1. Introduction
Toward elucidating information processing in the human brain, neuroscience research with a computational approach is being conducted. This approach understands the brain by considering its operating principles and expresses these processes with a mathematical model. If computational research advances the understanding of the mechanisms of the human brain, it will be possible to utilize the superior functions of the brain in various fields such as engineering, medical care, and rehabilitation.
David Marr, a pioneer in computational research, stated that three-level perspectives: Computational theory, representation and algorithm, and hardware implementation are important in understanding information processing in the human brain [
1]. Computational theory research focusing on trajectory planning in human motor control, has been actively conducted, and several models for trajectory generation based on optimization criteria have been proposed since the end of the 20th century. The primary optimization criteria for generating human movement trajectory are the minimum jerk criterion [
2], minimum commanded torque change criterion [
3,
4], minimum muscle-tension change criterion [
5], minimum motor command change criterion [
6], and minimum variance criterion [
7]. It has been reported that the trajectory generated by the minimum commanded torque change criterion is the closest to the human trajectory among movement criteria that can be quantitatively evaluated [
4,
8,
9]. Several neural network models have been proposed as representation, algorithm, and hardware that can satisfy these computational criteria [
10,
11,
12,
13]. Among them, Wada and Kawato’s computational model for trajectory formation based on the optimization principle is a model in which the minimum commanded torque change criterion is adopted as the computational theory, and the forward inverse relaxation model (FIRM) is introduced as the hardware and algorithm [
14,
15]. The FIRM can reproduce the features of human reaching movements as well as complicated human motion trajectories, such as handwriting [
16,
17]. The introduction of FIRM has overcome three problems of conventional neural network models: (1) The spatial representation of time is used, (2) back propagation is essential, and (3) too many iterations to obtain an optimal trajectory are required, by alternately and repeatedly calculating the forward and inverse dynamics models. The via-point representation of early FIRM had both spatial and temporal information, but with this mechanism, the time of passing through the via-point position could not be redetermined when the entire movement duration (the time from the start to the end) was changed. Therefore, Wada and Kawato proposed an algorithm that can optimally determine the via-point time without explicitly expressing the time information, using only spatial information as the via-point representation [
15]. Consequently, the current FIRM has been improved such that the movement trajectory, the motor command torque, and the position and time of the via-point can be optimally determined using the entire movement duration and spatial conditions given as inputs.
However, considering actual human movement, the entire movement duration is not always given as an input, and as such, it may be optimally determined based on certain criteria [
18,
19]. According to empirical facts, including Fitts’ law [
20,
21], the movement duration changes depending on task factors, such as required accuracy and movement distance. Indeed, how the movement duration is determined is one of the major issues in computational studies of reaching movement.
In order to achieve accurate, smooth, and low-energy (i.e., low-exerted-torque) movement, it is advisable to lengthen the movement duration and move slowly. Tanaka et al. proposed a minimum time model [
19], in which the shortest movement duration is determined under the constraint that endpoint accuracy meets a given tolerance in the presence of signal-dependent noise [
7]. However, Mazzoni et al. pointed out that patients with Parkinson’s disease are more likely to choose to move slowly, even though they can move as fast as control subjects [
22] thus, the minimum time criterion alone may not be sufficient. They also suggested that implicit motor motivation may be involved in determining movement duration, because Parkinson’s disease patients show symptoms such as bradykinesia, due to a decrease in dopamine, which is said to be involved in motor motivation. Moreover, Berret and Jean estimated the cost of time (CoT) from behavioral experimental data using the inverse optimal control approach, assuming that the CoT and the trajectory cost have an additive relationship [
18]. The results of their study demonstrated a striking sigmoidal shape of the CoT for self-paced reaching.
The primary purpose of our study is to elucidate the information processing mechanism of the human brain, especially during movement duration planning. Hence, we propose a model for trajectory planning that incorporates movement duration planning into the FIRM that considers the smoothness of motor commands and endpoint tolerance. Although the FIRM is an existing model, the module of the movement duration planning algorithm is the novelty of this research. The movement durations, hand paths, and velocity profiles estimated by our model well represented the features of human reaching movement. This suggests that it may explain motor information processing in the human brain. Furthermore, we estimated the distributions of the difference between the mean of the measured movement duration and model movement duration using Bayesian statistics and investigated how much difference there was between them. Finally, we described the limitations of the model.
2. Movement Duration Optimization Module
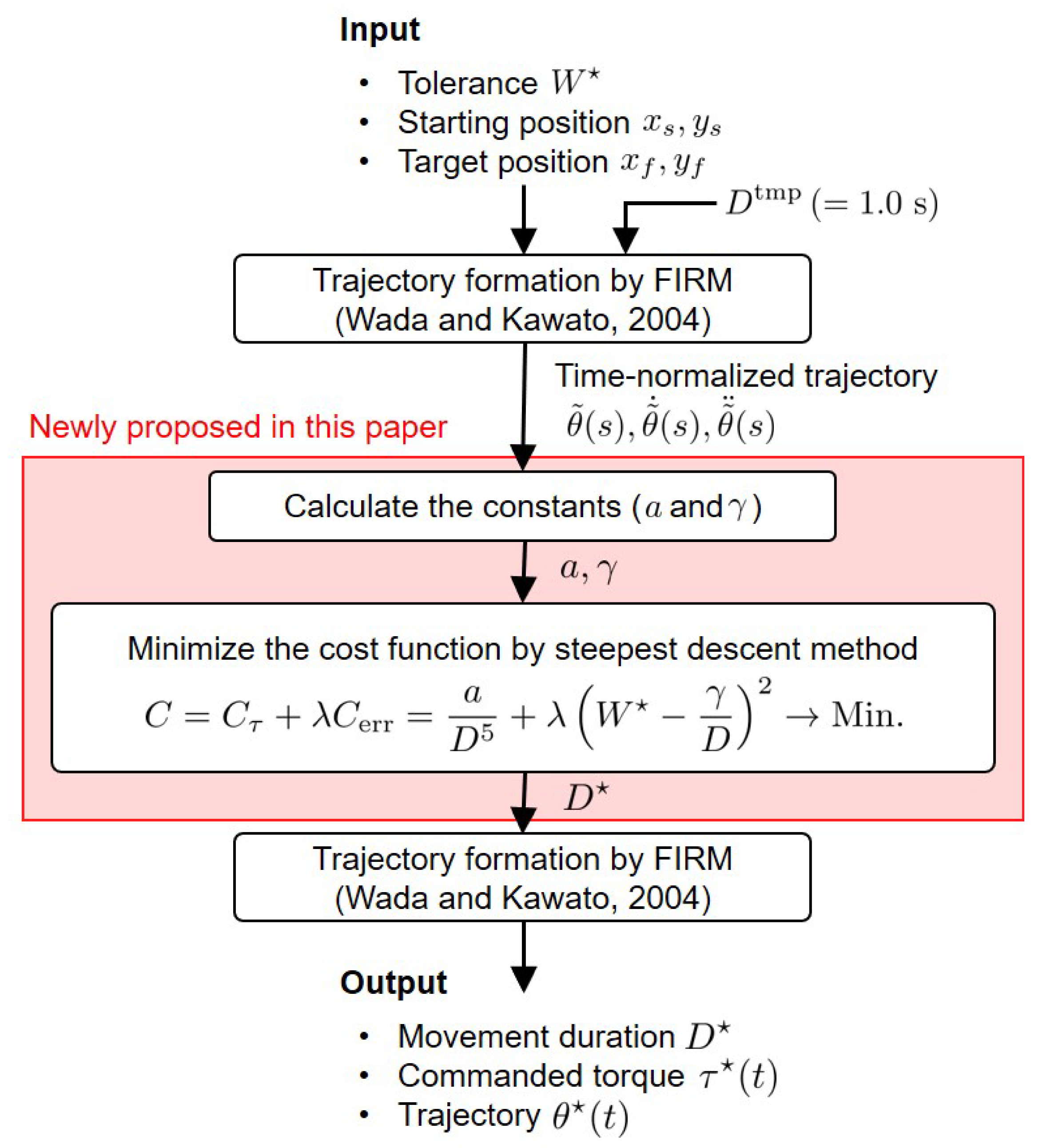
The computational model of the point-to-point arm reaching movement consists of the movement duration optimization model proposed in this paper and the FIRM. A schematic diagram of this model is shown in
Figure 1. The inputs of this model are the tolerance (
), which is the allowable error, such as the radius of the target, and the starting and target positions (
,
,
,
), which are given first as visual information. To find the movement duration
D that minimizes the cost function
C, it is necessary to calculate
a and
, which are determined based on the arm dynamics. To determine
a and
, the time-normalized trajectory is determined by the FIRM when
is the input. The steepest descent method finds the optimal solution
of the cost function
C (see
Section 3.4.5 for details of the engineering algorithm), and the optimal trajectory is generated by the FIRM using the
. Thus, the movement duration (
), commanded torque (
), and trajectory (
) are finally obtained as outputs.
We propose that the movement duration is determined by the optimization of the cost function which is expressed as a weighted sum of two terms related to smoothness and meet-tolerance as follows:
where
denotes the
i–th joint commanded torque at time
t,
t changes from 0 to movement duration
D, and
N represents the number of joints. The smoothness term
is the sum of the commanded torque change, and the tolerance term
is the squared error between the tolerance
, given as the task condition and the endpoint variability estimated in the brain
.
takes the minimum when
is equal to
, but the whole cost function
C should be minimized to consider trajectory smoothness. The weight
indicates how much more important the tolerance term is than the smoothness term.
Figure 2 shows a schematic diagram of this cost function.
and
can be approximately expressed as a function of the movement duration
D, as shown in the following equation:
The mathematical proof of these equations is given in the next section.
2.1. Proof of
The multi-joint human arm dynamics on the horizontal plane is as follows: [
3]
where
is the joint torque vector, and
,
, and
are the joint angular position, velocity, and acceleration, respectively. The first term is the inertial force given in terms of the inertia matrix
. The second term is the Coriolis force, which is given in terms of a Coriolis force coefficient matrix
. The third and fourth terms are the centrifugal and viscous forces in terms of the coefficient matrices of the centrifugal and viscous forces,
and
, respectively. Additionally,
and
are the vectors in the Coriolis and centrifugal forces,
and
, respectively (the superscript
denotes the transpose of a vector or matrix).
The above equation can be written as a function of the movement duration
D, by introducing the time-normalized variable
s (
), as follows (see [
23] for details):
where the tilde (
) means time-normalized, and
denotes the approximation coefficient that approximately holds when the time dependence of the viscous force is small.
Therefore, the commanded torque change is given as:
Substituting the above equation into the cost function of the minimum commanded torque change criterion gives:
where
a is a constant.
2.2. Proof of
The hand endpoint errors
and
in the
x- and
y-axes are expressed by the following equations [
23]:
where
denotes a signal-dependent noise parameter at the
i-th joint, and
and
are the time-normalized components, which are calculated based on arm dynamics.
is a pseudorandom variable that generates a different sequence every time, according to the standard normal distribution. Thus,
and
are random variables. The hand endpoint error
is defined as the Euclidean distance of
and
as follows:
where
is approximately represented as a function of
D with
as a constant, that is,
is inversely proportional to
D. Consequently,
is given as:
3. Methods
To examine the validity of the movement duration planning model, the behavioral experiment is performed to compare the movement duration estimated by the model with the actual human one. The distribution of the difference between the mean values of the measured and model movement duration was estimated based on Kruschke ’s Bayesian estimation supersedes the t-test (BEST) [
24,
25].
3.1. Subjects
A total of 6 healthy young adults (6 males; age range, 21–24 years) participated in this study. All subjects were right-handed according to the Edinburgh handedness test score (score range, 64.7–100%). Informed consent was obtained from all subjects, and the study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Nagaoka University of Technology (2019–2021 R1-1, 23 August 2019 approved).
3.2. Apparatus
The experimental setup is illustrated in
Figure 3A. The subject was asked to sit on a chair that was placed facing the table and display (PDP-504P, Pioneer, Tokyo, Japan). The subject placed his right forearm on the table, and the air-sled (a device used to reduce the frictional resistance between the table and the arm) was attached and fixed. Then, the chair height was adjusted such that the table and the subject’s arm were parallel. The chair was moved toward the table until the subject’s chest touched the table. Infra-red markers were affixed to the shoulder, elbow joints, and the hand, and the positions of the three markers were measured at a sampling frequency of 500 Hz using a three-dimensional optical position measurement digitiser (Optotrak Certus, Northern Digital Inc., Waterloo, ON, Canada). The measured current hand positions were projected onto the display installed in front of the subject. A starting point (a circle with a radius of 10.0 mm) and a target (a circle with a radius of 8.00, 15.0, or 25.0 mm) were also shown on the screen, and the subjects performed the experimental task while watching the display.
3.3. Task
We sought to investigate how the determined movement duration changes under a different tolerance, starting point, and final point conditions. To this end, the experimental task was designed to have the subject perform reaching movements toward large, medium, and small targets appearing in four directions: Front, back, left, and right, 150 mm away from the starting point. The endpoint accuracy was controlled by changing the tolerance, that is, target size. The target circle radius was 8.00, 15.0, or 25.0 mm. When the experimental program was run, the shoulder positions were defined as the origin of the task coordinate system. The starting circle was displayed in the center of the screen, and the coordinate of the starting circle was defined as a shoulder joint angle of
(
), and an elbow joint angle of
(
) therefore, the starting coordinates for the right shoulder varied depending on the subject. The targets were placed in four directions: Front (
), back (
), left (
), and right (
), based on the starting position. See
Figure 3B for details.
There were four directions and three target tolerances thus, a total of 12 types of patterns were displayed on the screen. The pattern of the four directions was pseudo-randomly proposed and switched to the next pattern when the trial was regarded as a success. Each pattern was performed in 32 trials, and the experiment continued until a total of 384 successful trials were conducted.
The subject received the following four instructions:
Meet your hand endpoint with the target circle;
Avoid corrective movements as much as possible;
Increase the number of consecutive successful trials displayed in the lower left of the screen as much as possible;
Reach to the target as quickly as possible.
With regard to item 3, we set this instruction to avoid too many failure trails. This also functions to control the success rate, which is defined as the percentage of success among a certain number of trials.
The definition of success was as follows:
The hand endpoint is within the target circle;
The tangential velocity from the start to the end of the reaching movement is bell-shaped, with one peak.
A corrective movement was considered to have been performed if there was more than one peak and in this case, the trial was regarded as a failure. All trials satisfying item 2 were used in the analysis, that is, even if the hand endpoint was not within the target circle, it was used in the analysis, as long as it was a trial without corrective movement. This was acceptable because the movement duration determined by our proposed model accounts for the movement duration of trials outside the circle, and not the movement duration obtained only including successful trials. This experiment was conducted after the subject had sufficient practice therefore, no clear learning effect was observed in the experimental data.
3.4. Data Analysis
3.4.1. Measured Movement Duration
The acquired positional data were low-pass filtered using a third-order, zero-phase-lag Butterworth filter with a cut-off frequency of 10 Hz. The start and end of the movement for each trial were determined based on the tangential velocity, which was calculated using numerical differentiation. The start of the movement was defined as the point following the start cue at which the tangential velocity first exceeded the threshold velocity, defined as 5% of the peak value of the tangential velocity. The end of the movement was defined as the point prior to the end cue at which the tangential velocity fell below the threshold. The time from the start to the end of the movement was then defined as the measured movement duration.
3.4.2. Model Movement Duration
The flow for obtaining the model movement duration is as follows. First, to calculate the constants a and , the time-normalized trajectory , , and the parameters of arm dynamics and signal-dependent noise were obtained. Subsequently, a and were calculated, and , the weight of the cost function, was determined. Finally, was determined using the steepest descent method. These details will be explained step-by-step in the next section.
3.4.3. Calculation of a and
First, the time-normalized trajectory , , and the parameters of arm dynamics and signal-dependent noise were obtained to calculate the constants a and .
The parameters of the arm dynamics consist of the
i-th link length (
), the distance from the
i-th joint to the center of mass (
),
i-th link mass (
), moment of inertia around the
i-th joint (
), and joint viscosity coefficient responsible for converting the
j-th joint angular velocity to the
i-th torque (
). The
,
, and
were obtained by the proportional calculation of
(see [
23] for details).
was calculated from the measured initial hand, elbow, and shoulder positions.
was estimated using the method in [
4], based on the approximate relationship between the joint torque and the measured viscosity coefficient during static force control.
was obtained for each tolerance condition, and then determined for each direction by taking the average over all the tolerance conditions.
Table 1 shows the parameters of the subject’s arm dynamics.
The method for estimating the parameters of the signal-dependent noise (
) is described.
was determined by searching for a pair of
and
when the 95% confidence ellipse of the hand endpoints generated by the simulation overlapped the measured one with a grid search algorithm. The hand endpoint error by simulation was obtained by the following procedure (see [
23] for details):
Joint torque is calculated using measured trajectory data and parameters of arm dynamics.
Generate signal-dependent noise at arbitrary and , and add to the joint torque, which is calculated as 1.
The hand endpoint error is generated by converting the noise-added torque from the joint space to the task space using the forward dynamics model.
Table 2 shows the obtained signal-dependent noise parameters of the subject.
Once the arm dynamics and noise parameters have been obtained, the time-normalized trajectory can be calculated. As a precondition for calculating the time-normalized trajectory, it is assumed that the human hand path does not change kinematically within a certain movement duration [
23]. The time-normalized trajectory was obtained by inputting the movement duration (
, because of time normalization) and the spatial information of the start and target points to the FIRM using the obtained parameters.
The approximation coefficient
of Equation (
8) was obtained by the least squares method, where the interval of
D is defined as [
,
]. The interval of
D was defined as [0.40, 1.50] s thus,
.
Finally, the constants
a in Equation (
4) and
in Equation (
5) were calculated using the above-obtained parameters. The value of
varies each time, since it includes the pseudo-normal random number
z. Therefore, we generated 1000 pseudorandom numbers to confirm the probability distribution of
more accurately.
3.4.4. Determination of
The weight
was determined by the grid search algorithm when the average value of the mean absolute error (MAE) between the average values of the measured and model movement duration was the smallest. The grid search range was from
to
. There are two assumptions about
: (1) There is one
per subject, regardless of tasks such as movement direction, and (2)
exists in each task, that is, each direction.
Table 3 shows the
determined for each direction, and the
determined as the mean value over the directions based on these assumptions. As shown in
Table 3, the determined
has values of the order of
and
, and the average value across subjects was
.
3.4.5. Optimization Algorithm
Several engineering methods can be used to obtain the optimal solution (i.e., movement duration
D) to minimize the cost function. In the current study, the steepest descent method, using the Rprop algorithm [
26], was adopted. The Rprop algorithm accelerates the convergence to the minimal solution, while also preventing divergence that can occur with the normal steepest descent method by adjusting the learning rate
according to the sign information of the gradient. The value of
increases slightly as
D changes in the desired direction if the gradients of the current and next step have the same signs (specifically,
is increased only when the value obtained by multiplying
by the coefficient
is smaller than the threshold
otherwise,
remains unchanged). On the contrary,
is small because the updated
D is oscillatory if the gradients of the current and next steps have opposite signs (specifically,
is reduced only when the value obtained by multiplying
by the coefficient
is larger than the threshold
otherwise,
remains unchanged). The parameters of the Rprop algorithm are listed in
Table 4. The
denotes the initial value of the learning rate.
3.4.6. Bayesian Estimation of the Difference between Two Mean Values
The distribution of the difference between the mean values of the measured and model movement duration was estimated based on Kruschke (2013, 2014)’s Bayesian estimation supersedes the
t-test (BEST) [
24,
25]. We used the MATLAB Toolbox for Bayesian Estimation [
27] which is a MATLAB implementation of Kruschke’s R code BEST package. In the posterior distribution estimation of the difference between the mean values, it is important that 95% HDI (highest density interval) is included in ROPE (region of practical equivalence). If the effect size is within ROPE, two mean values are considered to be equal. The ROPE was set to
0.1, 0.1]. For the prior distribution, a non-information prior distribution (uniform distribution) was used. The parameters used in the analysis are as follows:
Number of separate Markov chain Monte Carlo methods (MCMC) chains: 3;
Number of MCMC steps that are saved for each chain: 4000;
Number of steps that are thinned: 5.
5. Discussion
We propose that the movement duration is determined by a cost function that considers two things: To get smoother motor commands and to fit the hand endpoint variability in a given tolerance. Here, the movement duration optimization module was added to the FIRM, which is a computational model of human arm movement. The movement duration determined by the proposed model represented a universal phenomenon, such as the features of hand trajectory and velocity and the speed-accuracy trade-offs found in actual human movement (
Figure 4). The MAE between the average values of the measured and model movement duration was about 0.2 s at the maximum (
Figure 5). Furthermore, from the Bayesian estimation result of the difference between these mean values, it could be said that the average values of the measured and model movement duration were substantially equal under the condition of about one-third of the whole (
Table 5). The trajectory generated by the FIRM with this movement duration as the input reproduced the actual trajectory well (
Figure 6).
As mentioned in
Section 4.2, it is important to be able to reproduce not only the average value of the movement duration, but also its distribution with a model.
Figure 7 shows the distribution of the measured and model movement duration shown in
Figure 4A. The observed distribution and model distribution were not similar; the distribution of observed movement duration was likely to be normal, whereas the distribution of movement duration determined by the model was likely to be exponential distribution. In Equations (
15) and (
17),
and
follow a normal distribution, with an average of 0, such that the Euclidean distance
follows a Rayleigh distribution therefore,
in Equation (
19) also follows the Rayleigh distribution. It is considered that the movement duration
became an exponential because the
is obtained using Equation (
19). These findings suggest that further research is necessary to reproduce the distribution of actual human movement duration using the model.
As shown in
Figure 5, the leftward movement had the highest MAE among the four directions. Such an error is mainly due to the error caused by making some approximations and assumptions in modeling. Therefore, the error should be reduced by considering the approximation and assumptions more strictly in future work.
We investigated how the
–
D profile behaves when parameter
is changed. As shown in Equation (
1),
is a parameter indicating the extent to which the tolerance term should be emphasized with respect to the smoothness term.
was changed to
,
,
, and
, and the base value of
is shown in
Table 3.
Figure 8 shows that
is more important than
when
is increased thus, the movement duration becomes shorter.
One of the limitations of this study is the number of subjects. There are few subjects (six subjects) therefore, we could not rely on the power analysis, which requires a large number of samples. Instead, Bayesian statistics were used to estimate the difference in mean values.
Unfortunately, there is currently no clear rationale as to why the movement duration does not become too slow. If the shortest movement duration was determined under a certain condition, we found a sigmoidal shape of the CoT, as described by [
18] (the
area of the green dashed line in
Figure 2). Our findings represent the first step in clarifying the information processing mechanism of the brain during human-reaching movements.
As shown in
Figure 4,
Figure 5,
Figure 6 and
Figure 7, FIRM does reproduce the human motor trajectory well, but at the same time shows that the present distribution still needs improvement. This is the first time that FIRM has been extended to a stochastic model, and these will need further research. FIRM can be realized with a biologically plausible neural network model [
14,
15]. Therefore, it can be expected that a model incorporating this movement duration planning module into the FIRM is closer to the calculation performed in the human brain. We have incorporated the cost of exercise time to further improve this superior model. As explained in the Introduction section, it is the same idea as the model of Berret et al. (2016) in terms of CoT. In future, we would like to consider the possibility of multiple costs that incorporate time costs into FIRM. The possibility of multiple costs that incorporate CoT into FIRM will be considered in future.