Individual Alpha Peak Frequency, an Important Biomarker for Live Z-Score Training Neurofeedback in Adolescents with Learning Disabilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
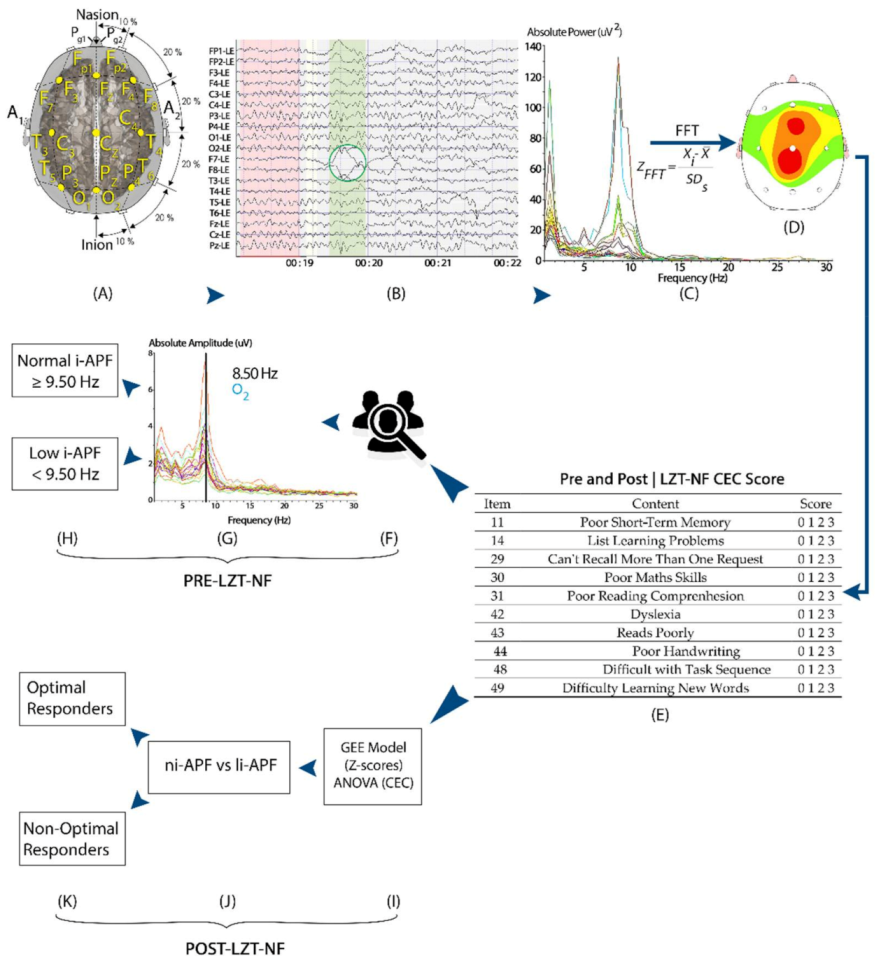
2.2. Cognitive and Emotional Checklist
2.3. EEG Collection and QEEG Analysis
2.4. Neurofeedback Intervention (Live Z-Score Training Neurofeedback)
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Item | Content | Score |
|---|---|---|
| 11 | Poor Short-Term Memory | 0 1 2 3 |
| 14 | List Learning Problems | 0 1 2 3 |
| 29 | Can’t Recall More Than One Request | 0 1 2 3 |
| 30 | Poor Maths Skills | 0 1 2 3 |
| 31 | Poor Reading Comprehension | 0 1 2 3 |
| 42 | Dyslexia | 0 1 2 3 |
| 43 | Reads Poorly | 0 1 2 3 |
| 44 | Poor Handwriting | 0 1 2 3 |
| 48 | Difficulty with Task Sequence | 0 1 2 3 |
| 49 | Difficulty Learning New Words | 0 1 2 3 |
Appendix B



Appendix C
Appendix D
| Parameter | li-APF Group (n = 28) | ni-APF Group (n = 12) | p-Value | ||
|---|---|---|---|---|---|
| I-APF | Mean | SD | Mean | SD | 0.000 |
| 8.54 Hz | 0.33 | 10 Hz | 0.31 | ||
| CEC-Total | Mean | SD | Mean | SD | p-Value |
| Pre | 51 | 6.88 | 49.96 | 8.24 | 0.850 |
| Post | 43.75 | 6.85 | 33.50 | 7.23 | 0.000 |
| CEC Learning | Mean | SD | Mean | SD | p-Value |
| Pre | 18.17 | 1.95 | 18.29 | 3.18 | 0.965 |
| Post | 15.08 | 1.93 | 11.46 | 2.66 | 0.000 |
| Z-Scores | Ni-APF | Li-APF | p-Value | |
|---|---|---|---|---|
| Pre/Post | Pre/Post | Pre/Post | ||
| F3 | Delta | 0.70 (0.49)/0.62 (0.58) | 0.72 (0.53)/0.62 (0.59) | 0.545/0.825 |
| Theta | 0.66 (0.61)/0.58 (0.38) | 0.80 (0.39)/0.92 (0.70) | 0.140/0.121 | |
| Alpha | 0.92 (0.63)/0.73 (0.50) | 0.80 (0.49)/0.86 (0.67) | 0.734/0.723 | |
| Beta-1 | 1.16 (0.95)/0.67 (0.62) | 0.71 (0.68)/0.98 (0.82) | 0.101/0.626 | |
| Beta-2 | 1.16 (0.73)/1.02 (0.55) | 0.77 (0.56)/0.98 (0.77) | 0.152/0.757 | |
| Beta-3 | 1.23 (0.81)/0.92 (0.55) | 1.10 (0.71)/1.34 (0.81) | 0.669/0.087 | |
| Hi-Beta | 1.52 (0.82)/1.11 (0.73) | 1.90 (1.23)/2.05 (1.19) | 0.479/0.007 | |
| F4 | Delta | 0.86 (0.62)/0.54 (0.35) | 0.72 (0.47)/0.61 (0.60) | 0.690/0.768 |
| Theta | 0.70 (0.68)/0.51 (0.33) | 0.76 (0.63)/0.68 (0.64) | 0.605/0.848 | |
| Alpha | 0.89 (0.68)/0.79 (0.52) | 0.87 (0.73)/0.75 (0.47) | 0.926/0.813 | |
| Beta-1 | 1.29 (0.97)/1.04 (0.79) | 0.85 (0.73)/0.84 (0.81) | 0.125/0.215 | |
| Beta-2 | 1.16 (0.89)/0.95 (0.69) | 1.00 (0.62)/0.84 (0.77) | 0.757/0.425 | |
| Beta-3 | 1.21 (0.79)/0.95 (0.53) | 1.16 (0.64)/1.20 (0.75) | 0.976/0.443 | |
| Hi-Beta | 1.49 (0.88)/1.00 (0.86) | 1.49 (1.02)/1.60 (1.42) | 0.906/0.148 | |
| P3 | Delta | 0.82 (0.82)/0.70 (0.54) | 0.89 (0.68)/0.71 (0.53) | 0.425/0.976 |
| Theta | 0.77 (0.68)/0.54 (0.32) | 0.76 (0.35)/0.67 (0.59) | 0.215/0.768 | |
| Alpha | 1.02 (0.63)/0.85 (0.54) | 0.96 (0.70)/0.86 (0.69) | 0.637/0.701 | |
| Beta-1 | 1.33 (0.91)/1.03 (0.61) | 0.79 (0.86)/0.86 (0.72) | 0.070/0.262 | |
| Beta-2 | 1.50 (0.74)/1.07 (0.55) | 0.96 (0.81)/1.83 (2.36) | 0.063/0.434 | |
| Beta-3 | 1.62 (0.78)/1.19 (0.59) | 1.14 (0.80)/1.23 (0.66) | 0.090/1.00 | |
| Hi-Beta | 1.94 (1.10)/1.29 (0.61) | 1.99 (0,99)/1.47 (0.89) | 0.779/0.352 | |
| P4 | Delta | 0.64 (0.43)/0.61 (0.50) | 0.65 (0.49)/0.79 (0.50) | 0.941/0.294 |
| Theta | 0.62 (0.60)/0.59 (0.37) | 0.87 (0.33)/0.74 (0.58) | 0.016/0.516 | |
| Alpha | 0.89 (0.57)/0.80 (0.48) | 1.00 (0.53)/1.64 (2.11) | 0.479/0.148 | |
| Beta-1 | 1.27 (0.91)/1.11 (0.76) | 0.82 (0.84)/0.80 (0.97) | 0.128/0.152 | |
| Beta-2 | 1.47 (0.88)/1.20 (0.73) | 0.98 (0.75)/1.11 (0.80) | 0.092/0.658 | |
| Beta-3 | 1.58 (0.83)/1.20 (0.64) | 1.14 (0.74)/1.30 (0.63) | 0.125/0.690 | |
| Hi-Beta | 1.82 (1.00)/1.39 (0.80) | 1.79 (0.85)/1.72 (0.89) | 0.918/0.256 |
References
- Abdalah, M.Q. Gender Difference in Learning Disabled Children Neuropsychological Review. Res. Rev. Healthc. Open Access J. 2018, 1. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association, Ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Bosch-Bayard, J.; Peluso, V.; Galan, L.; Valdes Sosa, P.; Chiarenza, G. Clinical and Electrophysiological Differences between Subjects with Dysphonetic Dyslexia and Non-Specific Reading Delay. Brain Sci. 2018, 8, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, T.; Harmony, T.; Bosch-Bayard, J.; Prado-Alcalá, R.; Otero-Ojeda, G.; Garcia, F.; Rodriguez, M.D.C.; Becerra, J. Optimization of the Neurofeedback protocol in children with Learning Disabilities and a lag in their EEG maturation. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Chiarenza, G.A. Quantitative EEG in Childhood Attention Deficit Hyperactivity Disorder and Learning Disabilities. Clin. EEG Neurosci. 2020, 155005942096234. [Google Scholar] [CrossRef] [PubMed]
- Fernández, T.; Harmony, T.; Fernández-Bouzas, A.; Silva, J.; Herrera, W.; Santiago-Rodríguez, E.; Sánchez, L. Sources of EEG activity in learning disabled children. Clin. EEG Electroencephalogr. 2002, 33, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T.; Rousson, V.; Schreiter Gasser, U. EEG power and coherence in children with educational problems. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2003, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Harmony, T.; Marosi, E.; Díaz de León, A.E.; Becker, J.; Fernández, T. Effect of sex, psychosocial disadvantages and biological risk factors on EEG maturation. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 482–491. [Google Scholar] [CrossRef]
- John, E.R.; Prichep, L.; Ahn, H.; Easton, P.; Fridman, J.; Kaye, H. Neurometric evaluation of cognitive dysfunctions and neurological disorders in children. Prog. Neurobiol. 1983, 21, 239–290. [Google Scholar] [CrossRef]
- Roca-Stappung, M.; Fernández, T.; Bosch-Bayard, J.; Harmony, T.; Ricardo-Garcell, J. Electroencephalographic characterization of subgroups of children with learning disorders. PLoS ONE 2017, 12, e0179556. [Google Scholar] [CrossRef] [Green Version]
- Angelakis, E.; Stathopoulou, S.; Frymiare, J.L.; Green, D.L.; Lubar, J.F.; Kounios, J. EEG Neurofeedback: A Brief Overview and an Example of Peak Alpha Frequency Training for Cognitive Enhancement in the Elderly. Clin. Neuropsychol. 2007, 21, 110–129. [Google Scholar] [CrossRef]
- Dickinson, A.; DiStefano, C.; Senturk, D.; Jeste, S.S. Peak alpha frequency is a neural marker of cognitive function across the autism spectrum. Eur. J. Neurosci. 2018, 47, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Suldo, S.M.; Olson, L.A.; Evans, J.R. Quantitative EEG Evidence of Increased Alpha Peak Frequency in Children with Precocious Reading Ability. J. Neurother. 2002, 5, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Demos, J.N. Getting Started with EEG Neurofeedback, 2nd ed.; W.W. Norton & Company: New York, NY, USA, 2019; ISBN 978-0-393-71253-7. [Google Scholar]
- Blum, A.S.; Rutkove, S.B. (Eds.) The Clinical Neurophysiology Primer; Humana Press: Totowa, NJ, USA, 2007; ISBN 978-0-89603-996-4. [Google Scholar]
- Bazanova, O.M. Alpha EEG Activity Depends on the Individual Dominant Rhythm Frequency. J. Neurother. 2012, 16, 270–284. [Google Scholar] [CrossRef]
- Arns, M.; Drinkenburg, W.H.; Fitzgerald, P.B.; Kenemans, J.L. Neurophysiological predictors of non-response to rTMS in depression. Brain Stimulat. 2012, 5, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Grandy, T.H.; Werkle-Bergner, M.; Chicherio, C.; Lövdén, M.; Schmiedek, F.; Lindenberger, U. Individual alpha peak frequency is related to latent factors of general cognitive abilities. NeuroImage 2013, 79, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Niedermeyer, E.; Lopes da Silva, F.H. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, 5th ed.; Lippincott Williams & Wilkins: London, UK, 2005; ISBN 978-0-7817-5126-1. [Google Scholar]
- Fernández, T.; Bosch-Bayard, J.; Harmony, T.; Caballero, M.I.; Díaz-Comas, L.; Galán, L.; Ricardo-Garcell, J.; Aubert, E.; Otero-Ojeda, G. Neurofeedback in Learning Disabled Children: Visual versus Auditory Reinforcement. Appl. Psychophysiol. Biofeedback 2016, 41, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Arns, M. EEG-Based Personalized Medicine in ADHD: Individual Alpha Peak Frequency as an Endophenotype Associated with Nonresponse. J. Neurother. 2012, 16, 123–141. [Google Scholar] [CrossRef]
- Carrobles, J.A. Bio/neurofeedback. Clin. Salud 2016, 27, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Groeneveld, K.M.; Mennenga, A.M.; Heidelberg, R.C.; Martin, R.E.; Tittle, R.K.; Meeuwsen, K.D.; Walker, L.A.; White, E.K. Z-Score neurofeedback and heart rate variability training for adults and children with symptoms of Attention-Deficit/Hyperactivity Disorder: A retrospective study. Appl. Psychophysiol. Biofeedback 2019, 44, 291–308. [Google Scholar] [CrossRef] [Green Version]
- Alkoby, O.; Abu-Rmileh, A.; Shriki, O.; Todder, D. Can We Predict Who Will Respond to Neurofeedback? A Review of the Inefficacy Problem and Existing Predictors for Successful EEG Neurofeedback Learning. Neuroscience 2018, 378, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Doehnert, M.; Brandeis, D.; Straub, M.; Steinhausen, H.-C.; Drechsler, R. Slow cortical potential neurofeedback in attention deficit hyperactivity disorder: Is there neurophysiological evidence for specific effects? J. Neural Transm. 2008, 115, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Hanslmayr, S.; Klimesch, W.; Sauseng, P.; Gruber, W.; Doppelmayr, M.; Freunberger, R.; Pecherstorfer, T. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci. Lett. 2005, 375, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Lubar, J.F.; Swartwood, M.O.; Swartwood, J.N.; O’Donnell, P.H. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self-Regul. 1995, 20, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Köberl, A.; Frank, S.; Doppelmayr, M. Predicting Successful Learning of SMR Neurofeedback in Healthy Participants: Methodological Considerations. Appl. Psychophysiol. Biofeedback 2011, 36, 37–45. [Google Scholar] [CrossRef]
- Zoefel, B.; Huster, R.J.; Herrmann, C.S. Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. NeuroImage 2011, 54, 1427–1431. [Google Scholar] [CrossRef]
- Collura, T. Technical Foundations of Neurofeedback; Routledge, Taylor & Francis Group: New York, NY, USA, 2014; ISBN 978-0-415-89901-7. [Google Scholar]
- Collura, T. Handbook of Clinical QEEG and Neurotherapy, 1st ed.; Includes bibliographical references and index; Routledge: New York, NY, USA, 2016; ISBN 978-1-315-75409-3. [Google Scholar]
- Collura, T.; Guan, J.; Tarrant, J.; Bailey, J.; Starr, F. EEG biofeedback case studies using live Z-score training and a normative database. J. Neurother. 2010, 14, 22–46. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.L. A father finds a solution: Z-Score Training. NeuroConnections 2008, 22–25. Available online: https://brainmaster.com/wp-content/uploads/2016/03/smith-nc.pdf (accessed on 28 January 2021).
- Thatcher, R.W. Handbook of Quantitative Electroencephalography and EEG Biofeedback, 2nd ed.; Anipublishing Co.: St. Petersburg, FL, USA, 2016; ISBN 978-0-9854692-0-7. [Google Scholar]
- Thatcher, R.W.; Lubar, J.F.; Koberda, J.L. Z-Score EEG Biofeedback: Past, Present, and Future. Biofeedback 2019, 47, 89–103. [Google Scholar] [CrossRef]
- Krigbaum, G.; Wigton, N.L. When discussing neurofeedback, does modality matter? NeuroRegulation 2014, 1, 48–60. [Google Scholar] [CrossRef]
- Krigbaum, G.; Wigton, N.L. A methodology of analysis for monitoring treatment progression with 19-Channel Z-Score neurofeedback (19ZNF) in a single-subject design. Appl. Psychophysiol. Biofeedback 2015, 40, 139–149. [Google Scholar] [CrossRef]
- Wigton, N.L.; Krigbaum, G. Attention, executive function, behavior, and electrocortical function, significantly improved with 19-Channel Z -Score Neurofeedback in a Clinical Setting: A Pilot Study. J. Atten. Disord. 2015, 23, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, R.Ł.; Ratomska, M.; Jaśkiewicz, M. The Neglected Problem of the Neurofeedback Learning (In) Ability. In Biomedical Engineering and Neuroscience; Hunek, W.P., Paszkiel, S., Eds.; Advances in Intelligent Systems and Computing; Springer International Publishing: Cham, Switzerland, 2018; Volume 720, pp. 45–58. ISBN 978-3-319-75024-8. [Google Scholar]
- Burde, W.; Blankertz, B. Is the locus of control of reinforcement a predictor of brain-computer interface performance? In Proceedings of the International Brain-Computer Interface Workshop and Training Course, Graz, Austria, 30 May–3 June 2006; Graz University of Technology: Graz, Austria, 2006; pp. 76–77. [Google Scholar]
- Daum, I.; Rockstroh, B.; Birbaumer, N.; Elbert, T.; Canavan, A.; Lutzenberger, W. Behavioural treatment of slow cortical potentials in intractable epilepsy: Neuropsychological predictors of outcome. J. Neurol. Neurosurg. Psychiatry 1993, 56, 94–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruzelier, J.H. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 2014, 44, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, M.E.J.; van Schie, H.T.; Gerrits, B.J.L.; Buitelaar, J.K.; de Moor, J.M.H. Is EEG-biofeedback an Effective Treatment in Autism Spectrum Disorders? A Randomized Controlled Trial. Appl. Psychophysiol. Biofeedback 2013, 38, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.E.; Birbaumer, N.; Rockstroh, B.; Lutzenberger, W.; Elbert, T. Self-Report During Feedback Regulation of Slow Cortical Potentials. Psychophysiology 1989, 26, 392–403. [Google Scholar] [CrossRef]
- Wangler, S.; Gevensleben, H.; Albrecht, B.; Studer, P.; Rothenberger, A.; Moll, G.H.; Heinrich, H. Neurofeedback in children with ADHD: Specific event-related potential findings of a randomized controlled trial. Clin. Neurophysiol. 2011, 122, 942–950. [Google Scholar] [CrossRef]
- Jafarova, O.; Mazhirina, K.; Sokhadze, E.; Shtark, M. Self-regulation Strategies and Heart Rate Biofeedback Training. Appl. Psychophysiol. Biofeedback 2020, 45, 87–98. [Google Scholar] [CrossRef]
- Blankertz, B.; Sannelli, C.; Halder, S.; Hammer, E.M.; Kübler, A.; Müller, K.-R.; Curio, G.; Dickhaus, T. Neurophysiological predictor of SMR-based BCI performance. NeuroImage 2010, 51, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Grosse-Wentrup, M.; Schölkopf, B. High gamma-power predicts performance in sensorimotor-rhythm brain–computer interfaces. J. Neural Eng. 2012, 9, 1–8. [Google Scholar] [CrossRef]
- Cantor, D.S.; Chabot, R. QEEG Studies in the Assessment and Treatment of Childhood Disorders. Clin. EEG Neurosci. 2009, 40, 113–121. [Google Scholar] [CrossRef]
- Holmes, G.L.; Solomon, M.; Royden, J. Clinical Neurophysiology of Infancy, Childhood, and Adolescence; Butterworth Heinemnn Elsevier: Philadelphia, PA, USA, 2006; ISBN 0-7506-7251-X. [Google Scholar]
- López-Ibor Aliño, J.J.; Valdés Miyar, M.; American Psychiatric Association. Manual Diagnóstico y Estadístico de los Trastornos Mentales; American Psychiatric Pub.: Washington, DC, USA, 2003; ISBN 978-84-458-1087-3. [Google Scholar]
- Kaufman, A.S.; Flanagan, D.P.; Alfonso, V.C.; Mascolo, J.T. Test Review: Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV). J. Psychoeduc. Assess. 2006, 24, 278–295. [Google Scholar] [CrossRef]
- Soutar, R.G. Holistic Neurointegration: The New Mind Model—A Bio-Psycho-Social qEEG Guided Neurofeedback Method; New Mind Academy: Roswell, GA, USA, 2018. [Google Scholar]
- Stoller, L. Z-Score Training, Combinatorics, and Phase Transitions. J. Neurother. 2011, 15, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Arns, M.; Gunkelman, J.; Breteler, M.; Spronk, D. EEG phenotipes predict treatment outcome to stimulants in children with ADHD. J. Integr. Neurosci. 2008, 7, 421–438. [Google Scholar] [CrossRef]
- Rubin, D.I.; Daube, J.R. Clinical Neurophysiology, 4th ed.; Oxford University Press: Oxford, UK, 2016; ISBN 978-0-19-025963-1. [Google Scholar]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A. Effects of QEEG normalization using 4-Channel Live Z-Score Training Neurofeedback for children with learning disabilities: Preliminary data. Behav. Psychol 2021, in press. [Google Scholar]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A. Comparing Live Z-Score Training and Theta/Beta Protocol to Reduce Theta-to-Beta Ratio: A Pilot Study. NeuroRegulation 2020, 7, 58. [Google Scholar] [CrossRef]
- Fisher, W.; Piazza, C.C.; Bowman, L.G.; Hagopian, L.P.; Owens, J.C.; Slevin, I. A comparison of two approaches for identifying reinforcers for persons with severe and profound disabilities. J. Appl. Behav. Anal. 1992, 25, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangum, A.; Fredrick, L.; Pabico, R.; Roane, H. The role of context in the evaluation of reinforcer efficacy: Implications for the preference assessment outcomes. Res. Autism Spectr. Disord. 2012, 6, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Piazza, C.C.; Fisher, W.W.; Hagopian, L.P.; Bowman, L.G.; Toole, L. Using a choice assessment to predict reinforcer effectiveness. J. Appl. Behav. Anal. 1996, 29, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höller, Y.; Thomschewski, A.; Schmid, E.V.; Höller, P.; Crone, J.S.; Trinka, E. Individual brain-frequency responses to self-selected music. Int. J. Psychophysiol. 2012, 86, 206–213. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis, 2nd ed.; Wiley series in probability and statistics; Wiley-Interscience: New York, NY, USA, 2002; ISBN 978-0-471-36093-3. [Google Scholar]
- Vittinghoff, E.; Glidden, D.V.; Shiboski, S.C.; McCulloch, C.E. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models; Statistics for Biology and Health; Springer: New York, NY, USA, 2005. [Google Scholar]
- Andreou, C.; Frielinghaus, H.; Rauh, J.; Mußmann, M.; Vauth, S.; Braun, P.; Leicht, G.; Mulert, C. Theta and high-beta networks for feedback processing: A simultaneous EEG–fMRI study in healthy male subjects. Transl. Psychiatry 2017, 7, e1016. [Google Scholar] [CrossRef]
- Güntensperger, D.; Thüring, C.; Kleinjung, T.; Neff, P.; Meyer, M. Investigating the Efficacy of an Individualized Alpha/Delta Neurofeedback Protocol in the Treatment of Chronic Tinnitus. Neural Plast. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arns, M.; Spronk, D.; Fitzgerald, P.B. Potential differential effects of 9 Hz rTMS and 10 Hz rTMS in the treatment of depression. Brain Stimulat. 2010, 3, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Bazanova, O.M.; Aftanas, L.I. Individual EEG Alpha Activity Analysis for Enhancement Neurofeedback Efficiency: Two Case Studies. J. Neurother. 2010, 14, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Kropotov, J. Methods of Neurotherapy. In Quantitative EEG, Event-Related Potentials and Neurotherapy; Elsevier: Amsterdam, The Netherlands, 2009; pp. 469–505. ISBN 978-0-12-374512-5. [Google Scholar]
- Kropotov, J. Functional Neuromarkers for Psychiatry; Elsevier: Boston, MA, USA, 2016; ISBN 978-0-12-410513-3. [Google Scholar]
- Johnstone, J.; Gunkelman, J. Use of Databases in QEEG Evaluation. J. Neurother. 2003, 7, 31–52. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Elvira, R.; López Bote, D.J.; Guarino, S.; Agudo Juan, M.; De León, R.J.; Feiner, T.; Perez, B. Neurometric results of a case series using Live Z-Scores neurofeedback. Int. J. Psychophysiol. 2018, 131, S139–S140. [Google Scholar] [CrossRef]
- Pérez-Elvira, R.; Carrobles, J.; López Bote, D.; Oltra-Cucarella, J. Efficacy of Live Z-Score neurofeedback training for chronic insomnia: A single-case study. NeuroRegulation 2019, 6, 93–101. [Google Scholar] [CrossRef]
- Azizi, A.; Drikvand, F.M.; Sepahvandi, M.A. Comparison of the Effect of Cognitive Rehabilitation and Neurofeedback on Sustained Attention Among Elementary School Students with Specific Learning Disorder: A Preliminary Randomized Controlled Clinical Trial. Appl. Psychophysiol. Biofeedback 2018, 43, 301–307. [Google Scholar] [CrossRef]
- Duarte Hernández, E.; González Marqués, J.; Alvarado, J.M. Effect of the Theta-Beta Neurofeedback Protocol as a Function of Subtype in Children Diagnosed with Attention Deficit Hyperactivity Disorder. Span. J. Psychol. 2016, 19, E30. [Google Scholar] [CrossRef] [Green Version]
- Hillard, B.; El-Baz, A.S.; Sears, L.; Tasman, A.; Sokhadze, E.M. Neurofeedback Training Aimed to Improve Focused Attention and Alertness in Children With ADHD: A Study of Relative Power of EEG Rhythms Using Custom-Made Software Application. Clin. EEG Neurosci. 2013, 44, 193–202. [Google Scholar] [CrossRef]
- Weber, L.A.; Ethofer, T.; Ehlis, A.-C. Predictors of neurofeedback training outcome: A systematic review. NeuroImage Clin. 2020, 27, 102301. [Google Scholar] [CrossRef]
- Krepel, N.; Egtberts, T.; Sack, A.T.; Heinrich, H.; Ryan, M.; Arns, M. A multicenter effectiveness trial of QEEG-informed neurofeedback in ADHD: Replication and treatment prediction. NeuroImage Clin. 2020, 28, 102399. [Google Scholar] [CrossRef]
- Martínez-Briones, B.J.; Fernández-Harmony, T.; Garófalo Gómez, N.; Biscay-Lirio, R.J.; Bosch-Bayard, J. Working Memory in Children with Learning Disorders: An EEG Power Spectrum Analysis. Brain Sci. 2020, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Breteler, M.H.M.; Arns, M.; Peters, S.; Giepmans, I.; Verhoeven, L. Improvements in Spelling after QEEG-based Neurofeedback in Dyslexia: A Randomized Controlled Treatment Study. Appl. Psychophysiol. Biofeedback 2010, 35, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koberda, J.L.; Moses, A.; Koberda, L.; Koberda, P. Cognitive Enhancement Using 19-Electrode Z -Score Neurofeedback. J. Neurother. 2012, 16, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Wigton, N.L. Clinical perspectives of 19-Channel Z-Score neurofeedback: Benefits and limitations. J. Neurother. 2013, 17, 259–264. [Google Scholar] [CrossRef]
- Miglioretti, D.L.; Heagerty, P.J. Marginal Modeling of Nonnested Multilevel Data using Standard Software. Am. J. Epidemiol. 2006, 165, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Akter, T.; Sarker, E.B.; Rahman, S. A Tutorial on GEE with Applications to Diabetes and Hypertension Data from a Complex Survey. J. Biomed. Anal. 2018, 1, 37–50. [Google Scholar] [CrossRef]








| Low i-APF Group (li-APF, n = 12) | Normal i-APF Group (ni-APF, n = 28) | |||
|---|---|---|---|---|
| Waves | Pre | Post | Pre | Post |
| Abs Z < 1.5 | 257 (76.49%) | 246 (73.21%) | 519 (66.19%) | 662 (84.44%) |
| Abs Z ≥ 1.5 | 79 (23.51%) | 90 (26.79%) | 265 (33.81%) | 122 (15.56%) |
| Total | 336 | 336 | 784 | 784 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A.; Teodoru, M.; Bacila, C.; Neamtu, B. Individual Alpha Peak Frequency, an Important Biomarker for Live Z-Score Training Neurofeedback in Adolescents with Learning Disabilities. Brain Sci. 2021, 11, 167. https://doi.org/10.3390/brainsci11020167
Pérez-Elvira R, Oltra-Cucarella J, Carrobles JA, Teodoru M, Bacila C, Neamtu B. Individual Alpha Peak Frequency, an Important Biomarker for Live Z-Score Training Neurofeedback in Adolescents with Learning Disabilities. Brain Sciences. 2021; 11(2):167. https://doi.org/10.3390/brainsci11020167
Chicago/Turabian StylePérez-Elvira, Rubén, Javier Oltra-Cucarella, José Antonio Carrobles, Minodora Teodoru, Ciprian Bacila, and Bogdan Neamtu. 2021. "Individual Alpha Peak Frequency, an Important Biomarker for Live Z-Score Training Neurofeedback in Adolescents with Learning Disabilities" Brain Sciences 11, no. 2: 167. https://doi.org/10.3390/brainsci11020167
APA StylePérez-Elvira, R., Oltra-Cucarella, J., Carrobles, J. A., Teodoru, M., Bacila, C., & Neamtu, B. (2021). Individual Alpha Peak Frequency, an Important Biomarker for Live Z-Score Training Neurofeedback in Adolescents with Learning Disabilities. Brain Sciences, 11(2), 167. https://doi.org/10.3390/brainsci11020167






