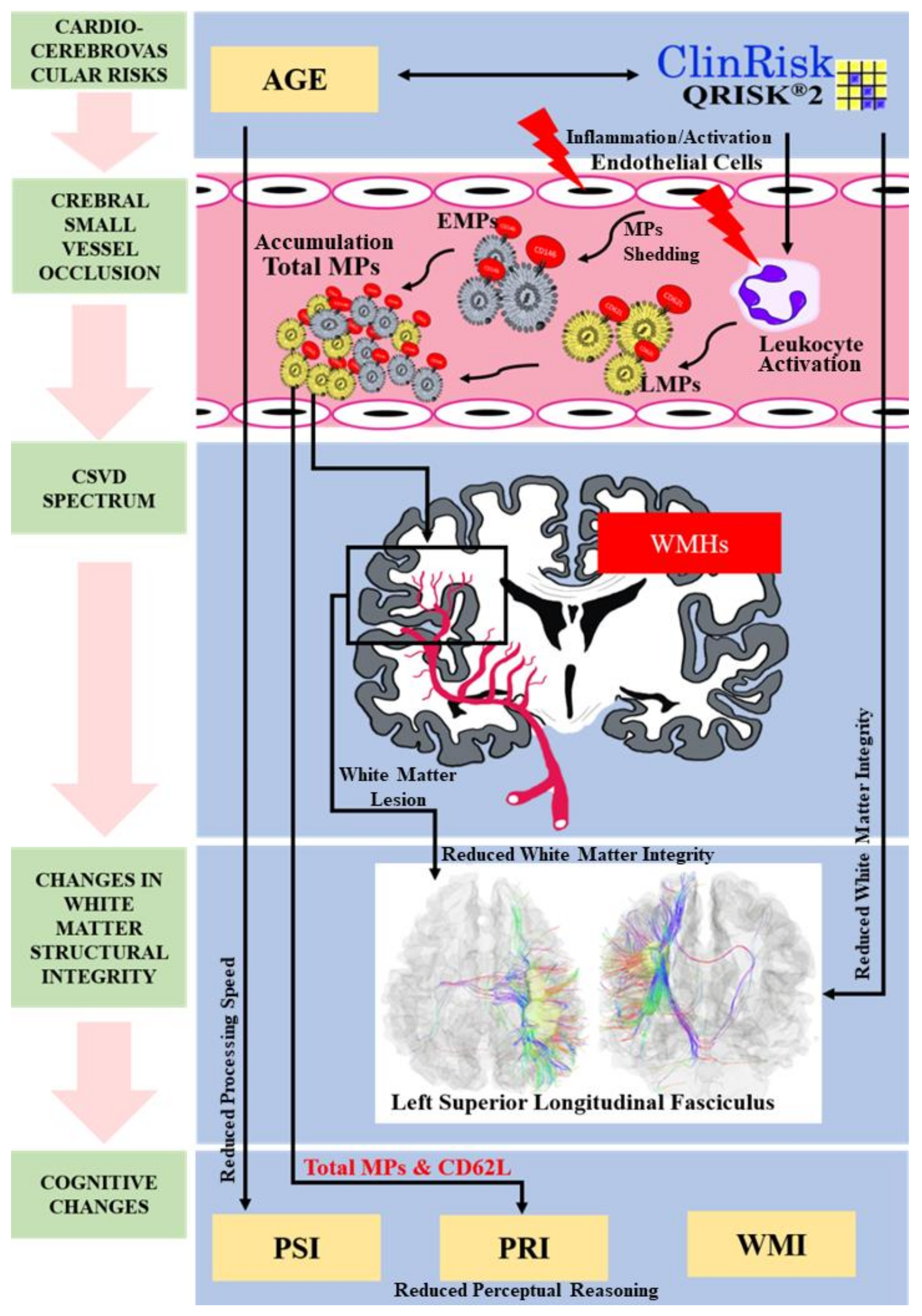
3.6. Multimodal Study for Cerebral White Matter Integrity among the Study Subjects
4. Discussion
4.1. Cardiocerebrovascular Risk Prediction and Age as Surrogate Markers of CSVD
4.2. Neurocognitive Profiles as Surrogate Markers to CSVD
4.3. Microparticles as Surrogate Markers for CSVD
4.4. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustapha, M.; Nassir, C.M.N.C.M.; Aminuddin, N.; Safri, A.A.; Ghazali, M.M. Cerebral small vessel disease (CSVD)—Lessons from the animal models. Front. Physiol. 2019, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Lammie, A.G. Small vessel disease. In Cerebrovascular Diseases; Kalimo, H., Ed.; ISN Neuropath Press: Basel, Switzerland, 2005; pp. 85–91. [Google Scholar]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Ogata, J.; Yamanishi, H.; Ishibashi-Ueda, H. Pathology of cerebral small vessel disease. In Cerebral Small Vessel Disease; Pantoni, L., Gorelick, P.B., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 4–15. [Google Scholar]
- Hachinski, V. World stroke Day 2008: “Little strokes, big trouble”. Stroke 2008, 39, 2407–2420. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.S.; Hakim, A.M. Living beyond our physiological means: Small vessel disease of the brain is an expression of a systemic failure in arteriolar function: A unifying hypothesis. Stroke 2009, 40, e322–e330. [Google Scholar] [CrossRef]
- Gunda, B.; Bereczki, D.; Várallyay, G. Multimodal MRI of cerebral small vessel disease. In Neuroimaging-Clinical Applications; Bright, P., Ed.; INTECH Open Access Publisher: London, UK, 2012; pp. 277–291. [Google Scholar]
- Das, A.S.; Regenhardt, R.W.; Vernooij, M.W.; Blacker, D.; Charidimou, A.; Viswanathan, A. Asymptomatic cerebral small vessel disease: Insights from population-based studies. J. Stroke 2019, 21, 121–138. [Google Scholar] [CrossRef]
- Smith, E.E. Leukoaraiosis and stroke. Stroke 2010, 41, S139–S143. [Google Scholar] [CrossRef]
- Patel, B.; Markus, H.S. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int. J. Stroke 2011, 6, 47–49. [Google Scholar] [CrossRef]
- Rost, N.S.; Rahman, R.M.; Biffi, A.; Smith, E.E.; Kanakis, A.; Fitzpatrick, K.; Lima, F.; Worrall, B.B.; Meschia, J.F.; Brown, R.D.; et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology 2010, 75, 1670–1677. [Google Scholar] [CrossRef]
- Huynh, T.J.; Murphy, B.; Pettersen, J.A.; Tu, H.; Sahlas, D.J.; Zhang, L.; Symons, S.P.; Black, S.; Lee, T.Y.; Aviv, R.I. CT perfusion quantification of small-vessel ischemic severity. AJNR. Am. J. Neuroradiol. 2008, 29, 1831–1836. [Google Scholar] [CrossRef]
- Kanjilal, S.; Rao, V.S.; Mukherjee, M.; Natesha, B.K.; Renuka, K.S.; Sibi, K.; Iyengar, S.S.; Kakkar, V.V. Application of cardiovascular disease risk prediction models and the relevance of novel biomarkers to risk stratification in Asian Indians. Vasc. Health Risk Manag. 2008, 4, 199–211. [Google Scholar] [CrossRef]
- Collins, G.S.; Altman, D.G. Predicting the 10-year risk of cardiovascular disease in the United Kingdom: Independent and external validation of an updated version of QRISK2. BMJ 2012, 344, e4181. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.E. Impaired phenotype of circulating endothelial-derived microparticles: Novel marker of cardiovascular risk. J. Cardiol. Ther. 2015, 2, 365–370. [Google Scholar] [CrossRef][Green Version]
- Dignat-George, F.; Boulanger, C.M. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 27–33. [Google Scholar] [CrossRef]
- Bebawy, M.; Roseblade, A.; Luk, F.; Rawling, T.; Ung, A.; Grau, G.E.R. Cell-derived microparticles: New targets in the therapeutic management of disease. J. Pharm. Pharm. Sci. 2013, 16, 238. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Suades, R.; Crespo, J.; Vilahur, G.; Arderiu, G.; Padró, T.; Corella, D.; Salas-Salvadó, J.; Arós, F.; Martínez-González, M.A. CD3/CD45 and SMA-α circulating microparticles are increased in individuals at high cardiovascular risk who will develop a major cardiovascular event. Int. J. Cardiol. 2016, 208, 147–149. [Google Scholar] [CrossRef]
- Nomura, S.; Shimizu, M. Clinical significance of procoagulant microparticles. J. Intensiv. Care 2015, 3, 2. [Google Scholar] [CrossRef]
- Owens, A.P.; Mackman, N. Microparticles in hemostasis and thrombosis. Circ. Res. 2011, 108, 1284–1297. [Google Scholar] [CrossRef]
- Andriantsitohaina, R.; Gaceb, A.; Vergori, L.; Martínez, M.C. Microparticles as regulators of cardiovascular inflammation. Trends Cardiovasc. Med. 2012, 22, 88–92. [Google Scholar] [CrossRef]
- Martinez, M.C.; Tual-Chalot, S.; Leonetti, D.; Andriantsitohaina, R. Microparticles: Targets and tools in cardiovascular disease. Trends Pharmacol. Sci. 2011, 32, 659–665. [Google Scholar] [CrossRef]
- Burger, D.; Touyz, R.M. Cellular biomarkers of endothelial health: Microparticles, endothelial progenitor cells, and circulating endothelial cells. J. Am. Soc. Hypertens. 2012, 6, 85–89. [Google Scholar] [CrossRef]
- Grammas, P.; Martinez, J.; Miller, B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev. Mol. Med. 2011, 13, e19. [Google Scholar] [CrossRef] [PubMed]
- Ogata, J.; Yamanishi, H.; Ishibashi-Ueda, H. Review: Role of cerebral vessels in ischaemic injury of the brain. Neuropathol. Appl. Neurobiol. 2011, 37, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Bueche, C.Z.; Garz, C.; Braun, H. Blood brain barrier breakdown as the starting point of cerebral small vessel disease?—New insights from a rat model. Exp. Transl. Stroke Med. 2013, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, P.; Robert, S.; Bailly, N.; Garnache-Ottou, F.; Bouriche, T.; Devalet, B.; Segatchian, J.H.; Saas, P.; Mullier, F. Tips and tricks for flow cytometry-based analysis and counting of microparticles. Transfus. Apher. Sci. 2015, 53, 110–126. [Google Scholar] [CrossRef]
- Lacroix, R.; Judicone, C.; Poncelet, P.; Robert, S.; Arnaud, L.; Sampol, J.; Dignat-George, F. Impact of pre-analytical parameters on the measurement of circulating microparticles: Towards standardization of protocol. J. Thromb. Haemost. 2012, 10, 437–446. [Google Scholar] [CrossRef]
- Mooberry, M.J.; Bradford, R.; Hobl, E.L.; Lin, F.C.; Jilma, B.; Key, N.S. Procoagulant microparticles promote coagulation in a factor XI-dependent manner in human endotoxemia. J. Thromb. Haemost. 2016, 14, 1031–1042. [Google Scholar] [CrossRef]
- Nielsen, M.H.; Beck-Nielsen, H.; Andersen, M.N.; Handberg, A. A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. J. Extracell. Vesicles 2014, 3, 20795. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Suades, R.; Crespo, J.; Peña, E.; Padró, T.; Jiménez-Xarrié, E.; Martí-Fàbregas, J.; Badimon, L. Microparticle Shedding from Neural Progenitor Cells and Vascular Compartment Cells Is Increased in Ischemic Stroke. PLoS ONE 2016, 11, e0148176. [Google Scholar] [CrossRef]
- Toussaint, N.; Souplet, J.C.; Fillard, P. MedINRIA: Medical image navigation and research tool by INRIA. In Proceedings of the MICCAI’07 Workshop on Interaction in Medical Image Analysis and Visualization, Brisbane, Australia, 29 October–2 November 2007. (inria-00616047). [Google Scholar]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimers dementia and normal aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Griffanti, L.; Jenkinson, M.; Suri, S.; Zsoldos, E.; Mahmood, A.; Filippini, N.; Sexton, C.E.; Topiwala, A.; Allan, C.; Kivimäki, M.; et al. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: A study in older adults. Neuroimage 2018, 170, 174–181. [Google Scholar] [CrossRef]
- Yeh, F.C.; Verstynen, T.D.; Wang, Y.; Fernández-Miranda, J.C.; Tseng, W.Y. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, C.; Jezzard, P.; Basser, P.J.; Barnett, A.; Di Chiro, G.D. Diffusion tensor MR imaging of the human brain. Radiology 1996, 201, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Basser, P.J. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995, 8, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Bennett, I.J.; Madden, D.J.; Vaidya, C.J.; Howard, D.V.; Howard, J.H. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum. Brain Mapp. 2010, 31, 378–390. [Google Scholar] [CrossRef]
- Breteler, M.; Van Swieten, J.C.; Bots, M.L.; Grobbee, D.E.; Claus, J.J.; Hout, J.H.V.D.; Van Harskamp, F.; Tanghe, H.L.; De Jong, P.T.; Van Gijn, J.; et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam Study. Neurology 1994, 44, 1246. [Google Scholar] [CrossRef]
- Raz, N.; Yang, Y.; Dahle, C.L.; Land, S. Volume of white matter hyperintensities in healthy adults: Contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim. Biophys. Acta 2012, 1822, 361–369. [Google Scholar] [CrossRef]
- Sam, K.; Peltenburg, B.; Conklin, J.; Sobczyk, O.; Poublanc, J.; Crawley, A.P.; Mandell, D.M.; Venkatraghavan, L.; Duffin, J.; Fisher, J.A.; et al. Cerebrovascular reactivity and white matter integrity. Neurology 2016, 87, 2333–2339. [Google Scholar] [CrossRef]
- Qiu, W.Q.; Himali, J.J.; Wolf, P.A.; Decarli, D.C.; Beiser, A.; Au, R. Effects of white matter integrity and brain volumes on late life depression in the Framingham Heart Study. Int. J. Geriatr. Psychiatry 2017, 32, 214–221. [Google Scholar] [CrossRef]
- Kilpatrick, E.P.; Buckley, R.F.; Marshall, G.A.; Klein, H.; Properzi, M.; Schultz, A.P.; Rao, V.; Rabin, J.S.; Hanseeuw, B.; Rentz, D.M.; et al. [P3-376]: Qrisk2 and framingham cardiovascular risk scores significantly correlate with imaging biomarkers of preclinical Ad: Findings from the harvard aging brain study. Alzheimers Dement. 2017, 13, P1103. [Google Scholar] [CrossRef]
- Armstrong, A.C.; Muller, M.; Ambale-Ventakesh, B.; Halstead, M.; Kishi, S.; Bryan, N.; Sidney, S.; Correia, L.C.; Gidding, S.S.; Launer, L.J. Association of early left ventricular dysfunction with advanced magnetic resonance white matter and gray matter brain measures: The CARDIA study. Echocardiography 2017, 34, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Hilal, S.; Mok, V.; Youn, Y.C.; Wong, A.; Ikram, M.K.; Chen, C.L.-H. Prevalence, risk factors and consequences of cerebral small vessel diseases: Data from three Asian countries. J. Neurol. Neurosurg. Psychiatry 2017, 88, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Hilal, S.; Tan, C.S.; Xin, X.; Amin, S.M.; Wong, T.Y.; Chen, C.; Venketasubramanian, N.; Ikram, M.K. Prevalence of Cognitive Impairment and Dementia in Malays – Epidemiology of Dementia in Singapore Study. Curr. Alzheimer Res. 2017, 14, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, P.A.; Bilgel, M.S.; Gottesman, R.; Yanek, L.R.; Moy, T.F.; Becker, L.C.; Cuzzocreo, J.; Prince, J.; Yousem, D.M.; Becker, D.M. Extreme deep white matter hyperintensity volumes are associated with African American race. Cerebrovasc. Dis. 2014, 37, 244–250. [Google Scholar] [CrossRef]
- Mok, V.C.T.; Srikanth, V.K.; Xiong, Y.; Phan, T.G.; Moran, C.; Chu, S.; Zhao, Q.; Chu, W.C.-W.; Wong, A.; Hong, Z.; et al. Race-Ethnicity and Cerebral Small Vessel Disease – Comparison between Chinese and White Populations. Int. J. Stroke 2014, 9, 36–42. [Google Scholar] [CrossRef]
- Sedaghat, S.; Cremers, L.G.; De Groot, M.; Hofman, A.; Van Der Lugt, A.; Niessen, W.; Franco, O.H.; Dehghan, A.; Ikram, M.K.; Vernooij, M.W. Lower microstructural integrity of brain white matter is related to higher mortality. Neurology 2016, 87, 927–934. [Google Scholar] [CrossRef]
- Gons, R.A.; Tuladhar, A.M.; de Laat, K.F.; van Norden, A.G.; van Dijk, E.J.; Norris, D.G.; Zwiers, M.P.; de Leeuw, F.E. Physical activity is related to the structural integrity of cerebral white matter. Neurology 2013, 81, 971–976. [Google Scholar] [CrossRef]
- Gons, R.A.; van Norden, A.G.; de Laat, K.F.; van Oudheusden, L.J.; van Uden, I.W.; Zwiers, M.P.; Norris, D.G.; de Leeuw, F.E. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain 2011, 134, 2116–2124. [Google Scholar] [CrossRef]
- Gons, R.A.; de Laat, K.F.; van Norden, A.G.; van Oudheusden, L.J.; van Uden, I.W.; Norris, D.G.; Zwiers, M.P.; de Leeuw, F.E. Hypertension and cerebral diffusion tensor imaging in small vessel disease. Stroke 2010, 41, 2801–2806. [Google Scholar] [CrossRef]
- Lyoubi-Idrissi, A.L.; Jouvent, E.; Poupon, C.; Chabriat, H. Diffusion magnetic resonance imaging in cerebral small vessel disease. Rev. Neurol. 2017, 173, 201–210. [Google Scholar] [CrossRef]
- Haight, T.; Bryan, R.N.; Erus, G.; Hsieh, M.-K.; Davatzikos, C.; Nasrallah, I.; D’Esposito, M.; Jacobs, D.R.; Lewis, C.; Schreiner, P.; et al. White matter microstructure, white matter lesions, and hypertension: An examination of early surrogate markers of vascular-related brain change in midlife. Neuroimage Clin. 2018, 18, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, F.J.; Gavrieli, A.; Saade-Lemus, P.; Lioutas, V.A.; Upadhyay, J.; Novak, V. White matter microstructure and cognitive decline in metabolic syndrome: A review of diffusion tensor imaging. Metab. Clin. Exp. 2018, 78, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.J.; Leritz, E.C.; Shepel, J.; McGlinchey, R.E.; Milberg, W.P.; Rudolph, J.L.; Lipsitz, L.A.; Salat, D.H. Interindividual variation in serum cholesterol is associated with regional white matter tissue integrity in older adults. Hum. Brain Mapp. 2013, 34, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Dempster, F.N. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Dev. Rev. 1992, 12, 45–55. [Google Scholar] [CrossRef]
- Greenwood, P.M. The frontal aging hypothesis evaluated. J. Int. Neuropsychol. Soc. 2000, 6, 705–726. [Google Scholar] [CrossRef]
- West, R.L. An application of prefrontal cortex function theory to cognitive aging. Psychol. Bull. 1996, 120, 272–292. [Google Scholar] [CrossRef]
- Abe, O.; Yamasue, H.; Aoki, S.; Suga, M.; Yamada, H.; Kasai, K.; Masutani, Y.; Kato, N.; Kato, N.; Ohtomo, K. Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiol. Aging 2008, 29, 102–116. [Google Scholar] [CrossRef]
- Lehmbeck, J.T.; Brassen, S.; Weber-Fahr, W.; Braus, D.F. Combining voxel-based morphometry and diffusion tensor imaging to detect age-related brain changes. Neuroreport 2006, 17, 467–470. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Adalsteinsson, E.; Sullivan, E.V. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage 2005, 26, 891–899. [Google Scholar] [CrossRef]
- Salat, D.H.; Tuch, D.; Greve, D.; Van Der Kouwe, A.; Hevelone, N.; Zaleta, A.; Rosen, B.; Fischl, B.; Corkin, S.; Rosas, H.D.; et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging 2005, 26, 1215–1227. [Google Scholar] [CrossRef]
- Stamatakis, E.A.; Shafto, M.A.; Williams, G.; Tam, P.; Tyler, L.K. White matter changes and word finding failures with increasing age. PLoS ONE 2011, 6, e14496. [Google Scholar] [CrossRef] [PubMed]
- Kynast, J.; Lampe, L.; Luck, T.; Frisch, S.; Arelin, K.; Hoffmann, K.-T.; Loeffler, M.; Riedel-Heller, S.G.; Villringer, A.; Schroeter, M. White matter hyperintensities associated with small vessel disease impair social cognition beside attention and memory. Br. J. Pharmacol. 2018, 38, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, D. The roles of working memory updating and processing speed in mediating age-related differences in fluid intelligence. Neuropsychology, Development, and Cognition. Sect. B Aging Neuropsychol. Cogn. 2007, 14, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Tsubota-Utsugi, M.; Satoh, M.; Tomita, N.; Hara, A.; Kondo, T.; Hosaka, M.; Saito, S.; Asayama, K.; Inoue, R.; Hirano, M.; et al. Lacunar Infarcts Rather than White Matter Hyperintensity as a Predictor of Future Higher Level Functional Decline: The Ohasama Study. J. Stroke Cerebrovasc. Dis. 2017, 26, 376–384. [Google Scholar] [CrossRef] [PubMed]
- De Groot, M.D.; Anderson, R.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. Association of Depression and Diabetes Complications: A meta-analysis. Psychosom. Med. 2001, 63, 619–630. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Lo, J.W.; Crawford, J.D.; Mellon, L.; Hickey, A.; Williams, D.; Bordet, R.; Mendyk, A.M.; Gelé, P.; Deplanque, D. STROKOG (stroke and cognition consortium): An international consortium to examine the epidemiology, diagnosis, and treatment of neurocognitive disorders in relation to cerebrovascular disease. Alzheimers Dement. 2017, 7, 11–13. [Google Scholar] [CrossRef]
- Dhamoon, M.S.; Cheung, Y.K.; Bagci, A.; Alperin, N.; Sacco, R.L.; Elkind, M.S.V.; Wright, C.B. Periventricular white matter hyperintensities and functional decline. J. Am. Geriatr. Soc. 2018, 66, 113–119. [Google Scholar] [CrossRef]
- Köhler, S.; Thomas, A.J.; Lloyd, A.; Barber, R.; Almeida, O.P.; O’Brien, J.T. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. Br. J. Psychiatry 2010, 196, 143–149. [Google Scholar] [CrossRef]
- Vannorsdall, T.D.; Waldstein, S.R.; Kraut, M.; Pearlson, G.D.; Schretlen, D.J. White matter abnormalities and cognition in a community sample. Arch. Clin. Neuropsychol. 2009, 24, 209–217. [Google Scholar] [CrossRef]
- Tuladhar, A.M.; van Norden, A.G.; de Laat, K.F.; Zwiers, M.P.; van Dijk, E.J.; Norris, D.G.; de Leeuw, F.E. White matter integrity in small vessel disease is related to cognition. Neuroimage Clin. 2015, 7, 518–624. [Google Scholar] [CrossRef]
- D’Souza, M.M.; Gorthi, S.P.; Vadwala, K.; Trivedi, R.; Vijayakumar, C.; Kaur, P.; Khushu, S. Diffusion tensor tractography in cerebral small vessel disease: Correlation with cognitive function. Neuroradiol. J. 2018, 31, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Van Norden, A.G.; de Laat, K.F.; Gons, R.A.; van Uden, I.W.; van Dijk, E.J.; van Oudheusden, L.J.; Esselink, R.A.; Bloem, B.R.; van Engelen, B.G.; Zwarts, M.J. Causes and consequences of cerebral small vessel disease. The RUN DMC study: A prospective cohort study. Study rationale and protocol. BMC Neurol. 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.J.; Patel, B.; Morris, R.G.; MacKinnon, A.D.; Rich, P.M.; Barrick, T.R.; Markus, H.S. Mechanisms of cognitive impairment in cerebral small vessel disease: Multimodal MRI results from the St George’s cognition and neuroimaging in stroke (SCANS) study. PLoS ONE 2013, 8, e61014. [Google Scholar] [CrossRef]
- Moonen, J.E.F.; Foster-Dingley, J.C.; Berg-Huijsmans, A.A.V.D.; De Ruijter, W.; De Craen, A.J.M.; Van Der Grond, J.; Van Der Mast, R. Influence of Small Vessel Disease and Microstructural Integrity on Neurocognitive Functioning in Older Individuals: The DANTE Study Leiden. Am. J. Neuroradiol. 2016, 38, 25–30. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tang, Y.; Qin, C. Increased circulating leukocyte-derived microparticles in ischemic cerebrovascular disease. Thromb. Res. 2017, 154, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.; Marlborough, F.; Doubal, F.; Webb, D.J.; Wardlaw, J. Blood Markers of Coagulation, Fibrinolysis, Endothelial Dysfunction and Inflammation in lacunar Stroke versus Non-Lacunar Stroke and Non-Stroke: Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2014, 37, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Padró, T.; Vilahur, G.; Badimon, L. C0074 Increased number of circulating and platelet-derived microparticles in human blood enhances thrombosis on atherosclerotic plaques. Thromb. Res. 2012, 130, S115. [Google Scholar] [CrossRef]
- Ueba, T.; Nomura, S.; Inami, N.; Nishikawa, T.; Kajiwara, M.; Iwata, R.; Yamashita, K. Plasma level of platelet-derived microparticles is associated with coronary heart disease risk score in healthy men. J. Atheroscler. Thromb. 2010, 17, 342–349. [Google Scholar] [CrossRef][Green Version]
- Viera, A.J.; Mooberry, M.; Key, N.S. Microparticles in cardiovascular disease pathophysiology and outcomes. J. Am. Soc. Hypertens. 2012, 6, 243–252. [Google Scholar] [CrossRef]
- Simak, J.; Gelderman, M.P.; Yu, H.; Wright, V.; Baird, A.E. Circulating endothelial microparticles in acute ischemic stroke: A link to severity, lesion volume and outcome. J. Thromb. Haemost. 2006, 4, 1296–1302. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Crespo, J.; Ramaiola, I.; Martin-Yuste, V.; Sabate, M.; Sans-Roselló, J.; Sionis, A.; Badimon, L. Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int. J. Cardiol. 2016, 202, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Marchisio, M.; Lanuti, P.; Boccatonda, A.; Miscia, S.; Davì, G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb. Haemost. 2016, 116, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Radum, C.M.; Duner, E.; Lombardi, A.M.; Spiezia, L.; Bendo, R.; Ferrari, S.; Simioni, P.; Fabris, F. Activated platelet-derived and leukocyte-derived circulating microparticles and the risk of thrombosis in heparin-induced thrombocytopenia: A role for PF4-bearing microparticles? Cytom. Part B Clin. Cytom. 2018, 94, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, F.; Barnea-Goraly, N.; Haas, B.W.; Golarai, G.; Ng, D.; Mills, D.; Korenberg, J.; Bellugi, U.; Galaburda, A.; Reiss, A.L. More Is Not Always Better: Increased Fractional Anisotropy of Superior Longitudinal Fasciculus Associated with Poor Visuospatial Abilities in Williams Syndrome. J. Neurosci. 2007, 27, 11960–11965. [Google Scholar] [CrossRef] [PubMed]
- Lawrenz, M.; Brassen, S.; Finsterbusch, J. Microscopic diffusion anisotropy in the human brain: Reproducibility, normal values, and comparison with the fractional anisotropy. Neuroimage 2015, 109, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Thomalla, G.; Glauche, V.; Koch, M.A.; Beaulieu, C.; Weiller, C.; Röther, J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage 2004, 22, 1767–1774. [Google Scholar] [CrossRef]
- Song, S.K.; Sun, S.W.; Ju, W.K.; Lin, S.J.; Cross, A.H.; Neufeld, A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003, 20, 1714–1722. [Google Scholar] [CrossRef]
- Davis, S.W.; Dennis, N.A.; Buchler, N.G.; White, L.E.; Madden, D.J.; Cabeza, R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 2009, 46, 530–541. [Google Scholar] [CrossRef]
| Variables | n (%) | |
|---|---|---|
| Age | <20 | 0 (0) |
| 21–40 | 31 (51.7) | |
| 41–60 | 26 (43.3) | |
| >60 | 3 (5) | |
| Gender | Male | 19 (31.7) |
| Female | 41 (68.3) | |
| Ethnic | Malay | 54 (90.0) |
| Chinese | 4 (6.7) | |
| Other | 2 (3.3) | |
| WMHs * | Absent (WMH−) | 40 (66.7) |
| Present (WMH+) | 20 (33.3) | |
| Smoking status | Nonsmoker | 52 (86.7) |
| Ex-smoker | 7 (11.7) | |
| Light smoker | 1 (1.7) | |
| Family history of coronary heart disease in 1st degree relative under 60 years old | Yes | 15 (25.0) |
| No | 45 (75.0) | |
| Treated Hypertension | Yes | 9 (15.0) |
| No | 51 (85.0) | |
| Atrial fibrillation | Yes | - |
| No | 60 (100) | |
| Type 2 Diabetes | Yes | - |
| No | 60 (100) | |
| Hyperlipidemia | Yes | - |
| No | 60 (100) | |
| Chronic kidney disease (stage 4 or 5) | Yes | - |
| No | 60 (100) |
| Variables | Mean ± SD | T-Statistics (df) | p-Value | |
|---|---|---|---|---|
| WMH- (n = 20) | WMH+ (n = 40) | |||
| Age | 36.75 ± 10.04 | 46.00 ± 12.00 | −2.98 (32.66) | 0.006 * |
| QRISK2 | 1.32 ± 1.54 | 5.84 ± 6.43 | −4.24 (58) | 0.000 * |
| PRI | 102.95 ± 13.30 | 101.30 ± 10.73 | 0.52 (46.12) | 0.607 |
| WMI | 108.53 ± 19.14 | 105.25 ± 14.55 | 0.74 (48.45) | 0.465 |
| PSI | 103.73 ± 14.31 | 98.05 ± 11.49 | 1.66 (46.33) | 0.104 |
| CD62L | 98.30 ± 42.43 | 163.85 ± 140.72 | −2.73 (58) | 0.008 * |
| CD235a | 33.05 ± 74.25 | 50.45 ± 42.10 | −1.16 (56.95) | 0.252 |
| CD62P | 150.45 ± 75.21 | 370.80 ± 330.271 | −4.05 (58) | 0.000 * |
| CD146 | 25.08 ± 20.27 | 194.80 ± 669.90 | −1.62 (58) | 0.112 |
| Total MPs | 306.88 ± 152.15 | 779.90 ± 930.04 | −3.16 (58) | 0.003 * |
| No. | Variables | r (β) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | Age | 1 | ||||
| 2 | QRISK2 | 0.75 * (0.57 *) | 1 | |||
| 3 | PRI | −0.27 * (0.02) | −0.23 * (−0.09) | 1 | ||
| 4 | WMI | −0.35 * (−0.01) | −0.32 * (−0.07) | 0.62 * (0.35 *) | 1 | |
| 5 | PSI | −0.56 * (−0.31*) | −0.40 * (−0.19) | 0.60 * (0.44 *) | 0.61 * (0.63 *) | 1 |
| 6 | CD62L | 0.51 * (0.19) | 0.58 * (0.41 *) | 0.09 (0.25 *) | −0.12 (0.12) | −0.19 (−0.18) |
| 7 | CD235a | 0.26 * (0.18) | 0.13 (0.02) | 0.10 (0.11) | 0.05 (0.19) | −0.16 (−0.15) |
| 8 | CD62P | 0.32 * (0.16) | 0.44 * (−0.08) | 0.08 (0.32) | −0.15 (−0.09) | −0.15 (0.01) |
| 9 | CD146 | 0.26 * (0.14) | 0.41 * (0.28) | −0.18 (−0.16) | −0.14 (−0.42) | −0.07 (0.05) |
| 10 | Total MPs | 0.40 * (−0.02) | 0.54 * (0.30) | −0.06 (−0.34 *) | −0.16 (−0.12) | −0.15 (0.00) |
| Tracts Parameters | (Mean ± SD) | T-Statistics (df) | p-Value | |
|---|---|---|---|---|
| WMH− | WMH+ | |||
| RACR | ||||
| FA | 0.42 ± 0.03 | 0.42 ± 0.03 | 0.35 (37.94) | 0.727 |
| MD | 0.82 ± 0.07 | 0.84 ± 0.08 | −0.59 (33.83) | 0.560 |
| AD | 1.23 ± 0.13 | 1.24 ± 0.14 | −0.34 (35.39) | 0.733 |
| RD | 0.63 ± 0.05 | 0.64 ± 0.06 | −0.86 (32.21) | 0.395 |
| LACR | ||||
| FA | 0.42 ± 0.02 | 0.42 ± 0.27 | 0.45 (34.42) | 0.649 |
| MD | 0.82 ± 0.08 | 0.83 ± 0.08 | −0.24 (38.36) | 0.812 |
| AD | 1.23 ± 0.13 | 1.23 ± 0.13 | −0.05 (38.38) | 0.957 |
| RD | 0.62 ± 0.05 | 0.63 ± 0.05 | −0.46 (37.81) | 0.645 |
| RSCR | ||||
| FA | 0.46 ± 0.03 | 0.46 ± 0.02 | −0.06 (48.00) | 0.955 |
| MD | 0.81 ± 0.07 | 0.81 ± 0.07 | −0.05 (37.40) | 0.958 |
| AD | 1.26 ± 0.13 | 1.26 ± 0.12 | −0.02 (40.09) | 0.987 |
| RD | 0.59 ± 0.04 | 0.59 ± 0.05 | −0.09 (34.21) | 0.923 |
| LSCR | ||||
| FA | 0.46 ± 0.02 | 0.46 ± 0.02 | 0.17 (41.85) | 0.866 |
| MD | 0.82 ± 0.07 | 0.83 ± 0.08 | −0.30 (34.89) | 0.765 |
| AD | 1.26 ± 0.12 | 1.27 ± 0.13 | −0.16 (36.26) | 0.875 |
| RD | 0.59 ± 0.05 | 0.60 ± 0.06 | −0.45 (33.64) | 0.655 |
| RSLF | ||||
| FA | 0.40 ± 0.02 | 0.40 ± 0.03 | 0.68 (34.32) | 0.499 |
| MD | 0.77 ± 0.07 | 0.78 ± 0.08 | −0.24 (34.15) | 0.813 |
| AD | 1.13 ± 0.11 | 1.14 ± 0.12 | −0.00 (35.76) | 0.998 |
| RD | 0.59 ± 0.05 | 0.60 ± 0.06 | −0.49 (32.15) | 0.627 |
| LSLF | ||||
| FA | 0.41 ± 0.02 | 0.41 ± 0.03 | 0.94 (34.30) | 0.355 |
| MD | 0.78 ± 0.07 | 0.78 ± 0.08 | −0.13 (33.43) | 0.897 |
| AD | 1.15 ± 0.11 | 1.15 ± 0.12 | 0.14 (34.98) | 0.892 |
| RD | 0.59 ± 0.05 | 0.60 ± 0.06 | −0.41 (31.77) | 0.683 |
| Variables & Tracts | r (β) | |||
|---|---|---|---|---|
| FA | MD | AD | RD | |
| Age | ||||
| RACR | −0.43 * (1.23) | −0.13 (−0.78) | −0.23 * (−2.52) | 0.00 (1.46) |
| LACR | −0.42 * (1.15) | −0.14 (−1.96) | −0.23 * (−2.44) | −0.01 (2.74) |
| RSCR | −0.38 * (0.17) | −0.11 (−0.53) | −0.20 * (0.43) | 0.03 (0.16) |
| LSCR | −0.40 * (−0.80) | −0.13 (−0.26) | −0.21 * (0.74) | −0.02 (−1.26) |
| RSLF | −0.46 * (−0.32) | −0.14 (−0.85) | −0.23 * (−0.72) | −0.02 (0.27) |
| LSLF | −0.37 * (2.76) | −0.16 (1.18) | −0.24 * (−5.73) | −0.07 (4.81) |
| QRISK2 | ||||
| RACR | −0.28 * (3.90) | 0.05 (−0.67) | −0.04 (−7.32) | 0.16 (4.84) |
| LACR | −0.26 * (−1.87) | 0.05 (−1.41) | −0.03 (4.26) | 0.15 (−2.42) |
| RSCR | −0.24 * (0.47) | 0.05 (1.44) | −0.03 (−1.86) | 0.16 (0.63) |
| LSCR | −0.30 * (−0.41) | 0.05 (1.81) | −0.03 (0.69) | 0.14 (−0.97) |
| RSLF | −0.35 * (−2.94) | 0.07 (−0.75) | −0.02 (5.14) | 0.18 (−3.47) |
| LSLF | −0.35 * (4.09 *) | 0.06 (3.07 *) | −0.03 (−9.09 *) | 0.17 (8.11 *) |
| PRI | ||||
| RACR | 0.45 * (−3.55) | 0.15 (−0.21) | 0.24 * (8.21) | 0.03 (−4.79) |
| LACR | 0.45 * (1.69) | 0.14 (0.67) | 0.23 * (−3.15) | 0.03 (1.75) |
| RSCR | 0.33* (2.61) | 0.14 (−0.07) | 0.21 * (−7.64) | 0.05 (4.25) |
| LSCR | 0.39* (−1.03) | 0.16 (0.76) | 0.23 * (5.11) | 0.07 (−2.65) |
| RSLF | 0.45* (−0.06) | 0.12 (0.41) | 0.21 * (0.49) | 0.02 (−1.47) |
| LSLF | 0.42* (0.57) | 0.16 (1.27) | 0.24 * (−1.67) | 0.07 (1.49) |
| WMI | ||||
| RACR | 0.28* (−0.80) | 0.03 (0.08) | 0.09 (1.20) | −0.06 (0.30) |
| LACR | 0.32* (−0.14) | 0.01 (0.21) | 0.08 (2.30) | −0.09 (−2.20) |
| RSCR | 0.14 (−1.17) | 0.00 (−0.92) | 0.05 (0.44) | −0.06 (−0.17) |
| LSCR | 0.25 * (1.95) | 0.00 (0.14) | 0.06 (−4.05) | −0.07 (3.13) |
| RSLF | 0.36 * (−1.85) | −0.00 (−0.07) | 0.08 (5.10) | −0.11 (−4.89) |
| LSLF | 0.28 * (0.23) | 0.00 (0.98) | 0.07 (−0.27) | −0.06 (0.59) |
| PSI | ||||
| RACR | 0.28 * (0.49) | −0.04 (−0.79) | 0.04 (−0.79) | −0.14 (1.47) |
| LACR | 0.27 * (−1.68) | −0.05 (0.82) | 0.03 (3.85) | −0.15 (−3.95) |
| RSCR | 0.20 * (−0.73) | −0.06 (0.51) | 0.01 (0.35) | −0.15 (0.24) |
| LSCR | 0.31 * (2.10) | −0.06 (0.90) | 0.02 (−4.71) | −0.16 (3.25) |
| RSLF | 0.35 * (−0.14) | −0.07 (0.54) | 0.02 (0.81) | −0.18 (−1.22) |
| LSLF | 0.33 * (−2.02) | −0.03 (0.99) | 0.05 (6.11) | −0.13 (−4.10) |
| CD62L | ||||
| RACR | −0.03 (1.36) | 0.17 (−0.70) | 0.13 (−1.48) | 0.21 * (1.05) |
| LACR | −0.04 (−1.08) | 0.16 (−0.89) | 0.12 (1.42) | 0.21 * (−0.49) |
| RSCR | −0.03 (−0.02) | 0.17 (0.07) | 0.13 (−0.46) | 0.22 * (0.04) |
| LSCR | −0.05 (−0.19) | 0.17 (0.57) | 0.14 (1.15) | 0.21 * (−0.69) |
| RSLF | −0.08 (−2.96) | 0.16 (0.36) | 0.11 (5.48) | 0.21 * (−4.42) |
| LSLF | −0.03 (5.05 *) | 0.13 (2.28 *) | 0.10 (−11.61 *) | 0.16 (9.45 *) |
| CD235a | ||||
| RACR | 0.00 (−4.68) | 0.00 (−0.79) | −0.00 (7.79) | 0.00 (−5.04) |
| LACR | 0.03 (2.40) | 0.00 (1.31) | 0.00 (−2.55) | 0.00 (2.43) |
| RSCR | 0.00 (−1.24) | 0.04 (1.05) | 0.03 (4.03) | 0.06 (−1.63) |
| LSCR | 0.07 (1.99) | 0.01 (0.73) | 0.02 (−5.03) | 0.01 (3.15) |
| RSLF | −0.07 (2.49) | 0.00 (−0.45) | −0.02 (−7.30) | 0.03 (4.12) |
| LSLF | 0.06 (1.15) | −0.04 (1.05) | −0.03 (−2.73) | −0.05 (2.07) |
| CD62P | ||||
| RACR | 0.10 (1.99) | 0.11 (−0.06) | 0.11 (−3.68) | 0.11 (2.82) |
| LACR | 0.09 (−1.42) | 0.11 (1.46) | 0.10 (4.12) | 0.11 (−2.47) |
| RSCR | 0.10 (−1.23) | 0.08 (0.92) | 0.09 (1.36) | 0.07 (−1.98) |
| LSCR | 0.09 (2.23) | 0.14 (−0.40) | 0.13 (−3.38) | 0.15 (3.99) |
| RSLF | −0.03 (−2.35) | 0.08 (−0.01) | 0.06 (2.97) | 0.12 (−3.12) |
| LSLF | 0.04 (3.61) | 0.07 (2.02) | 0.06 (−8.37) | 0.08 (6.87) |
| CD146 | ||||
| RACR | 0.02 (2.12) | 0.02 (0.77) | 0.02 (−5.13) | 0.02 (2.89) |
| LACR | 0.06 (−2.21) | 0.03 (1.26) | 0.03 (6.05) | 0.02 (−3.16) |
| RSCR | −0.00 (2.29) | 0.02 (1.91) | 0.02 (−6.25) | 0.03 (2.79) |
| LSCR | 0.01 (−1.95) | 0.00 (0.12) | 0.00 (5.04) | 0.00 (−3.31) |
| RSLF | 0.0 (−3.16) | 0.06 (−0.98) | 0.05 (8.12) | 0.06 (−4.57) |
| LSLF | 0.03 (5.06 *) | −0.02 (2.26 *) | −0.02 (−12.01 *) | −0.03 (8.50 *) |
| Total MPs | ||||
| RACR | 0.07 (2.31) | 0.09 (0.07) | 0.08 (−5.08) | 0.09 (3.26) |
| LACR | 0.08 (−2.00) | 0.10 (1.91) | 0.09 (5.99) | 0.10 (−3.33) |
| RSCR | 0.06 (−0.53) | 0.08 (1.50) | 0.08 (0.18) | 0.08 (−1.11) |
| LSCR | 0.04 (1.09) | 0.12 (−0.09) | 0.10 (−1,67) | 0.13 (2.23) |
| RSLF | −0.04 (−2.66) | 0.09 (−0.29) | 0.06 (4.45) | 0.12 (−3.43) |
| LSLF | 0.04 (4.82 *) | 0.06 (2.77 *) | 0.04 (−11.17 *) | 0.07 (8.71 *) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Mohd Nassir, C.M.N.; Mohamad Ghazali, M.; Ahmad Safri, A.; Jaffer, U.; Abdullah, W.Z.; Idris, N.S.; Muzaimi, M. Elevated Circulating Microparticle Subpopulations in Incidental Cerebral White Matter Hyperintensities: A Multimodal Study. Brain Sci. 2021, 11, 133. https://doi.org/10.3390/brainsci11020133
Che Mohd Nassir CMN, Mohamad Ghazali M, Ahmad Safri A, Jaffer U, Abdullah WZ, Idris NS, Muzaimi M. Elevated Circulating Microparticle Subpopulations in Incidental Cerebral White Matter Hyperintensities: A Multimodal Study. Brain Sciences. 2021; 11(2):133. https://doi.org/10.3390/brainsci11020133
Chicago/Turabian StyleChe Mohd Nassir, Che Mohd Nasril, Mazira Mohamad Ghazali, Amanina Ahmad Safri, Usman Jaffer, Wan Zaidah Abdullah, Nur Suhaila Idris, and Mustapha Muzaimi. 2021. "Elevated Circulating Microparticle Subpopulations in Incidental Cerebral White Matter Hyperintensities: A Multimodal Study" Brain Sciences 11, no. 2: 133. https://doi.org/10.3390/brainsci11020133
APA StyleChe Mohd Nassir, C. M. N., Mohamad Ghazali, M., Ahmad Safri, A., Jaffer, U., Abdullah, W. Z., Idris, N. S., & Muzaimi, M. (2021). Elevated Circulating Microparticle Subpopulations in Incidental Cerebral White Matter Hyperintensities: A Multimodal Study. Brain Sciences, 11(2), 133. https://doi.org/10.3390/brainsci11020133





