Obsessive-Compulsive Disorder and Decision Making under Ambiguity: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
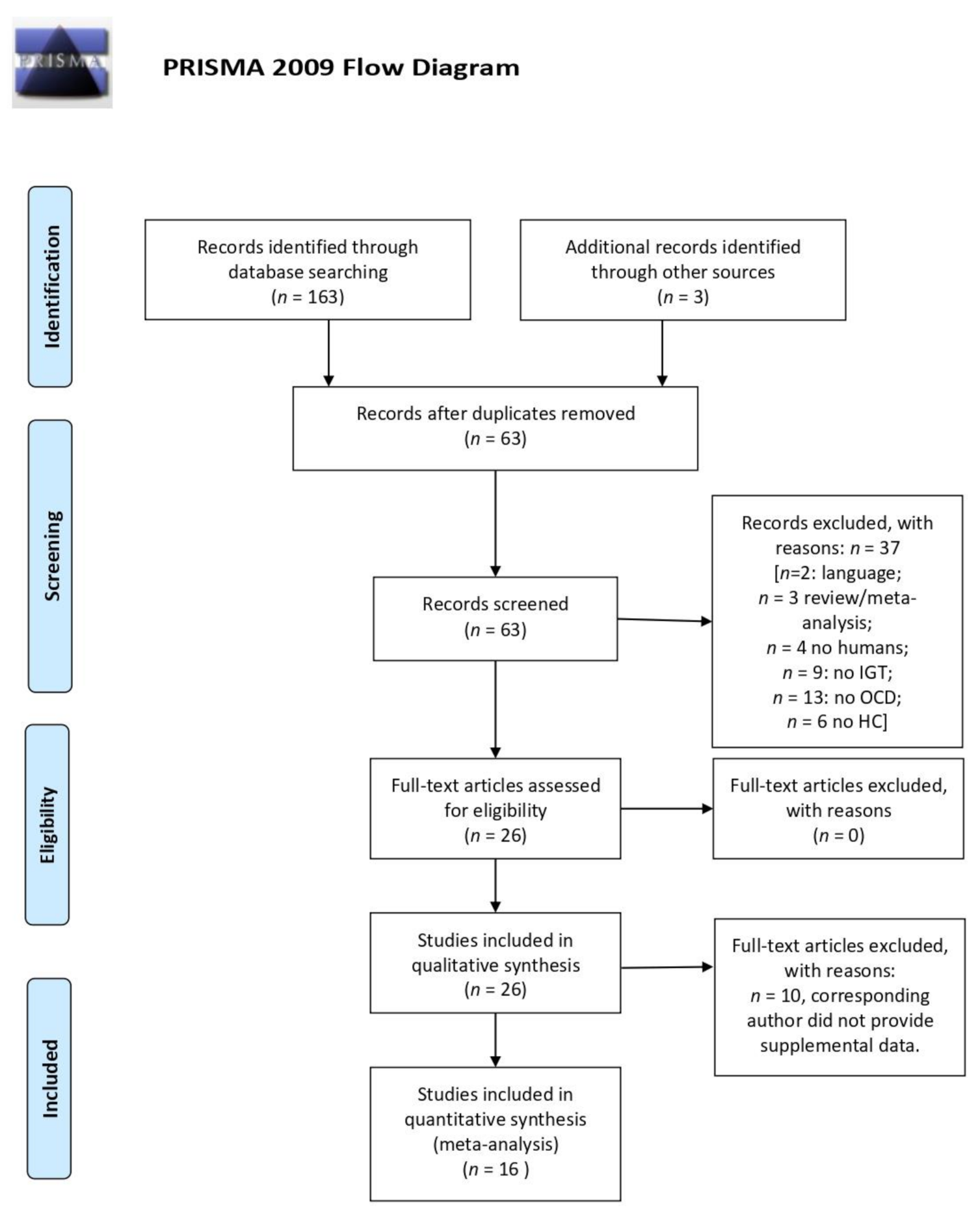
2. Materials and Methods
3. Results
3.1. Systematic Review
3.1.1. Performance at IGT
3.1.2. Correlation with Symptoms and Severity
3.1.3. OCD Clusters
3.1.4. Differences between OCD and Other Behavioural Addictions in IGT Performance
3.1.5. Gender
3.1.6. Pathological Doubt
3.2. Meta-Analysis
3.2.1. Sensitivity Analysis
Age
Medications
3.2.2. IGT Blocks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013; p. 5. [Google Scholar]
- Ruscio, A.M.; Stein, D.J.; Chiu, W.T.; Kessler, R.C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloch, M.H.; Landeros-Weisenberger, A.; Rosário, M.C.; Pittenger, C.; Leckman, J.F. Meta-analysis of the symptom structure of obsessive-compulsive disorder. Am. J. Psychiatry 2010, 165, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Pauls, D.L.; Abramovitch, A.; Rauch, S.L.; Geller, D.A. Obsessive–compulsive disorder: An integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 2014, 15, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Neale, M.C. Endophenotype: A conceptual analysis. Mol. Psychiatry 2010, 15, 789–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yang, X.; Yang, Q. Neuropsychological dysfunction in adults with early-onset obsessive-compulsive disorder: The search for a cognitive endophenotype. Rev. Bras. Psiquiatr. 2015, 37, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottesman, I.I.; Gould, T.D. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef]
- Reus, V.; Freimer, N.B. Understanding the genetic basis of mood disorders: Where do we stand? Am. J. Hum. Genet. 1997, 60, 1283–1288. [Google Scholar] [CrossRef] [Green Version]
- Benzina, N.; Mallet, L.; Burguière, E.; N’Diaye, K.; Pelissolo, A. Cognitive dysfunction in obsessive-compulsive disorder. Curr. Psychiatry Rep. 2016, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, D. Decision making: From neuroscience to psychiatry. Neuron 2013, 78, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, S.R.; Blackwell, A.D.; Fineberg, N.A.; Robbins, T.W.; Sahakian, B.J. The neuropsychology of obsessive compulsive disorder: The importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci. Biobehav. Rev. 2005, 29, 399–419. [Google Scholar] [CrossRef]
- Brand, M.; Labudda, K.; Markowitsch, H.J. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Netw. 2006, 19, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Fujiwara, E.; Borsutzky, S.; Kalbe, E.; Kessler, J.; Markowitsch, H.J. Decision-Making Deficits of Korsakoff patients in a new gambling task with explicit rules: Associations with executive functions. Neuropsychology 2005, 19, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, L.H.; Sahakian, B.J.; Robertson, M.M.; Veale, D.M.; Rogers, R.D.; Pickard, K.M.; Aitken, M.R.F.; Robbins, T.W. Executive function in Tourette’s syndrome and obsessive–compulsive disorder. Psychol. Med. 2005, 35, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Starcke, K.; Tuschen-Caffier, B.; Markowitsch, H.-J.; Brand, M. Skin conductance responses during decisions in ambiguous and risky situations in obsessive-compulsive disorder. Cogn. Neuropsychiatry 2009, 14, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Starcke, K.; Tuschen-Caffier, B.; Markowitsch, H.J.; Brand, M. Dissociation of decisions in ambiguous and risky situations in obsessive–compulsive disorder. Psychiatry Res. 2010, 175, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Morein-Zamir, S.; Papmeyer, M.; Pertusa, A.; Chamberlain, S.R.; Fineberg, N.A.; Sahakian, B.J.; Mataix-Cols, D.; Robbins, T.W. The profile of executive function in OCD hoarders and hoarding disorder. Psychiatry Res. 2014, 215, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.W.; Kang, J.I.; Namkoong, K.; Jhung, K.; Ha, R.Y.; Kim, S.J. Further evidence of a dissociation between decision-making under ambiguity and decision-making under risk in obsessive–compulsive disorder. J. Affect. Disord. 2015, 176, 118–124. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Ji, Y.; Tao, R.; Chen, X.; Ye, J.; Zhang, L.; Yu, F.; Zhu, C.; Wang, K. Trait-related decision making impairment in obsessive-compulsive disorder: Evidence from decision making under ambiguity but not decision making under risk. Sci. Rep. 2015, 5, 17312. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Dong, Y.; Ji, Y.; Zhu, C.; Yu, F.; Ma, H.; Chen, X.; Wang, K. Dissociation of decision making under ambiguity and decision making under risk: A neurocognitive endophenotype candidate for obsessive–compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 57, 60–68. [Google Scholar] [CrossRef]
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1. The Cochrane Collaboration. 2011. Available online: https://handbook-5-1.cochrane.org/ (accessed on 1 September 2020).
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Review Manager (RevMan) [Computer Program], Version 5.4; The Cochrane Collaboration: London, UK, 2020.
- Cavedini, P.; Riboldi, G.; D’Annucci, A.; Belotti, P.; Cisima, M.; Bellodi, L. Decision-making heterogeneity in obsessive-compulsive disorder: Ventromedial prefrontal cortex function predicts different treatment outcomes. Neuropsycholgia 2002, 40, 205–211. [Google Scholar] [CrossRef]
- Boisseau, C.L.; Thompson-Brenner, H.; Pratt, E.M.; Farchione, T.J.; Barlow, D.H. The relationship between decision-making and perfectionism in obsessive-compulsive disorder and eating disorders. J. Behav. Ther. Exp. Psychiatry 2013, 44, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Bottesi, G.; Ghisi, M.; Ouimet, A.J.; Tira, M.D.; Sanavio, E. Compulsivity and impulsivity in pathological gambling: Does a dimensional–transdiagnostic approach add clinical utility to DSM-5 classification? J. Gambl. Stud. 2014, 31, 825–847. [Google Scholar] [CrossRef]
- Grassi, G.; Makris, N.; Pallanti, S. Addicted to compulsion: Assessing three core dimensions of addiction across obsessive-compulsive disorder and gambling disorder. CNS Spectr. 2019, 25, 392–401. [Google Scholar] [CrossRef]
- Blom, R.M.; Samuels, J.; Grados, M.A.; Chen, Y.; Bienvenu, O.J.; Riddle, M.A.; Liang, K.-Y.; Brandt, J.; Nestadt, G. Cognitive functioning in compulsive hoarding. J. Anxiety Disord. 2011, 25, 1139–1144. [Google Scholar] [CrossRef]
- Norman, L.J.; Carlisi, C.O.; Christakou, A.; Murphy, C.M.; Chantiluke, K.; Giampietro, V.; Simmons, A.; Brammer, M.; Mataix-Cols, D.; Rubia, K. Frontostriatal dysfunction during decision making in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 694–703. [Google Scholar] [CrossRef]
- Cavallaro, R.; Cavedini, P.; Mistretta, P.; Bassi, T.; Angelone, S.M.; Ubbiali, A.; Bellodi, L. Basal-corticofrontal circuits in schizophrenia and obsessive-compulsive disorder: A controlled, double dissociation study. Biol. Psychiatry 2003, 54, 437–443. [Google Scholar] [CrossRef]
- Cavedini, P.; Zorzi, C.; Piccinni, M.; Cavallini, M.C.; Bellodi, L. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: Searching for a new intermediate phenotype. Biol. Psychiatry 2010, 67, 1178–1184. [Google Scholar] [CrossRef]
- Cavedini, P.; Zorzi, C.; Baraldi, C.; Patrini, S.; Salomoni, G.; Bellodi, L.; Freire, R.C.; Perna, G. The somatic marker affecting decisional processes in obsessive-compulsive disorder. Cogn. Neuropsychiatry 2012, 17, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, F.F.; Alvarenga, N.B.; Malloy-Diniz, L.; Corrêa, H. Decision-making impairment in obsessive-compulsive disorder as measured by the Iowa Gambling Task. Arq. Neuropsiquiatr. 2011, 69, 642–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, G.; Pallanti, S.; Righi, L.; Figee, M.; Mantione, M.; Denys, D.; Piccagliani, D.; Rossi, A.; Stratta, P. Think twice: Impulsivity and decision making in obsessive–compulsive disorder. J. Behav. Addict. 2015, 4, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Figee, M.; Ooms, P.; Righi, L.; Nakamae, T.; Pallanti, S.; Schuurman, R.; Denys, D. Impulsivity and decision-making in obsessive-compulsive disorder after effective deep brain stimulation or treatment as usual. CNS Spectr. 2018, 23, 333–339. [Google Scholar] [CrossRef]
- Kashyap, H.; Kumar, J.K.; Kandavel, T.; Reddy, Y.J. Neuropsychological functioning in obsessive-compulsive disorder: Are executive functions the key deficit? Compr. Psychiatry 2013, 54, 533–540. [Google Scholar] [CrossRef]
- Kodaira, M.; Iwadare, Y.; Ushijima, H.; Oiji, A.; Kato, M.; Sugiyama, N.; Sasayama, D.; Usami, M.; Watanabe, K.; Saito, K. Poor performance on the Iowa gambling task in children with obsessive-compulsive disorder. Ann. Gen. Psychiatry 2012, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Martoni, R.M.; Brombin, C.; Nonis, A.; Salgari, G.C.; Buongiorno, A.; Cavallini, M.C.; Galimberti, E.; Bellodi, L. Evaluating effect of symptoms heterogeneity on decision-making ability in obsessive-compulsive disorder. Psychiatry Clin. Neurosci. 2015, 69, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chen, Y.; Tian, S.; Wang, T.; Xie, Y.; Jin, H.; Lin, G.; Gong, H.; Zeljic, K.; Sun, B.; et al. Effects of Anterior capsulotomy on decision making in patients with refractory obsessive–compulsive disorder. Front. Psychol. 2017, 8, 1814. [Google Scholar] [CrossRef] [Green Version]
- Borges, M.C.; Braga, D.T.; Iêgo, S.; D’Alcante, C.C.; Sidrim, I.; Machado, M.C.; Pinto, P.S.P.; Cordioli, A.V.; Rosário, M.C.; Petribú, K.; et al. Cognitive dysfunction in post-traumatic obsessive–compulsive disorder. Aust. N. Z. J. Psychiatry 2011, 45, 76–85. [Google Scholar] [CrossRef]
- Krishna, R.; Udupa, S.; George, C.M.; Kumar, K.J.; Viswanath, B.; Kandavel, T.; Venkatasubramanian, G.; Reddy, Y.J. Neuropsychological performance in OCD: A study in medication-naïve patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1969–1976. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Wooderson, S.; Mataix-Cols, D.; David, R.; Speckens, A.; Phillips, M.L. Decision making and set shifting impairments are associated with distinct symptom dimensions in obsessive-compulsive disorder. Neuropsychology 2006, 20, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Nielen, M.; Veltman, D.; De Jong, R.; Mulder, G.; Boer, J.D. Decision making performance in obsessive compulsive disorder. J. Affect. Disord. 2002, 69, 257–260. [Google Scholar] [CrossRef]
- Tolin, D.F.; Villavicencio, A. An exploration of economic reasoning in hoarding disorder patients. Behav. Res. Ther. 2011, 49, 914–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittrich, W.; Johansen, T. Cognitive deficits of executive functions and decision-making in obsessive-compulsive disorder. Scand. J. Psychol. 2013, 54, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Nestadt, G.; Kamath, V.; Maher, B.S.; Krasnow, J.; Nestadt, P.; Wang, Y.; Bakker, A.; Samuels, J. Doubt and the decision-making process in obsessive-compulsive disorder. Med. Hypotheses 2016, 96, 1–4. [Google Scholar] [CrossRef]
- Foa, E.B.; Mathews, A.; Abramowitz, J.S.; Amir, N.; Przeworski, A.; Riggs, D.S.; Filip, J.C.; Alley, A. Do Patients with obsessive–compulsive disorder have deficits in decision-making? Cogn. Ther. Res. 2003, 27, 431–445. [Google Scholar] [CrossRef]
- Sachdev, P.; Malhi, G.S. Obsessive–compulsive behaviour: A disorder of decision-making. Aust. N. Z. J. Psychiatry 2005, 39, 757–763. [Google Scholar] [CrossRef]
- Abbruzzese, M.; Ferri, S.; Scarone, S. The selective breakdown of frontal functions in patients with obsessive-compulsive disorder and in patients with schizophrenia: A double dissociation experimental finding. Neuropsychologia 1997, 35, 907–912. [Google Scholar] [CrossRef]
- Aycicegi, A.; Dinn, W.M.; Harris, C.L.; Erkmen, H. Neuropsychological function in obsessive-compulsive disorder: Effects of comorbid conditions on task performance. Eur. Psychiatry 2003, 18, 241–248. [Google Scholar] [CrossRef]
- Remijnse, P.; Nielen, M.; van Balkom, A.; Cath, D.; van Oppen, P.; Uylings, H.; Veltman, D. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch. Gen. Psychiatry 2006, 63, 1225–1236. [Google Scholar] [CrossRef]
- Cauffman, E.; Shulman, E.P.; Steinberg, L.; Claus, E.; Banich, M.T.; Graham, S.; Woolard, J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Dev. Psychol. 2010, 46, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, J.C.N.; Cardoso, C.D.O.; Shneider-Bakos, D.; Kristensen, C.; Fonseca, R.P. The effect of age on decision making according to the Iowa gambling task. Span. J. Psychol. 2012, 15, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.J.; Luciana, M.; Conklin, H.M.; Yarger, R.S. Adolescents’ performance on the Iowa Gambling Task: Implications for the development of decision making and ventromedial prefrontal cortex. Dev. Psychol. 2004, 40, 1148–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvan, A.; Hare, T.A.; Parra, C.E.; Penn, J.; Voss, H.; Glover, G.; Casey, B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006, 26, 6885–6892. [Google Scholar] [CrossRef] [Green Version]
- Ernst, M.; Jazbec, S.; McClure, E.B.; Monk, C.S.; Blair, R.J.R.; Leibenluft, E.; Pine, D.S. Amygdala and nucleus accumbens activation in response to receipt and omission of gains in adults and adolescents. NeuroImage 2005, 25, 1279–1291. [Google Scholar] [CrossRef]
- Gardner, M.; Steinberg, L. Peer Influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Dev. Psychol. 2005, 41, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Mancini, C.; Cardona, F.; Baglioni, V.; Panunzi, S.; Pantano, P.; Suppa, A.; Mirabella, G. Inhibition is impaired in children with obsessive-compulsive symptoms but not in those with tics. Mov. Disord. 2018, 33, 950–959. [Google Scholar] [CrossRef]
- Mirabella, G.; Mancini, C.; Valente, F.; Cardona, F. Children with primary complex motor stereotypies show impaired reactive but not proactive inhibition. Cortex 2020, 124, 250–259. [Google Scholar] [CrossRef]
- Noël, X.; Bechara, A.; Dan, B.; Hanak, C.; Verbanck, P. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology 2007, 21, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Kovács, I.; Richman, M.J.; Janka, Z.; Maraz, A.; Ando, B. Decision making measured by the Iowa Gambling Task in alcohol use disorder and gambling disorder: A systematic review and meta-analysis. Drug Alcohol Depend. 2017, 181, 152–161. [Google Scholar] [CrossRef]
- Rotge, J.-Y.; Poitou, C.; Fossati, P.; Aron-Wisnewsky, J.; Oppert, J.-M. Decision-making in obesity without eating disorders: A systematic review and meta-analysis of Iowa gambling task performances. Obes. Rev. 2017, 18, 936–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Qiu, X.; Zhu, X.; Zou, X.; Chen, L. Decision-making in patient s with epilepsy: A systematic review and meta-analysis. Epilepsy Res. 2018, 148, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Betz, L.T.; Brambilla, P.; Ilankovic, A.; Premkumar, P.; Kim, M.-S.; Raffard, S.; Bayard, S.; Hori, H.; Lee, K.-U.; Lee, S.J.; et al. Deciphering reward-based decision-making in schizophrenia: A meta-analysis and behavioral modeling of the Iowa Gambling Task. Schizophr. Res. 2019, 204, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| First Author, Year [Reference] | Included in Meta-Analysis? | Experimental Groups | Age | Gender | Disease Duration | N° of Patients Receiving Medication | Method—Diagnosis and Psychiatric Assessment | Method—Neuropsychological Assessment | IGT Findings | Medication Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| Blom et al., 2011 [30] | no | OCD non Hoarding (n = 17) vs. HC (n = 19) vs. HD (n = 24, of whom 14 with past OCD) | 43 [range: 19–63] | Majority F | N/A | HD: 13; OCD: 16 | Clinical interview (DSM-IV criteria); OCI-R | SI-R, The vocabulary scale from the Shipley-2, SRTT, SSRTT, IGT | OCD < HC OCD < HD | N/A |
| Boisseau et al., 2013 [27] | no | OCD (n = 19) vs. ED (n = 17) vs. HC (n = 21) | OCD: 22.32 ± 4.24, ED: 23.12 ± 4.80, HC: 24.24 ± 3.47 | F = 100% | OCD: 6.74 ± 4.92, Eds: 6.79 ± 4.95 | OCD: 5, ED: 3 | Clinical interview (DSM-IV criteria); Y-BOCS and Y-BOCS-SC, EDE-Q, FMPS, MINI | IGT | No significant differences between OCD and HC. | No medication effect |
| Borges et al., 2011 [42] | yes | pst-OCD (n = 16) vs. prt-OCD (n = 18) vs. ntOCD (n = 67) vs. HC (n = 27) | Pst-OCD: 39.2 ± 12.4, Prt-OCD: 41.2 ± 12.3, NonT-OCD: 33.0 ± 13.2, HC: 29.9 ± 7.9 | Pst-OCD: F = 9, M = 7; Prt-OCD: F = 11, M = 7; NonT-OCD: F = 39, M = 28; HC: F = 12, M = 5 | N/A | Most patients were under psychological and/or pharmacological treatment | Clinical interview (DSM-IV criteria), SCID-I for DSM-IV criteria, Y-BOCS, BDI, BAI | WCST, IGT, WMS-R LM, BVMT-R, WASI | No significant differences between OCD and HC. | N/A |
| Bottesi et al., 2014 [28] | yes | PG (n = 40) vs. AD (n = 40) vs. OCD (n = 22) vs. HC (n = 47) | PG: 40.01 ± 12.05, AD: 31.27 ± 11.58, OCD: 47.15 ± 10.43, HC: 43.06 ± 11.94 | PG: F = 5%, M = 95%, AD: F = 27.3%, M = 72.7%, OCD: F = 50%, M = 50%, HC: F = 21.3%, M = 78.7% | N/A | PG: 17, AD: 33, OCD: 12 | Y-BOCS, BDI-II, BAI, SOGS, PSWQ | AUDIT, PI, OBQ-87, BIS-11, the Go/No-go task, IGT | No significant differences between OCD and HC, nor between PG and OCD. | No medication effect |
| Cavallaro et al., 2003 [32] | yes | OCD (n = 67) vs. SKZ (n = 110) vs. HC (n = 56) | OCD: 30.5 ± 8.9, SKZ: 33 ± 9.5, HC: 31.2 ± 6.0 | OCD: F = 50.8%, M = 49.2%, SKZ: F = 40%, M = 60%, HC: F = 60.8%, M = 39.2% | OCD: 10.4 ± 8.1; SKZ: 9.4 ± 7.2 | all patients: medication-free for at least 2 weeks. | Clinical interview (DSM-IV criteria) | the Gambling Task, WCST, ToH | OCD < HC OCD < SKZ | N/A |
| Cavedini et al., 2002 [26] | yes | OCD (n = 34) vs. PD (n = 16) vs. HC (n = 34) | OCD: 33.7 ± 11.5, PD: 36.3 ± 10.9, HC: 29.5 ± 8.9 | OCD: F = 47%, M = 53%, F = 56.2%, M = 43.8%, HC: F = 55.8%, M = 43.2% | N/A | all patients: medication-free for at least 2 weeks. | Diagnostic Interview Schedule III R, RY–BOCS | IGT | OCD < HC. OCD patients made significantly more selections from the disadvantageous decks than PD and HC. | Poor neuropsychological task performance predicted poor outcome of pharmacological treatment |
| Cavedini et al., 2010 [33] | no | OCD probands and UFDR (35 pairs) vs. HC (31 pairs) | OCD probands 35.6 ± 2.9, relatives 45 ± 3, HC:probands 34.7 ± 2.9, relatives 43.2 ± 2.5 | OCD: probands F = 15, M = 20, relatives F = 22, M = 13, HC: probands F = 22, M = 9, relatives F = 23, M = 8 | N/A | Clinical interview (DSM-IV criteria); Y-BOCS, MINI-DIS | Y-BOCS, IGT, ToH, WCST | OCD < HC. HC probands chose more frequently from the advantageous decks than OCD probands. | N/A | |
| Cavedini et al., 2012 [34] | no | OCD (n = 20) vs. HC (n = 18) | OCD: 36.05 ± 11.05, HC: 27 ± 4.73 | OCD: F = 7, M = 13, HC: F = 5, M = 13 | N/A | all patients: medication-free for at least 1 month | Clinical interview (DSM-IV-TR criteria), Y-BOCS, MINI-PLUS | IGT, SCR | OCD < HC. OCD showed no significant differences of SCRs activation according to card selections, while HC did. | N/A |
| da Rocha 2011 [35] | yes | OCD (n = 107) vs. HC (n = 107) | OCD: 28.40 ± 14.12, HC: 29.33 ± 13.22 | OCD: F = 49, M = 58, HC: F = 51, M = 56 | Average duration of illness 111.54 ± 94.36 months; Average duration of untreated illness 78.40 ± 43.01 months | OCD: 85 | MINI-PLUS interview, Y-BOCS, DY-BOCS, BID, BAI, review of medical records, interview with minimum 1 close relative | Raven Progressive Matrices, IGT, CPT-II | OCD < HC | N/A |
| Grassi et al., 2015 [36] | yes | OCD (n = 38) vs. HC (n = 39) | OCD: 36.29 ± 12.73, HC: 34.10 ± 11.18 | OCD: F = 39.47%, M = 60,53%, HC: F = 51.28%, M = 48.72% | N/A | OCD: 32 | SCID-I for DSM-IV criteria; Y-BOCS, Y-BOCS-SC | BIS-11, IGT, the beads task | OCD < HC. OCD did not improve across the blocks. No difference between groups in performance under ambiguity and under risk emerged. | No medication effect |
| Grassi G, 2018 [37] | yes | DBS-OCD (n = 20) vs. TAU-OCD (n = 40) vs. HC (n = 40) | DBS-OCD: 45.65 ± 12.7, TAU-OCD: 44.75 ± 11.5, HC: 44.08 ± 9.96 | DBS-OCD: F = 55%, M = 45%; TAU-OCD: F = 45%, M = 55%; HC: F = 52.5% M = 47.5% | DBS-OCD: 26.5 [range: 20–38]; TAU-OCD: 30 [range: 17–35] | 20 OCD treated with DBS targeting the ventral limb of the internal capsule, 40 OCD TAU: medication and/or CBT | SCID-I and SCID-II for DSM-IV criteria; Y-BOCS, Y-BOCS-SC | IGT, the beads task. | OCD < HC | No differences were found between OCD patients treated with DBS or TAU |
| Grassi G, 2019 [29] | yes | OCD (n = 44) vs. GD (n = 26) vs. HC (n = 40) | OCD: 33 [range: 42.75; 26]; GD: 39 [57.25; 33.75]; HC: 34 [48; 27] | OCD: F = 11.5%; GD: F = 31.8%; HC: F = 20% | OCD: 15.6 ± 10.4; GD: 12.1 ± 9.9 | OCD: 93.2%; GD: 61.5% | SCID-I and SCID-II for DSM-IV criteria; Y-BOCS, Y-BOCS-SC, PG-YBOCS, HDRS, HARS, SHAPS | TIB, BIS-11, IGT, FTND, Burghart’s Sniffin’ Sticks Screening Test | OCD < HC GD < HC OCD = GD | N/A |
| Kashyap et al., 2013 [38] | yes | OCD (n = 150) vs. HC (n = 205 of whom 75 performed the IGT) | OCD: 27.56 ± 7.35, HC: 27.42 ± 6.57. | OCD: F = 56, M = 94; HC: N/A | 8.39 ± 5.69 | 80% of OCD: SRIs. 29.9% CBT. 44.5% augmentation, either with an antipsychotic (13.9%) or a benzodiazepine (25.5%) | Clinical interview (DSM-IV criteria), MINI Plus, Y-BOCS, CGI, BABS, STAI, HDRS- 17. | CTT, Digit Span (WMS III), Matrix Test (WAIS III), AVLT, CFT, ToH, WCST, OAT, IGT, SCWT, COWA, Five-point Test, Verbal N-Back, Spatial Span, BGT | OCD < HC | N/A |
| Kim et al., 2015 [18] | yes | OCD (n = 65) vs. HC (n = 58) | OCD: 26.62 ± 9.12, HC: 26.56 ± 6.28 | OCD: F = 14, M = 51, HC: F = 22, M = 36 | 9.57 ± 7.46 | OCD: 63 | SCID for DSM-IV criteria. Y-BOCS, MADRS | IGT, GDT, SRLT, WCST | OCD < HC at the netscore and at the last three blocks. | N/A |
| Kodaira et al., 2013 [39] | yes | OCD (n = 22) vs. HC (n = 22) | OCD: 163.5 ± 22.1 months, HC: 161.8 ± 20.6 months | OCD: F = 10, M = 12, HC: F = 10, M = 12 | 23.9 ± 21.3 months | OCD: 10 | Clinical interview (DSM-IV-TR criteria), NIMH-OCS, CY-BOCS, IGT, WISC-III, WCST | IGT, WISC-III, WCST | OCD < HC. OCD selected a higher number of disadvantageous cards than HC in the last block; this number was associated with CY-BOCS score. | N/A |
| Krishna et al., 2011 [43] | yes | OCD (n = 31) vs. HC (n = 31) | OCD: 26.0 ± 6.1, HC: 26.3 ± 6.2. | OCD: F = 7, M = 24, HC: F = 7, M = 24 | 48 months | all patients: medication-naïve | Clinical interview (DSM-IV criteria), MMSE, MINI, YBOCS | WCST, CPT, TMT, TOL, COWA, WMS, IGT, RCFT, OAT, BGT, fMRI, DST, Design fluency test, SCWT, Rey’s AVLT, Matrix reasoning test | No significant differences between OCD and HC. | N/A |
| Lawrence et al., 2006 [44] | yes | OCD (n = 39) vs. HC (n = 40) | OCD: 36.1 ± 11.07, HC: 33.48 ± 10.4 | OCD: F = 19, M = 20, HC: F = 20, M = 20 | 20.7 ± 12 | OCD: 30 | SCID I and II for DSM-IV criteria; Y-BOCS, Y-BOCS-SC, SI–R, BDI, STAI | IGT, WCST, NART, SCR | No significant differences between OCD and HC. IGT performance and SCR were impaired in patients with prominent hoarding symptoms. | N/A |
| Martoni et al., 2015 [40] | no | OCD (n = 269) vs. HC (n = 120) | OCD: 34.43 ± 11.70, HC: 32.14 ± 11.02 | OCD: F = 141, M = 129, HC: F = 78, M = 42 | 12.68 ± 9.98 | OCD: 58.4% | Clinical interview (DSM-IV-TR criteria), Y-BOCS | WAIS-R, IGT | OCD < HC. Patients with high scores in ‘Washing’ and ‘Symmetry’ factors showed an improving performance across the blocks; patients with high scores in ‘Hoarding’, ‘Rituals’ and ‘Forbidden thoughts’ did not. | N/A |
| Nielen et al., 2002 [45] | no | OCD (n = 27) vs. HC (n = 26) | OCD: 34.9 ± 9.9, HC: 31.2 ± 8.3 | OCD: F = 20, M = 7, H: F = 18, M = 8 | 18.4 ± 12.3 | all patients: medication-free | Clinical interview (DSM-IV-TR criteria), Y-BOCS, HARS, HDRS | the Gambling Task, RSPM | No significant differences between OCD and HC Within OCD group, IGT performance was associated with anxiety and OCD severity. | N/A |
| Norman LJ, 2018 [31] | yes | OCD (n = 20) vs. ADHD (n = 16) vs. HC (n = 20) | HC: 15.15 ± 1.99, ADHD: 14.61 ± 1.87, OCD: 15.76 ± 1.43 | M = 100% | N/A | OCD: 16 medication naïve, 4 SSRI, 1 risperidone augmentation treatment | clinical interview with patients and parents (following ICD-10 criteria); CY-BOCS | WASI-R, IGT, fMRI, SDQ | No significant differences between OCD and HC. | N/A |
| Starcke et al., 2009 [15] | no | OCD (n = 14) vs. HC (n = 15) | OCD: 36.36 ± 8.54, HC: 36.60 ± 10.84 | OCD: F = 7, M = 7, HC: F = 5, M = 10 | N/A | OCD: 9 | SCID for DSM-IV criteria | LPS-4, IGT, GDT, SCR | OCD < HC. HC showed higher SCR elevations after losses than after gain, OCD did not | N/A |
| Starcke et al., 2010 [6] | yes | OCD (n = 23) vs. HC (n = 22) | OCD: 35.25 ± 7.35, HC: 36.50 ± 10.23 | OCD: F = 10, M = 13, HC: F = 10, M = 12 | N/A | OCD: 14 | SCID for DSM-IV criteria | GDT, IGT, AFLT, LPS-4, mWCST, TMT A and B, ToH, F-A-S-test, the Word Color Interference Test. | OCD < HC at netscore, block 3, block 5. | No significant differences for medicated and unmedicated patients on any of the decision-making tasks |
| Tolin and Villavicencioa 2011 [46] | no | HD (n = 42) vs. OCD (n = 20) vs. HC (n = 36) | HD: 51.14 ± 8.33, OCD: 31.21 ± 11.80, HC: 47.00 ± 12.29 | HD: F = 31, M = 11, OCD: F = 8, M = 12, HC: F = 29, M = 7 | N/A | HD: 23; OCD: 22 | CGI, ADIS-IV, HRS-I, HRSD-17, SIGH-D, SI-R, OCI-R, FIS | IGT, The dissonance reduction task | No significant differences between OCD and HC. | N/A |
| Zhang et al., 2015 [6] | yes | umOCD (n = 57) vs. mOCD (n = 77) vs. rOCD (n = 48) vs. HC (n = 115). | umOCD: 28.07 ± 7.73, mOCD: 27.92 ± 7.07, rOCD: 28.50 ± 7.61, HC: 27.32 ± 7.81 | OCD: F = 87, M = 95 [um = OCD: F = 30, M = 27, mOCD: F = 42, M = 35, rOCD: F = 23, M = 25], HC: F = 60, M = 55. | umOCD: 75.95 ± 45.69 months, mOCD: 65.83 ± 46.35 months, rOCD: 63.60 ± 36.73 months | overall, 125 | SCID for DSM-IV criteria, HARS-14, HDRS-17, Y-BOCS | DST, TMT, WCST, IGT, GDT. | umOCD, mOCD and rOCD < HC at netscore and at the last three blocks. | DM deficits under ambiguity persisted regardless the medication status and symptom remittance. |
| Zhang et al., 2015 [19] | no | OCD (n = 55) vs. UFDR (n = 55), HC (n = 55) | OCD: 26.51 ± 7.84, UFDR: 28.42 ± 7.37, HC: 27.85 ± 7.32 | OCD: F = 33, M = 22, UFDR: F = 29, M = 26, HC: F = 31, M = 24 | 4.33 ± 3.66 | all patients: medication-naïve | Clinical interview with the patient at minimum 1 relative (DSM-IV-TR criteria), Y-BOCS, HDRS-17, HARS | SCWT, DST, TMT, WCST, IGT, GDT, ToL. | OCD and UFDRs < HC | N/A |
| Zhang et al., 2017 [41] | no | preop-OCD (n = 51) vs. postST-OCD (n = 24) vs. postLT-OCD (n = 32) vs. HC (n = 31) | preop-OCD: 30.71 ± 7.62, postST: 29.29 ± 5.72, postLT: 33.41 ± 7.86, HC: 37.77 ± 10.83 | preop-OCD: F = 15, M = 36, postST-OCD: F = 8, M = 16, postLT: F = 12, M = 20, HC: F = 14, M = 17 | preop-OCD: 10.66 ± 7.12; postST: 8.92 ± 3.55; postLT: 10.50 ± 4.85 | N/A | MINI for DSM-IV-TR, Y-BOCS, HDRS-17, HARS | IGT | preopOCD < HC postLT-OCD = HC | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisticò, V.; De Angelis, A.; Erro, R.; Demartini, B.; Ricciardi, L. Obsessive-Compulsive Disorder and Decision Making under Ambiguity: A Systematic Review with Meta-Analysis. Brain Sci. 2021, 11, 143. https://doi.org/10.3390/brainsci11020143
Nisticò V, De Angelis A, Erro R, Demartini B, Ricciardi L. Obsessive-Compulsive Disorder and Decision Making under Ambiguity: A Systematic Review with Meta-Analysis. Brain Sciences. 2021; 11(2):143. https://doi.org/10.3390/brainsci11020143
Chicago/Turabian StyleNisticò, Veronica, Andrea De Angelis, Roberto Erro, Benedetta Demartini, and Lucia Ricciardi. 2021. "Obsessive-Compulsive Disorder and Decision Making under Ambiguity: A Systematic Review with Meta-Analysis" Brain Sciences 11, no. 2: 143. https://doi.org/10.3390/brainsci11020143
APA StyleNisticò, V., De Angelis, A., Erro, R., Demartini, B., & Ricciardi, L. (2021). Obsessive-Compulsive Disorder and Decision Making under Ambiguity: A Systematic Review with Meta-Analysis. Brain Sciences, 11(2), 143. https://doi.org/10.3390/brainsci11020143




