Conflict between Threat Sensitivity and Sensation Seeking in the Adolescent Brain: Role of the Hippocampus, and Neurobehavioural Plasticity Induced by Pleasurable Early Enriched Experience
Abstract
:1. Introduction
2. Reward- and Threat-Processing Neural Circuits, and Regulatory Systems during Adolescence: A “Tetradic” Model to Account for Behavioural Outputs under Approach–Avoidance Conflict?
2.1. Summary of Maturational Aspects of Neural Circuits Processing Reward and Threat during Adolescence
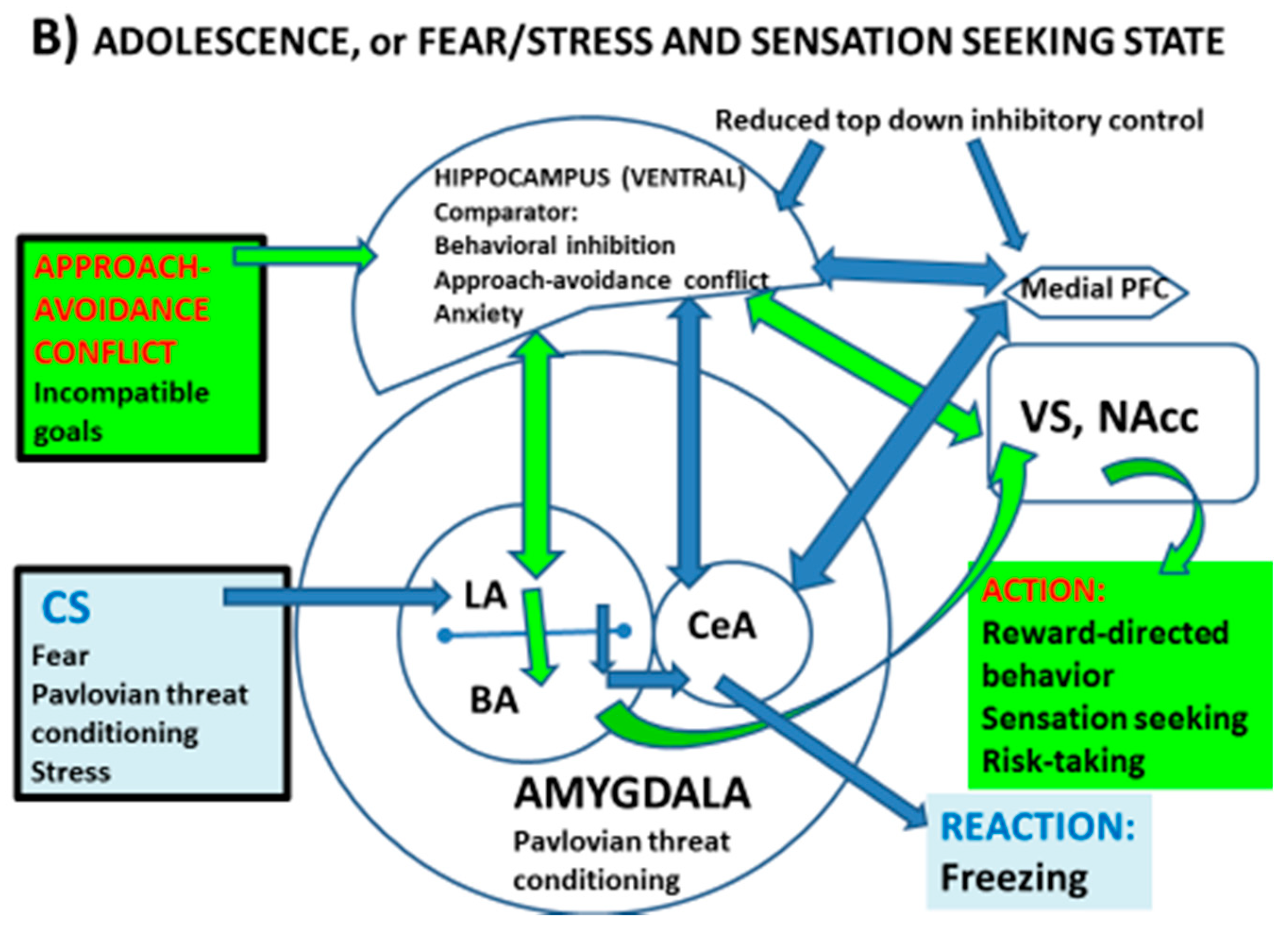
2.2. Shifting the Focus to “Threat–Reward” Conflict
3. Approach–Avoidance Conflict in Adolescence: The HPC as a Comparator and Regulator within the “Tetradic” Model
4. How Does Early-Life Pleasurable-Enriched Experience Influence Threat and Reward Circuitry?
5. Conclusions
Funding
Conflicts of Interest
References
- Baker, A.E.; Galván, A. Threat or thrill? the neural mechanisms underlying the development of anxiety and risk taking in adolescence. Dev. Cogn. Neurosci. 2020, 45, 100841. [Google Scholar] [CrossRef]
- Baker, A.E.; Tashjian, S.M.; Goldenberg, D.; Galván, A. Neural activity moderates the association between sleep and risky driving behaviors in adolescence. Dev. Cogn. Neurosci. 2020, 43, 100790. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Romeo, R.D.; Andersen, S.L. Neurobiology of the development of motivated behaviors in adolescence: A window into a neural systems model. Pharmacol. Biochem. Behav. 2009, 93, 199–211. [Google Scholar] [CrossRef]
- Spear, L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Caseya, B.J.; Getza, S.; Adriana Galvan, A. The adolescent brain. Dev. Rev. 2008, 28, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Lago, T.; Davis, A.; Grillon, C.; Ernst, M. Striatum on the anxiety map: Small detours into adolescence. Brain Res. 2017, 1654, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Spielberg, J.M.; Olino, T.M.; Forbes, E.E.; Dahl, R.E. Exciting fear in adolescence: Does pubertal development alter threat processing? Dev. Cogn. Neurosci. 2014, 8, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.S.; Richardson, R.; Baker, K.D. Maturational Changes in Prefrontal and Amygdala Circuits in Adolescence: Implications for Understanding Fear Inhibition during a Vulnerable Period of Development. Brain Sci. 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Teruel, A.; Tobeña, A. Revisiting the role of anxiety in the initial acquisition of two-way active avoidance: Pharmacological, behavioural and neuroanatomical convergence. Neurosci. Biobehav. Rev. 2020, 118, 739–758. [Google Scholar] [CrossRef]
- Gray, J.A.; McNaughton, N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- McNaughton, N.; Corr, P.J. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004, 28, 285–305. [Google Scholar] [CrossRef]
- Bannerman, D.M.; Rawlins, J.N.; McHugh, S.B.; Deacon, R.M.; Yee, B.K.; Bast, T.; Zhang, W.N.; Pothuizen, H.H.; Feldon, J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef]
- Ernst, M.; Pine, D.S.; Hardin, M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006, 36, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Birnie, M.T.; Kooiker, C.L.; Short, A.K.; Bolton, J.L.; Chen, Y.; Baram, T.Z. Plasticity of the Reward Circuitry After Early-Life Adversity: Mechanisms and Significance. Biol. Psychiatry 2020, 87, 875–884. [Google Scholar] [CrossRef]
- Romeo, R.D. The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain Res. 2017, 1654, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, M.; Fudge, J.L. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neurosci. Biobehav. Rev. 2009, 33, 367–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, J.M.; Plate, R.C.; Ernst, M. Neural systems underlying motivated behavior in adolescence: Implications for preventive medicine. Prev. Med. 2012, 55, S7–S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keresztes, A.; Chi, T.; Ngo, C.T.; Lindenberger, U.; Werkle-Bergner, M.; Newcombe, N.S. Hippocampal maturation drives memory from generalization to specificity. Trends Cogn. Sci. 2018, 22, 676–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvan, A.; Hare, T.A.; Parra, C.E.; Penn, J.; Voss, H.; Glover, G.; Casey, B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006, 26, 6885–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, K.Y.; Chambers, R.A.; Lipska, B.K. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav. Brain Res. 2009, 204, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Helfinstein, S.M.; Fox, N.A.; Pine, D.S. Approach-withdrawal and the role of the striatum in the temperament of behavioral inhibition. Dev. Psychol. 2012, 48, 815–826. [Google Scholar] [CrossRef]
- Porter, J.N.; Roy, A.K.; Benson, B.; Carlisi, C.; Collins, P.F.; Leibenluft, E.; Pine, D.S.; Luciana, M.; Ernst, M. Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Dev. Cog. Neurosci. 2015, 11, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laviola, G.; Macri, S.; Morley-Fletcher, S.; Adriani, W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 2003, 27, 19–31. [Google Scholar] [CrossRef]
- Schwarting, R.K.; Busse, S. Behavioral facilitation after hippocampal lesion: A review. Behav. Brain Res. 2017, 317, 401–414. [Google Scholar] [CrossRef]
- Thomason, M.E.; Marusak, H.A. Within-subject neural reactivity to reward and threat is inverted in young adolescents. Psychol. Med. 2017, 47, 1549–1560. [Google Scholar] [CrossRef]
- Gray, J.A. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System; Oxford University Press: Oxford, UK, 1982. [Google Scholar]
- McNaughton, N.; DeYoung, C.G.; Corr, P.J. Approach/Avoidance. In Neuroimaging Personality, Social Cognition, and Character; Absher, J.R., Cloutier, J., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2016; pp. 25–49. [Google Scholar]
- Bannerman, D.M.; Sprengel, R.; Sanderson, D.J.; McHugh, S.B.; Rawlins, J.N.; Monyer, H.; Seeburg, P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014, 15, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.A.; McNaughton, N. Comparison between the behavioural effects of septal and hippocampal lesions: A review. Neurosci. Biobehav. Rev. 1983, 7, 119–188. [Google Scholar] [CrossRef]
- Ito, R.; Lee, A.C.H. The role of the hippocampus in approach-avoidance conflict decision-making: Evidence from rodent and human studies. Behav. Brain Res. 2016, 313, 345–357. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Anxious: Using the Brain to Understand and Treat Fear and Anxiety; Penguin Books: New York, NY, USA, 2015. [Google Scholar]
- LeDoux, J.E.; Moscarello, J.; Sears, R.; Campese, V. The birth, death and resurrection of avoidance: A reconceptualization of a troubled paradigm. Mol. Psychiatr. 2017, 22, 24–36. [Google Scholar] [CrossRef]
- Bach, D.R.; Guitart-Masip, M.; Packard, P.A.; Miró, J.; Falip, M.; Fuentemilla, L.; Dolan, R.J. Human Hippocampus Arbitrates Approach-Avoidance Conflict. Curr. Biol. 2014, 24, 1435. [Google Scholar] [CrossRef] [Green Version]
- Fung, B.J.; Qi, S.; Hassabis, D.; Daw, N.; Mobbs, D. Slow escape decisions are swayed by trait anxiety. Nat. Hum. Behav. 2019, 3, 702–708. [Google Scholar] [CrossRef]
- Korn, C.W.; Bach, D.R. Minimizing threat via heuristic and optimal policies recruits hippocampus and medial prefrontal cortex. Nat Hum Behav. 2019, 3, 733–745. [Google Scholar] [CrossRef]
- McNaughton, N. Brain maps of fear and anxiety. Nat Hum Behav. 2019, 3, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Loh, E.; Kurth-Nelson, Z.; Berron, D.; Dayan, P.; Duzel, E.; Dolan, R.; Guitart-Masip, M. Parsing the Role of the Hippocampus in Approach-Avoidance Conflict. Cereb. Cortex 2017, 27, 201–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neil, E.B.; Newsome, R.N.; Li, I.H.N.; Thavabalasingam, S.; Ito, R.; Lee, A.C.H. Examining the role of the human hippocampus in approach-avoidance decision making using a novel conflict paradigm and multivariate functional magnetic resonance imaging. J. Neurosci. 2015, 35, 15039–15049. [Google Scholar] [CrossRef] [Green Version]
- Corda, M.G.; Piras, G.; Giorgi, O. Neonatal ventral hippocampal lesions potentiate amphetamine-induced increments in dopamine efflux in the core, but not the shell, of the nucleus accumbens. Biol. Psychiatry 2006, 60, 1188–1195. [Google Scholar] [CrossRef]
- Giorgi, O.; Piras, G.; Corda, M.G. The psychogenetically selected Roman high- and low-avoidance rat lines: A model to study the individual vulnerability to drug addiction. Neurosci. Biobehav. Rev. 2007, 31, 148–163. [Google Scholar] [CrossRef]
- Giorgi, O.; Corda, M.G.; Fernández-Teruel, A. A Genetic Model of Impulsivity, Vulnerability to Drug Abuse and Schizophrenia-Relevant Symptoms with Translational Potential: The Roman High- vs. Low-Avoidance Rats. Front. Behav. Neurosci. 2019, 13, 145. [Google Scholar] [CrossRef]
- Elfving, B.; Müller, H.K.; Oliveras, I.; Østerbøg, T.B.; Rio-Alamos, C.; Sanchez-Gonzalez, A.; Tobeña, A.; Fernandez-Teruel, A.; Aznar, S. Differential expression of synaptic markers regulated during neurodevelopment in a rat model of schizophrenia-like behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109669. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, A.; Thougaard, E.B.; Tapias-Espinosa, C.; Cañete, T.; Sampedro-Viana, D.; Saunders, J.M.; Toneatti, R.; Tobeña, A.; Gónzalez-Maeso, J.; Aznar, S.; et al. Increased thin-spine density in frontal cortex pyramidal neurons in a genetic rat model of schizophrenia-relevant features. Eur. Neuropsychopharmacol. 2021. [Google Scholar] [CrossRef]
- Meyza, K.Z.; Boguszewski, P.M.; Nikolaev, E.; Zagrodzka, J. Diverse sensitivity of RHA/Verh and RLA/Verh rats to emotional and spatial aspects of a novel environment as a result of a distinct pattern of neuronal activation in the fear/anxiety circuit. Behav. Genet. 2009, 39, 48–61. [Google Scholar] [CrossRef]
- Tapias-Espinosa, C.; Río-Álamos, C.; Sánchez-González, A.; Oliveras, I.; Sampedro-Viana, D.; Castillo-Ruiz, M.D.M.; Cañete, T.; Tobeña, A.; Fernández-Teruel, A. Schizophrenia-like reduced sensorimotor gating in intact inbred and outbred rats is associated with decreased medial prefrontal cortex activity and volume. Neuropsychopharmacology 2019, 44, 1975–1984. [Google Scholar] [CrossRef]
- McClelland, S.; Korosi, A.; Cope, J.; Ivy, A.; Baram, T.Z. Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol. Learn. Mem. 2011, 96, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Malter Cohen, M.; Tottenham, N.; Casey, B.J. Translational developmental studies of stress on brain and behavior: Implications for adolescent mental health and illness? Neuroscience 2013, 249, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, R. Infantile experience and play motivation. Soc. Neurosci. 2010, 5, 422–440. [Google Scholar] [CrossRef]
- Aguilar, R.; Caramés, J.M.; Espinet, A. Effects of neonatal handling on playfulness by means of reversal of the desire to play in rats (Rattus norvegicus). J. Comp. Psychol. 2009, 123, 347–356. [Google Scholar] [CrossRef]
- Burgdorf, J.; Colechio, E.M.; Stanton, P.; Panksepp, J. Positive Emotional Learning Induces Resilience to Depression: A Role for NMDA Receptor-mediated Synaptic Plasticity. Curr. Neuropharmacol. 2017, 15, 3–10. [Google Scholar] [CrossRef]
- Perez-Sepulveda, J.A.; Flagel, S.B.; Garcia-Fuster, M.J.; Slusky, R.J.; Aldridge, J.W.; Watson, S.; Akil, H. Differential impact of a complex environment on positive affect in an animal model of individual differences in emotionality. Neuroscience 2013, 248, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Schloesser, R.J.; Lehmann, M.; Martinowich, K.; Manji, H.K.; Herkenham, M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry 2010, 15, 1152–1163. [Google Scholar] [CrossRef] [Green Version]
- Maaswinkel, H.; Gispen, W.H.; Spruijt, B.M. Executive function of the hippocampus in social behavior in the rat. Behav. Neurosci. 1997, 111, 777–784. [Google Scholar] [CrossRef]
- Fernández-Teruel, A.; Escorihuela, R.M.; Castellano, B.; González, B.; Tobeña, A. Neonatal handling and environmental enrichment effects on emotionality, novelty/reward seeking, and age-related cognitive and hippocampal impairments: Focus on the Roman rat lines. Behav Genet. 1997, 27, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Teruel, A.; Giménez-Llort, L.; Escorihuela, R.M.; Gil, L.; Aguilar, R.; Steimer, T.; Tobeña, A. Early-life handling stimulation and environmental enrichment: Are some of their effects mediated by similar neural mechanisms? Pharmacol. Biochem. Behav. 2002, 73, 233–245. [Google Scholar] [CrossRef]
- Raineki, C.; Lucion, A.B.; Weinberg, J. Neonatal handling: An overview of the positive and negative effects. Dev. Psychobiol. 2014, 56, 1613–1625. [Google Scholar] [CrossRef] [Green Version]
- Río-Álamos, C.; Piludu, M.A.; Gerbolés, C.; Barroso, D.; Oliveras, I.; Sánchez-González, A.; Cañete, T.; Tapias-Espinosa, C.; Sampedro-Viana, D.; Torrubia, R.; et al. Volumetric brain differences between the Roman rat strains: Neonatal handling effects, sensorimotor gating and working memory. Behav. Brain Res. 2019, 361, 74–85. [Google Scholar] [CrossRef]
- Meaney, M.J.; Aitken, D.H.; Van Berkel, C.; Bhatnagar, S.; Sapolsky, R.M. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science 1988, 239, 766–768. [Google Scholar] [CrossRef]
- Peters, S.L.; Gray, J.A.; Joseph, M.H. Preweaning non-handling of rats disrupts latent inhibition in males, and results in persisting sex- and area-dependent increases in dopamine and serotonin turnover. Behav. Pharmacol. 1991, 2, 215–223. [Google Scholar] [CrossRef]
- Núñez, J.F.; Ferré, P.; Escorihuela, R.M.; Tobeña, A.; Fernández-Teruel, A. Effects of postnatal handling of rats on emotional, HPA-axis, and prolactin reactivity to novelty and conflict. Physiol. Behav. 1996, 60, 1355–1359. [Google Scholar] [CrossRef]
- Park, M.K.; Hoang, T.A.; Belluzzi, J.D.; Leslie, F.M. Gender specific effect of neonatal handling on stress reactivity of adolescent rats. J. Neuroendocrinol. 2003, 15, 289–295. [Google Scholar] [CrossRef]
- Río-Alamos, C.; Oliveras, I.; Cañete, T.; Blázquez, G.; Martínez-Membrives, E.; Tobeña, A.; Fernández-Teruel, A. Neonatal handling decreases unconditioned anxiety, conditioned fear, and improves two-way avoidance acquisition: A study with the inbred Roman high (RHA-I)- and low-avoidance (RLA-I) rats of both sexes. Front. Behav. Neurosci. 2015, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Río-Álamos, C.; Oliveras, I.; Piludu, M.A.; Gerbolés, C.; Cañete, T.; Blázquez, G.; Lope-Piedrafita, S.; Martínez-Membrives, E.; Torrubia, R.; Tobeña, A.; et al. Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in a genetic model of differential anxiety: Behavioral-volumetric associations in the Roman rat strains. Eur. Neuropsychopharmacol. 2017, 27, 146–158. [Google Scholar] [CrossRef]
- Vey, L.T.; Rosa, H.Z.; Silva Barcelos, R.C.; Tironi Dias, V.; Ugalde Marques da Rocha, M.I.; Escobar Burger, M. Neonatal handling increases neurogenesis, BDNF and GR in the hippocampus favoring memory acquisition in rats. Brain Res. 2020, 1745, 146921. [Google Scholar] [CrossRef]
- Vey, L.T.; Rosa, H.Z.; Barcelos, R.C.S.; Segat, H.J.; Metz, V.G.; Dias, V.T.; Duarte, T.; Duarte, M.M.F.; Burger, M.E. Stress during the gestational period modifies pups’ emotionality parameters and favors preference for morphine in adolescent rats. Behav. Brain Res. 2015, 296, 408–417. [Google Scholar] [CrossRef]
- Colorado, E.A.; Shumake, J.; Conejo, N.M.; Gonzalez-Pardo, H.; Gonzalez-Lima, F. Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behav. Process. 2006, 71, 51–58. [Google Scholar] [CrossRef]
- Antoniazzi, C.T.D.; Boufleur, N.; Dolci, G.S.; Roversi, K.; Kuhn, F.T.; Pase, C.S.; Dias, V.T.; Roversi, K.; Barcelos, R.C.S.; Benvegnu, D.M.; et al. Influence of neonatal tactile stimulation on amphetamine preference in young rats: Parameters of addiction and oxidative stress. Pharmacol. Biochem. Behav. 2014, 124, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Antoniazzi, C.T.D.; Boufleur, N.; Pase, C.S.; Kuhn, F.T.; Dias, V.T.; Segat, H.J.; Roversi, K.; Roversi, K.; Benvegnú, D.M.; Bürger, M.E. Tactile stimulation and neonatal isolation affect behavior and oxidative status linked to cocaine administration in young rats. Behav. Process. 2014, 103, 297–305. [Google Scholar] [CrossRef]
- Brake, W.G.; Zhang, T.Y.; Diorio, J.; Meaney, M.J.; Gratton, A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur. J. Neurosci. 2004, 19, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Monroy, E.; Hernandez-Torres, E.; Flores, G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J. Chem. Neuroanat. 2010, 40, 93–101. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Groenewegen, H.J. The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev. 2003, 27, 555–579. [Google Scholar] [CrossRef]
- Rule, L.; Yang, J.; Watkin, H.; Hall, J.; Brydges, N.M. Environmental enrichment rescues survival and function of adult-born neurons following early life stress. Mol. Psychiatry 2020. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Whittle, S.; Simmons, J.G.; Dennison, M.; Vijayakumar, N.; Schwartz, O.; Yap, M.B.H.; Sheeber, L.; Allen, N.B. Positive parenting predicts the development of adolescent brain structure: A longitudinal study. Dev. Cog. Neurosci. 2014, 8, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittle, S.; Vijayakumar, N.; Simmons, J.G.; Dennison, M.; Schwartz, O.; Pantelis, C.; Franzcp, H.M.D.; Sheeber, L.; Byrne, M.L.; Allen, N.B. Role of Positive Parenting in the Association Between Neighborhood Social Disadvantage and Brain Development Across Adolescence. JAMA Psychiatry 2017, 74, 824–832. [Google Scholar] [CrossRef]
- Caldji, C.; Tannenbaum, B.; Sharma, S.; Francis, D.; Plotsky, P.M.; Meaney, M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. USA 1998, 95, 5335–5340. [Google Scholar] [CrossRef] [Green Version]
- Meaney, M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef] [PubMed]
- Van Hasselt, F.N.; Tieskens, J.M.; Trezza, V.; Krugers, H.J.; Vanderschuren, L.J.M.J.; Joëls, M. Within-litter variation in maternal care received by individual pups correlates with adolescent social play behavior in male rats. Physiol. Behav. 2012, 106, 701–706. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.M.J.; Niesink, R.J.M.; van Ree, J.M. The Neurobiology of Social Play Behavior in Rats. Neurosci. Biobehav. Rev. 1997, 21, 309–326. [Google Scholar] [CrossRef]
- Trezza, V.; Baarendse, P.J.J.; Vanderschuren, L.J.M.J. The pleasures of play: Pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010, 31, 463–469. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Teruel, A. Conflict between Threat Sensitivity and Sensation Seeking in the Adolescent Brain: Role of the Hippocampus, and Neurobehavioural Plasticity Induced by Pleasurable Early Enriched Experience. Brain Sci. 2021, 11, 268. https://doi.org/10.3390/brainsci11020268
Fernández-Teruel A. Conflict between Threat Sensitivity and Sensation Seeking in the Adolescent Brain: Role of the Hippocampus, and Neurobehavioural Plasticity Induced by Pleasurable Early Enriched Experience. Brain Sciences. 2021; 11(2):268. https://doi.org/10.3390/brainsci11020268
Chicago/Turabian StyleFernández-Teruel, Alberto. 2021. "Conflict between Threat Sensitivity and Sensation Seeking in the Adolescent Brain: Role of the Hippocampus, and Neurobehavioural Plasticity Induced by Pleasurable Early Enriched Experience" Brain Sciences 11, no. 2: 268. https://doi.org/10.3390/brainsci11020268




