Effects of Transcranial Direct Current Stimulation (tDCS) on Go/NoGo Performance Using Food and Non-Food Stimuli in Patients with Prader–Willi Syndrome
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Participants
2.2. Go/NoGo Task
2.3. tDCS Intervention
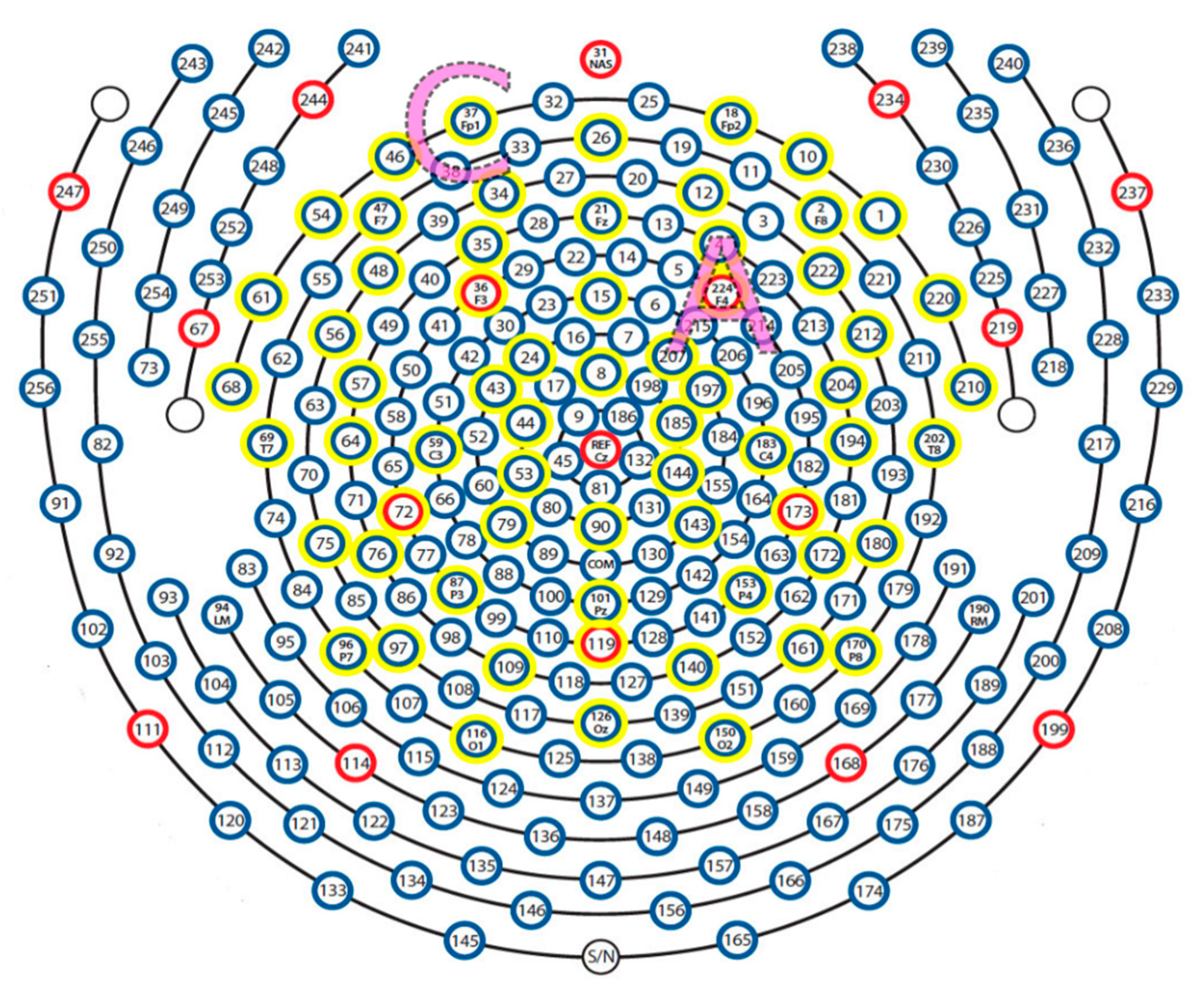
2.4. EEG Recording and Analysis
3. Results
3.1. Task Performance
3.2. ERP Findings
3.2.1. Go vs. NoGo Condition Effects
3.2.2. PWS Genetic Subtype Differences
3.2.3. Treatment Effects Using tDCS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, M.G.; Lee, P.D.K.; Whitman, B. Management of Prader-Willi Syndrome, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2011, 14, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.G.; Hartin, S.N.; Hossain, W.A.; Manzardo, A.M.; Kimonis, V.; Dykens, E.; Gold, J.A.; Kim, S.-J.; Weisensel, N.; Tamura, R.; et al. Molecular genetic classification in Prader-Willi syndrome: A multisite cohort study. J. Med. Genet. 2019, 56, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Miller, J.L.; Forster, J.L. Prader-Willi Syndrome—Clinical Genetics, Diagnosis and Treatment Approaches: An Update. Curr. Pediatr. Rev. 2019, 15, 207–244. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Thompson, T. Prader-Willi Syndrome. Endocrinologist 2000, 10, 3S–16S. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G. Management of obesity in Prader-Willi syndrome. Nat. Clin. Pr. Endocrinol. Metab. 2006, 2, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The orbitofrontal cortex and reward. Cereb. Cortex 2000, 10, 284–294. [Google Scholar] [CrossRef]
- Rolls, E.T. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol. Behav. 2005, 85, 45–56. [Google Scholar] [CrossRef]
- Holsen, L.M.; Savage, C.R.; Martin, L.E.; Bruce, A.S.; Lepping, R.J.; Ko, E.; Brooks, W.M.; Butler, M.G.; Zarcone, J.R.; Goldstein, J.M. Importance of reward and prefrontal circuitry in hunger and satiety: Prader–Willi syndrome vs simple obesity. Int. J. Obes. 2011, 36, 638–647. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.E.; Wang, J.; Zhang, G.; Zhu, Q.; Cai, W.; Tian, J.; Miller, J.L.; Wen, X.; Ding, M.; Gold, M.S.; et al. The neurobiological drive for overeating implicated in Prader–Willi syndrome. Brain Res. 2015, 1620, 72–80. [Google Scholar] [CrossRef]
- Honea, R.A.; Holsen, L.M.; Lepping, R.J.; Perea, R.; Butler, M.G.; Brooks, W.M.; Savage, C.R. The neuroanatomy of genetic subtype differences in Prader-Willi syndrome. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2012, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fregni, F.; Pascual-Leone, A. Technology Insight: Noninvasive brain stimulation in neurology—Perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pr. Neurol. 2007, 3, 383–393. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Vöröslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernández-Ruiz, A.; Kozák, G.; Kincses, Z.T.; Iványi, B.; Buzsáki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Boekholdt, L.; Kerstens, S.; Khatoun, A.; Asamoah, B.; Mc Laughlin, M. tDCS peripheral nerve stimulation: A neglected mode of action? Mol. Psychiatry 2021, 26, 456–461. [Google Scholar] [CrossRef]
- Adair, D.; Truong, D.; Esmaeilpour, Z.; Gebodh, N.; Borges, H.; Ho, L.; Bremner, J.D.; Badran, B.W.; Napadow, V.; Clark, V.P.; et al. Electrical stimulation of cranial nerves in cognition and disease. Brain Stimul. 2020, 13, 717–750. [Google Scholar] [CrossRef]
- Fregni, F.; Ligouri, P.; Fecteau, S.; Nitsche, M.A.; Pascual-Leone, A.; Boggio, P.S. Cortical Stimulation of the Prefrontal Cortex With Transcranial Direct Current Stimulation Reduces Cue-Provoked Smoking Craving. J. Clin. Psychiatry 2008, 69, 32–40. [Google Scholar] [CrossRef]
- Boggio, P.S.; Liguori, P.; Sultani, N.; Rezende, L.; Fecteau, S.; Fregni, F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci. Lett. 2009, 463, 82–86. [Google Scholar] [CrossRef]
- Fregni, F.; Orsati, F.; Pedrosa, W.; Fecteau, S.; Tome, F.A.; Nitsche, M.A.; Mecca, T.; Macedo, E.C.; Pascual-Leone, A.; Boggio, P.S. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 2008, 51, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Goldman, R.L.; Borckardt, J.J.; Frohman, H.A.; O’Neil, P.M.; Madan, A.; Campbell, L.K.; Budak, A.; George, M.S. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite 2011, 56, 741–746. [Google Scholar] [CrossRef]
- Bravo, G.L.; Poje, A.B.; Perissinotti, I.; Marcondes, B.F.; Villamar, M.F.; Manzardo, A.M.; Luque, L.; Lepage, J.-F.; Stafford, D.; Fregni, F.; et al. Transcranial direct current stimulation reduces food-craving and measures of hyperphagia behavior in participants with Prader-Willi syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 171, 266–275. [Google Scholar] [CrossRef]
- Azevedo, C.C.; Trevizol, A.P.; Gomes, J.S.; Akiba, H.; Franco, R.R.; Simurro, P.B.; Ianni, R.M.; Grigolon, R.B.; Blumberger, D.M.; Dias, A.M. Transcranial Direct Current Stimulation for Prader-Willi Syndrome. J. ECT 2020. [Google Scholar] [CrossRef] [PubMed]
- Szabo-Reed, A.N.; Breslin, F.J.; Lynch, A.M.; Patrician, T.M.; Martin, L.E.; Lepping, R.J.; Powell, J.N.; Yeh, H.-W.; Befort, C.A.; Sullivan, D.; et al. Brain function predictors and outcome of weight loss and weight loss maintenance. Contemp. Clin. Trials 2015, 40, 218–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo-Reed, A.N.; Martin, L.E.; Hu, J.; Yeh, H.-W.; Powell, J.; Lepping, R.J.; Patrician, T.M.; Breslin, F.J.; Donnelly, J.E.; Savage, C.R. Modeling interactions between brain function, diet adherence behaviors, and weight loss success. Obes. Sci. Pr. 2020, 6, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual; Technical Report, 1997 A-8; University of Florida: Gainesville, FL, USA, 1997. [Google Scholar]
- Palm, U.; Reisinger, E.; Keeser, D.; Kuo, M.-F.; Pogarell, O.; Leicht, G.; Mulert, C.; Nitsche, M.A.; Padberg, F. Evaluation of Sham Transcranial Direct Current Stimulation for Randomized, Placebo-Controlled Clinical Trials. Brain Stimul. 2013, 6, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Calderon, J.; Luck, S.J. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-Y.; Hsu, S.-H.; Pion-Tonachini, L.; Jung, T.-P. Evaluation of Artifact Subspace Reconstruction for Automatic Artifact Components Removal in Multi-Channel EEG Recordings. IEEE Trans. Biomed. Eng. 2020, 67, 1114–1121. [Google Scholar] [CrossRef]
- Delorme, A.; Palmer, J.; Onton, J.; Oostenveld, R.; Makeig, S. Independent EEG Sources Are Dipolar. PLoS ONE 2012, 7, e30135. [Google Scholar] [CrossRef]
- Chaumon, M.; Bishop, D.V.; Busch, N.A. A practical guide to the selection of independent components of the electroencephalogram for artifact correction. J. Neurosci. Methods 2015, 250, 47–63. [Google Scholar] [CrossRef]
- Nolan, H.; Whelan, R.; Reilly, R. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J. Neurosci. Methods 2010, 192, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Donkers, F.C.; Van Boxtel, G.J. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004, 56, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Key, A.P.F.; Dykens, E.M. ‘Hungry Eyes’: Visual processing of food images in adults with Prader-Willi syndrome. J. Intellect. Disabil. Res. 2008, 52, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Stauder, J.E.A.; Boer, H.; Gertis, R.H.A.; Tummers, A.; Whittington, J.; Curfs, L.M.G. Differences in behavioral phenotype between parental deletion and maternal uniparental disomy in Prader-Willi syndrome: An ERP study. Clin. Neurophysiol. 2005, 116, 1464–1470. [Google Scholar] [CrossRef]
- Lapenta, O.M.; Di Sierve, K.; De Macedo, E.C.; Fregni, F.; Boggio, P.S. Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite 2014, 83, 42–48. [Google Scholar] [CrossRef]
- Rafi, S.K.; Butler, M.G. The 15q11.2 BP1-BP2 Microdeletion (Burnside–Butler) Syndrome: In Silico Analyses of the Four Coding Genes Reveal Functional Associations with Neurodevelopmental Disorders. Int. J. Mol. Sci. 2020, 21, 3296. [Google Scholar] [CrossRef]
- Writing Committee for the ENIGMA-CNV Working Group; Van Der Meer, D.; Sønderby, I.E.; Kaufmann, T.; Walters, G.B.; Abdellaoui, A.; Ames, D.; Amunts, K.; Andersson, M.; Armstrong, N.J.; et al. Association of Copy Number Variation of the 15q11.2 BP1-BP2 Region With Cortical and Subcortical Morphology and Cognition. JAMA Psychiatry 2020, 77, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lin, Y.; Gao, M.; Jin, X. Effect of Modulating Activity of DLPFC and Gender on Search Behavior: A tDCS Experiment. Front. Hum. Neurosci. 2018, 12, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Before tDCS or Sham Intervention | After tDCS or Sham Intervention | ||||||
|---|---|---|---|---|---|---|---|
| Group | Reaction Time (ms) | Hit Rate (n) | False Alarm (n) | Group | Reaction Time (ms) | Hit Rate (n) | False Alarm (n) |
| Active | 633.7 (106.6) * | 75.3 (3.3) | 3.8 (4.1) | Active | 563.8 (67.0) | 75.0 (8.8) | 3.8 (6.0) |
| Sham | 865.2 (160.4) * | 70.3 (11.84) | 0.8 (0.4) | Sham | 802.3 (254.7) | 77.8 (3.3) | 4.3 (4.0) |
| Group | Reaction Time (ms) | Hit Rate (n) | False Alarm (n) |
|---|---|---|---|
| DEL | 714.6 (39.1) | 76.0 (2.94) | 4.0 (6.1) |
| UPD | 734.1 (226.7) | 71.5 (11.1) | 1.7 (0.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poje, A.B.; Manzardo, A.; Gustafson, K.M.; Liao, K.; Martin, L.E.; Butler, M.G. Effects of Transcranial Direct Current Stimulation (tDCS) on Go/NoGo Performance Using Food and Non-Food Stimuli in Patients with Prader–Willi Syndrome. Brain Sci. 2021, 11, 250. https://doi.org/10.3390/brainsci11020250
Poje AB, Manzardo A, Gustafson KM, Liao K, Martin LE, Butler MG. Effects of Transcranial Direct Current Stimulation (tDCS) on Go/NoGo Performance Using Food and Non-Food Stimuli in Patients with Prader–Willi Syndrome. Brain Sciences. 2021; 11(2):250. https://doi.org/10.3390/brainsci11020250
Chicago/Turabian StylePoje, Albert B., Ann Manzardo, Kathleen M. Gustafson, Ke Liao, Laura E. Martin, and Merlin G. Butler. 2021. "Effects of Transcranial Direct Current Stimulation (tDCS) on Go/NoGo Performance Using Food and Non-Food Stimuli in Patients with Prader–Willi Syndrome" Brain Sciences 11, no. 2: 250. https://doi.org/10.3390/brainsci11020250
APA StylePoje, A. B., Manzardo, A., Gustafson, K. M., Liao, K., Martin, L. E., & Butler, M. G. (2021). Effects of Transcranial Direct Current Stimulation (tDCS) on Go/NoGo Performance Using Food and Non-Food Stimuli in Patients with Prader–Willi Syndrome. Brain Sciences, 11(2), 250. https://doi.org/10.3390/brainsci11020250







