Neuroprotective Benefits of Exercise and MitoQ on Memory Function, Mitochondrial Dynamics, Oxidative Stress, and Neuroinflammation in D-Galactose-Induced Aging Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Drug Administration
2.3. Treadmill Exercise (TE)
2.4. Passive Avoidance Task
2.5. Morris Water Maze Test
2.6. Tissue Preparation
2.7. Mitochondria Isolation
2.8. Western Blotting
2.9. Immunohistochemistry (IHC)
2.10. Statistical Analyses
3. Results
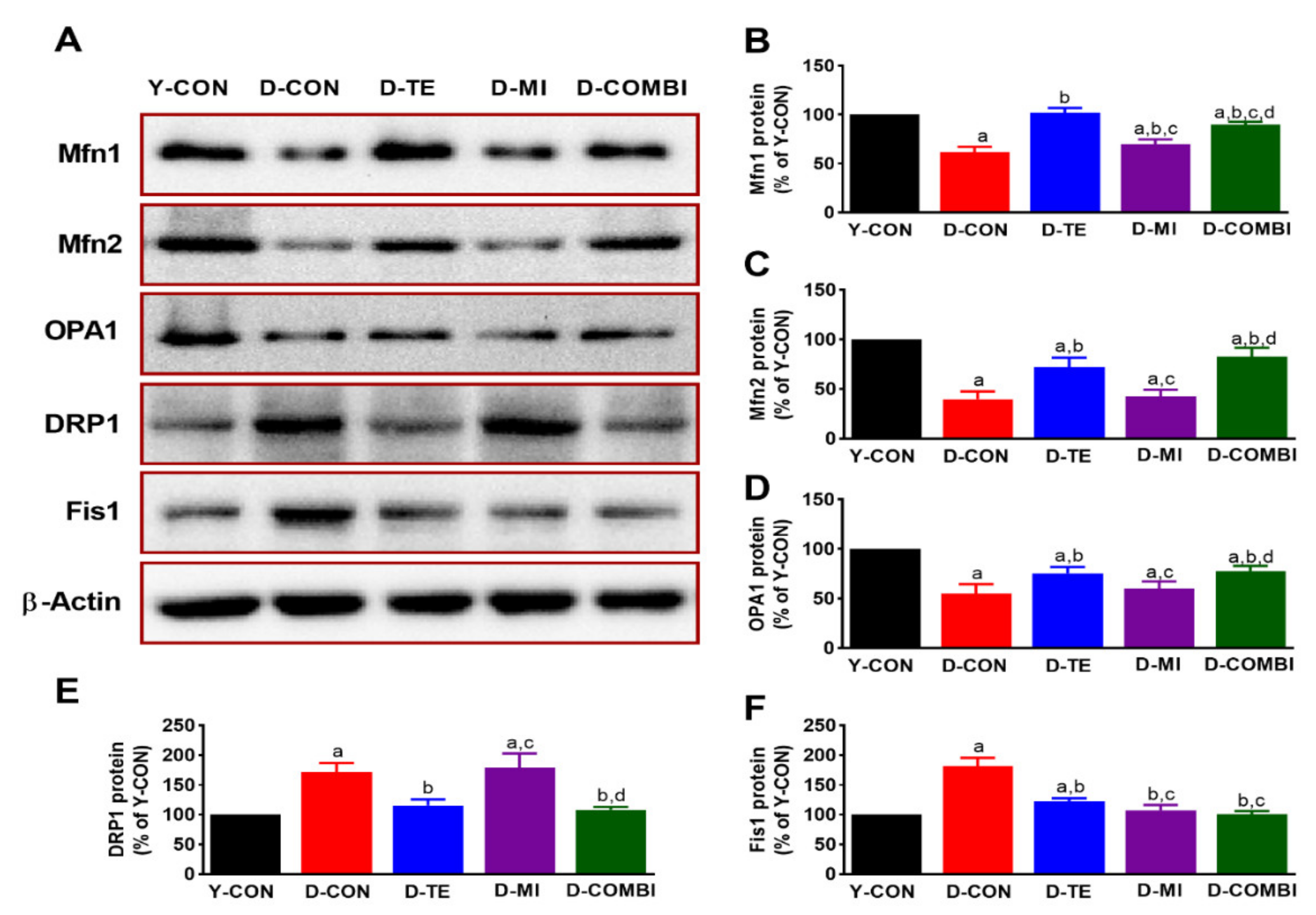
3.1. Effects of Treadmill Exercise and MitoQ on the Expression of Mitochondrial Dynamics-Related Proteins in the Hippocampus of D-Gal-Induced Aging Rats
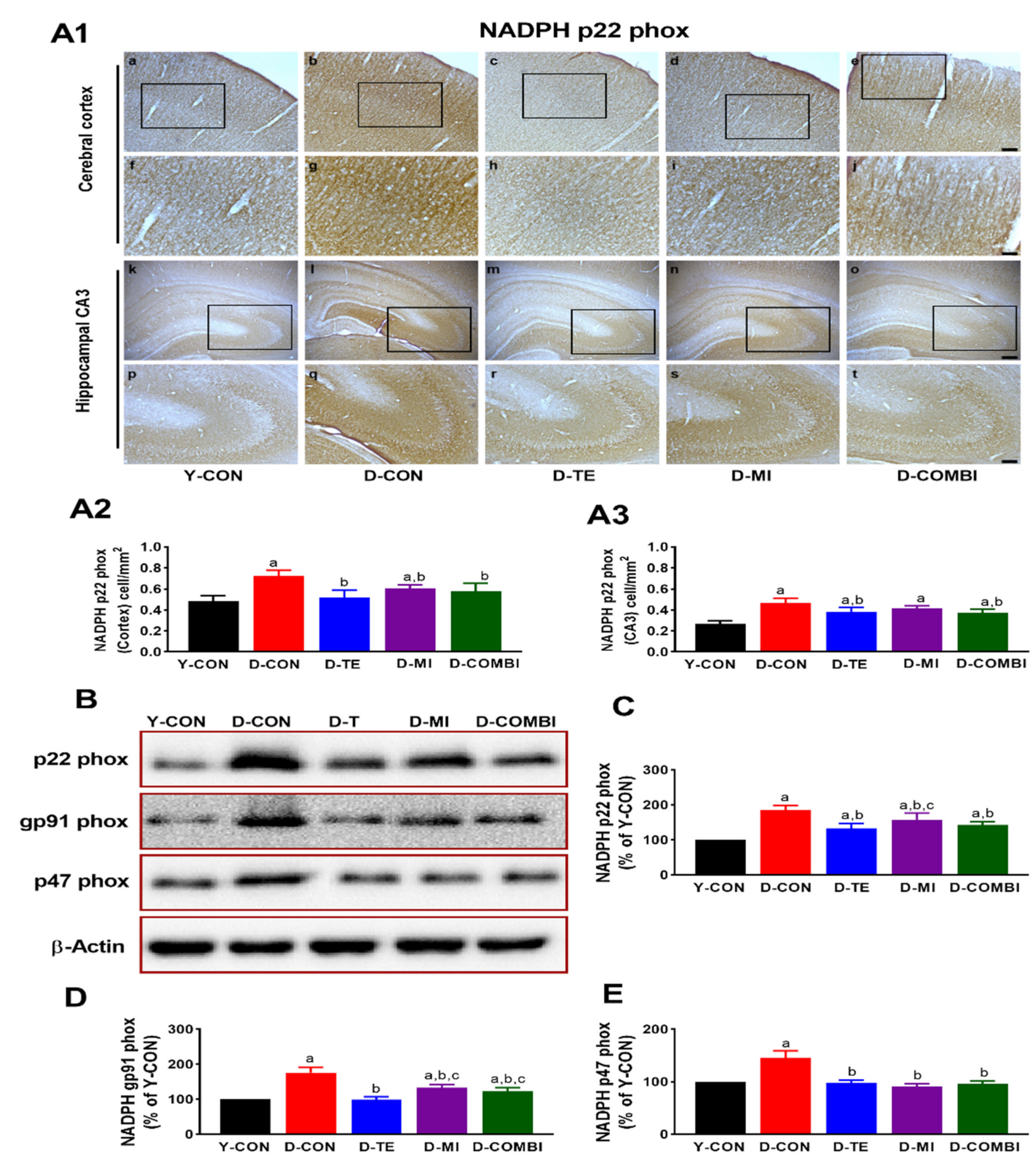
3.2. Effects of Treadmill Exercise and MitoQ on the Expression of NADPH Oxidase Subunits in the Cerebral Cortex and Hippocampus of D-Gal-Induced Aging Rats
3.3. Effect of Treadmill Exercise and MitoQ on GFAP Expression in the Cerebral Cortex and Hippocampal Dentate Gyrus of D-Gal-Induced Aging Rats
3.4. Effect of Treadmill Exercise and MitoQ on the Expression of Inflammatory Proteins and Antioxidant Enzymes in the Hippocampus of D-Gal-Induced Aging Rats
3.5. Effect of Treadmill Exercise and MitoQ on Learning and Memory in D-Gal-Induced Aging Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, J.N. Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing Res. Rev. 2006, 5, 1–13. [Google Scholar] [CrossRef]
- Rowe, J.W.; Kahn, R.L. Successful aging. Gerontologist 1997, 37, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Lucero, M.J.; Yeo, G.W.; Stecker, K.; Heivand, N.; Zhao, C.; Yip, E.; Afanador, M.; Schroeter, H.; Hammerstone, J.; et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J. Neurosci. 2007, 27, 5869–5878. [Google Scholar] [CrossRef] [PubMed]
- Yook, J.S.; Rakwal, R.; Shibato, J.; Takahashi, K.; Koizumi, H.; Shima, T.; Ikemoto, M.J.; Oharomari, L.K.; McEwen, B.S.; Soya, H. Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc. Natl. Acad. Sci. USA 2019, 116, 10988–10993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, T.K.; Pence, B.D.; Ossyra, J.M.; Gibbons, T.E.; Perez, S.; McCusker, R.H.; Kelley, K.W.; Johnson, R.W.; Woods, J.A.; Rhodes, J.S. Exercise but not (-)-epigallocatechin-3-gallate or β-alanine enhances physical fitness, brain plasticity, and behavioral performance in mice. Physiol. Behav. 2015, 145, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbons, T.E.; Pence, B.D.; Petr, G.; Ossyra, J.M.; Mach, H.C.; Bhattacharya, T.K.; Perez, S.; Martin, S.A.; McCusker, R.H.; Kelley, K.W.; et al. Voluntary wheel running, but not a diet containing (-)-epigallocatechin-3-gallate and β-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behav. Brain Res. 2014, 272, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.M.; Seo, M.; Seo, J.S.; Rhim, H.; Nahm, S.S.; Cho, I.H.; Chang, B.J.; Kim, H.J.; Choi, S.H.; Nah, S.Y. Ascorbic Acid Mitigates D-galactose-Induced Brain Aging by Increasing Hippocampal Neurogenesis and Improving Memory Function. Nutrients 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, S.L. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.R.; Burke, N.; Dongworth, R.K.; Hausenloy, D.J. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 2014, 171, 1890–1906. [Google Scholar] [CrossRef]
- Zhan, M.; Brooks, C.; Liu, F.; Sun, L.; Dong, Z. Mitochondrial dynamics: Regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013, 83, 568–581. [Google Scholar] [CrossRef] [Green Version]
- Held, N.M.; Houtkooper, R.H. Mitochondrial quality control pathways as determinants of metabolic health. Bioessays 2015, 37, 867–876. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Pan, Y.; Kao, S.Y.; Li, C.; Kohane, I.; Chan, J.; Yankner, B.A. Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429, 883–891. [Google Scholar] [CrossRef]
- Poon, H.F.; Calabrese, V.; Scapagnini, G.; Butterfield, D.A. Free radicals and brain aging. Clin. Geriatr. Med. 2004, 20, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Cevenini, E.; Monti, D.; Franceschi, C. Inflamm-ageing. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 14–20. [Google Scholar] [CrossRef]
- Fuller, S.; Steele, M.; Münch, G. Activated astroglia during chronic inflammation in Alzheimer’s disease--do they neglect their neurosupportive roles? Mutat. Res. 2010, 690, 40–49. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; White, C.L.; Gupta, S.; Knight, A.G.; Pistell, P.J.; Ingram, D.K.; Morrison, C.D.; Keller, J.N. NOX activity in brain aging: Exacerbation by high fat diet. Free Radic. Biol. Med. 2010, 49, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Hernandes, M.S.; D’Avila, J.C.; Trevelin, S.C.; Reis, P.A.; Kinjo, E.R.; Lopes, L.R.; Castro-Faria-Neto, H.C.; Cunha, F.Q.; Britto, L.R.; Bozza, F.A. The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J. Neuroinflamm. 2014, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Serrano, F.; Kolluri, N.S.; Wientjes, F.B.; Card, J.P.; Klann, E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003, 988, 193–198. [Google Scholar] [CrossRef]
- Niu, Y.L.; Zhang, W.J.; Wu, P.; Liu, B.; Sun, G.T.; Yu, D.M.; Deng, J.B. Expression of the apoptosis-related proteins caspase-3 and NF-kappaB in the hippocampus of Tg2576 mice. Neurosci. Bull. 2010, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rohn, T.T. The role of caspases in Alzheimer’s disease; potential novel therapeutic opportunities. Apoptosis 2010, 15, 1403–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, S.; Ma, G.; Ye, M.; Lu, G. Involvement of proinflammatory factors, apoptosis, caspase-3 activation and Ca2+ disturbance in microglia activation-mediated dopaminergic cell degeneration. Mech. Ageing Dev. 2005, 126, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Vaynman, S.; Souda, P.; Whitelegge, J.P.; Gomez-Pinilla, F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur. J. Neurosci. 2006, 24, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Kumagai, S.; Taylor, A.W.; Naito, H.; Goto, S. Effects of exercise on brain function: Role of free radicals. Appl. Physiol. Nutr. Metab. 2007, 32, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Murphy, M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef]
- Gioscia-Ryan, R.A.; LaRocca, T.J.; Sindler, A.L.; Zigler, M.C.; Murphy, M.P.; Seals, D.R. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. 2014, 592, 2549–2561. [Google Scholar] [CrossRef]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef]
- Vergeade, A.; Mulder, P.; Vendeville-Dehaudt, C.; Estour, F.; Fortin, D.; Ventura-Clapier, R.; Thuillez, C.; Monteil, C. Mitochondrial impairment contributes to cocaine-induced cardiac dysfunction: Prevention by the targeted antioxidant MitoQ. Free Radic. Biol. Med. 2010, 49, 748–756. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Nagulan, R.; Somerville, V. The Effect of MitoQ on Aging-Related Biomarkers: A Systematic Review and Meta-Analysis. Oxid. Med. Cell Longev. 2018, 2018, 8575263. [Google Scholar] [CrossRef]
- Lei, M.; Hua, X.; Xiao, M.; Ding, J.; Han, Q.; Hu, G. Impairments of astrocytes are involved in the d-galactose-induced brain aging. Biochem. Biophys. Res. Commun. 2008, 369, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zuo, P.; Zhang, Q.; Li, X.; Hu, Y.; Long, J.; Packer, L.; Liu, J. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of R-alpha-lipoic acid. J. Neurosci. Res. 2006, 84, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Lei, M.; Zhang, Y.; Ding, J.; Han, Q.; Hu, G.; Xiao, M. Long-term D-galactose injection combined with ovariectomy serves as a new rodent model for Alzheimer’s disease. Life Sci. 2007, 80, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Liu, J.H.; Wu, R.Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003, 4, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.P.; Lee, H.C.; Kim, H.T. Treadmill exercise after social isolation increases the levels of NGF, BDNF, and synapsin I to induce survival of neurons in the hippocampus, and improves depression-like behavior. J. Exerc. Nutr. Biochem. 2015, 19, 11–18. [Google Scholar] [CrossRef]
- Carelli, V.; Chan, D.C. Mitochondrial DNA: Impacting central and peripheral nervous systems. Neuron 2014, 84, 1126–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lightowlers, R.N.; Taylor, R.W.; Turnbull, D.M. Mutations causing mitochondrial disease: What is new and what challenges remain? Science 2015, 349, 1494–1499. [Google Scholar] [CrossRef]
- Solesio, M.E.; Prime, T.A.; Logan, A.; Murphy, M.P.; Del Mar Arroyo-Jimenez, M.; Jordán, J.; Galindo, M.F. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson’s disease. Biochim. Biophys. Acta 2013, 1832, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Kang, L.; Liu, S.; Li, J.; Tian, Y.; Xue, Y.; Liu, X. The mitochondria-targeted anti-oxidant MitoQ protects against intervertebral disc degeneration by ameliorating mitochondrial dysfunction and redox imbalance. Cell Prolif. 2020, 53, e12779. [Google Scholar] [CrossRef]
- Du, Z.; Yang, Q.; Liu, L.; Li, S.; Zhao, J.; Hu, J.; Liu, C.; Qian, D.; Gao, C. NADPH oxidase 2-dependent oxidative stress, mitochondrial damage and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging rats. Neuroscience 2015, 286, 281–292. [Google Scholar] [CrossRef]
- Cunha, T.F.; Bechara, L.R.; Bacurau, A.V.; Jannig, P.R.; Voltarelli, V.A.; Dourado, P.M.; Vasconcelos, A.R.; Scavone, C.; Ferreira, J.C.; Brum, P.C. Exercise training decreases NADPH oxidase activity and restores skeletal muscle mass in heart failure rats. J. Appl. Physiol. 2017, 122, 817–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touati, S.; Montezano, A.C.; Meziri, F.; Riva, C.; Touyz, R.M.; Laurant, P. Exercise training protects against atherosclerotic risk factors through vascular NADPH oxidase, extracellular signal-regulated kinase 1/2 and stress-activated protein kinase/c-Jun N-terminal kinase downregulation in obese rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, B.V.; Izyumov, D.S.; Lyamzaev, K.G.; Pashkovskaya, A.A.; Pletjushkina, O.Y.; Antonenko, Y.N.; Sakharov, D.V.; Wirtz, K.W.; Skulachev, V.P. Production of reactive oxygen species in mitochondria of HeLa cells under oxidative stress. Biochim. Biophys. Acta 2006, 1757, 525–534. [Google Scholar] [CrossRef] [Green Version]
- La Favor, J.D.; Dubis, G.S.; Yan, H.; White, J.D.; Nelson, M.A.; Anderson, E.J.; Hickner, R.C. Microvascular endothelial dysfunction in sedentary, obese humans is mediated by NADPH oxidase: Influence of exercise training. Arter. Thromb. Vasc. Biol. 2016, 36, 2412–2420. [Google Scholar] [CrossRef] [Green Version]
- Chao, P.C.; Yin, M.C.; Mong, M.C. Anti-apoptotic and anti-glycative effects of asiatic acid in the brain of D-galactose treated mice. Food Funct. 2015, 6, 542–548. [Google Scholar] [CrossRef]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Colbert, L.H.; Visser, M.; Simonsick, E.M.; Tracy, R.P.; Newman, A.B.; Kritchevsky, S.B.; Pahor, M.; Taaffe, D.R.; Brach, J.; Rubin, S.; et al. Physical activity, exercise, and inflammatory markers in older adults: Findings from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2004, 52, 1098–1104. [Google Scholar] [CrossRef]
- Navarro, A.; Gomez, C.; López-Cepero, J.M.; Boveris, A. Beneficial effects of moderate exercise on mice aging: Survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R505–R511. [Google Scholar] [CrossRef] [Green Version]
- Gioscia-Ryan, R.A.; Battson, M.L.; Cuevas, L.M.; Eng, J.S.; Murphy, M.P.; Seals, D.R. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J. Appl. Physiol. 2018, 124, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, B.; Squire, L.R.; Kandel, E.R. Cognitive neuroscience and the study of memory. Neuron 1998, 20, 445–468. [Google Scholar] [CrossRef] [Green Version]
- Jolkkonen, J.; Gallagher, N.P.; Zilles, K.; Sivenius, J. Behavioral deficits and recovery following transient focal cerebral ischemia in rats: Glutamatergic and GABAergic receptor densities. Behav. Brain Res. 2003, 138, 187–200. [Google Scholar] [CrossRef]
- Fordyce, D.E.; Wehner, J.M. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993, 619, 111–119. [Google Scholar] [CrossRef]
- Rodrigues, L.; Dutra, M.F.; Ilha, J.; Biasibetti, R.; Quincozes-Santos, A.; Leite, M.C.; Marcuzzo, S.; Achaval, M.; Gonçalves, C.A. Treadmill training restores spatial cognitive deficits and neurochemical alterations in the hippocampus of rats submitted to an intracerebroventricular administration of streptozotocin. J. Neural. Transm. 2010, 117, 1295–1305. [Google Scholar] [CrossRef]
- Russo-Neustadt, A.; Ha, T.; Ramirez, R.; Kesslak, J.P. Physical activity-antidepressant treatment combination: Impact on brain-derived neurotrophic factor and behavior in an animal model. Behav. Brain Res. 2001, 120, 87–95. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-H.; Koo, J.-H.; Yook, J.S.; Cho, J.-Y.; Kang, E.-B. Neuroprotective Benefits of Exercise and MitoQ on Memory Function, Mitochondrial Dynamics, Oxidative Stress, and Neuroinflammation in D-Galactose-Induced Aging Rats. Brain Sci. 2021, 11, 164. https://doi.org/10.3390/brainsci11020164
Jeong J-H, Koo J-H, Yook JS, Cho J-Y, Kang E-B. Neuroprotective Benefits of Exercise and MitoQ on Memory Function, Mitochondrial Dynamics, Oxidative Stress, and Neuroinflammation in D-Galactose-Induced Aging Rats. Brain Sciences. 2021; 11(2):164. https://doi.org/10.3390/brainsci11020164
Chicago/Turabian StyleJeong, Jae-Hoon, Jung-Hoon Koo, Jang Soo Yook, Joon-Yong Cho, and Eun-Bum Kang. 2021. "Neuroprotective Benefits of Exercise and MitoQ on Memory Function, Mitochondrial Dynamics, Oxidative Stress, and Neuroinflammation in D-Galactose-Induced Aging Rats" Brain Sciences 11, no. 2: 164. https://doi.org/10.3390/brainsci11020164
APA StyleJeong, J. -H., Koo, J. -H., Yook, J. S., Cho, J. -Y., & Kang, E. -B. (2021). Neuroprotective Benefits of Exercise and MitoQ on Memory Function, Mitochondrial Dynamics, Oxidative Stress, and Neuroinflammation in D-Galactose-Induced Aging Rats. Brain Sciences, 11(2), 164. https://doi.org/10.3390/brainsci11020164




