Characterization of Motor-Evoked Responses Obtained with Transcutaneous Electrical Spinal Stimulation from the Lower-Limb Muscles after Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
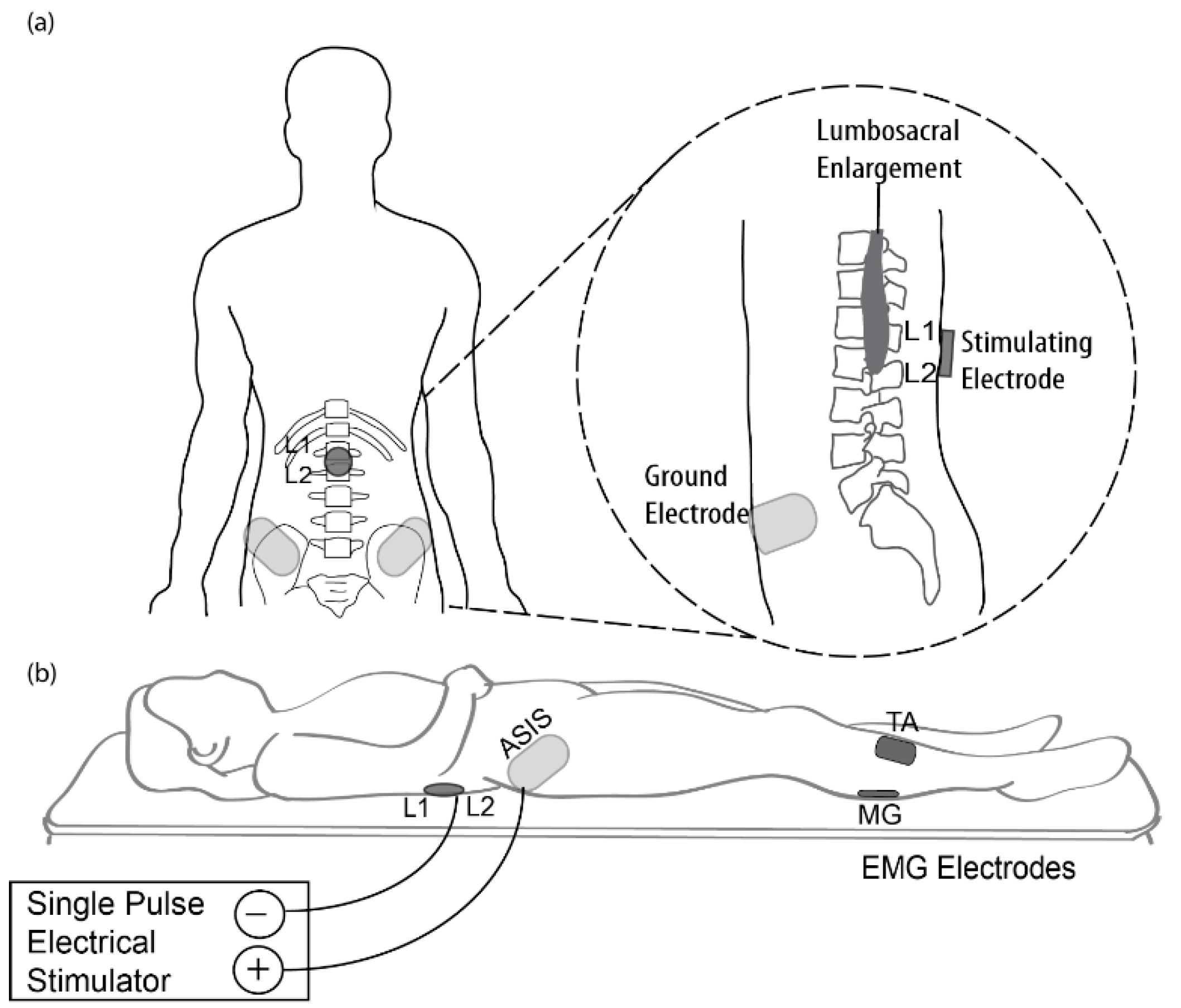
2.2. Transcutaneous Electrical Spinal Cord Stimulation (tSCS) Procedure
2.3. Electromyographic (EMG) Recordings
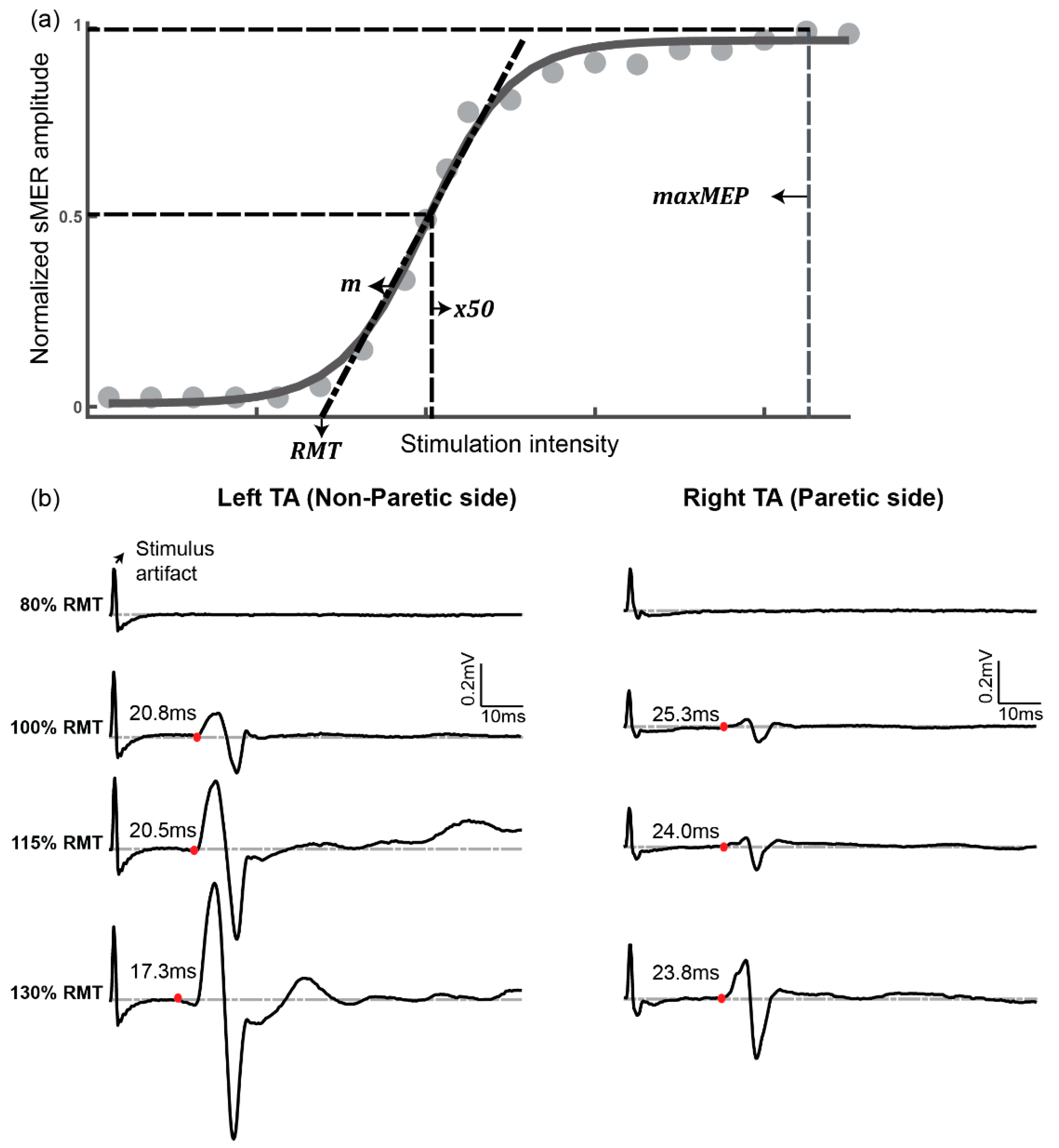
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographics
3.2. Participants’ Tolerance to a Single Pulse of tSCS
3.3. Variability of Responses to Single-Pulse tSCS
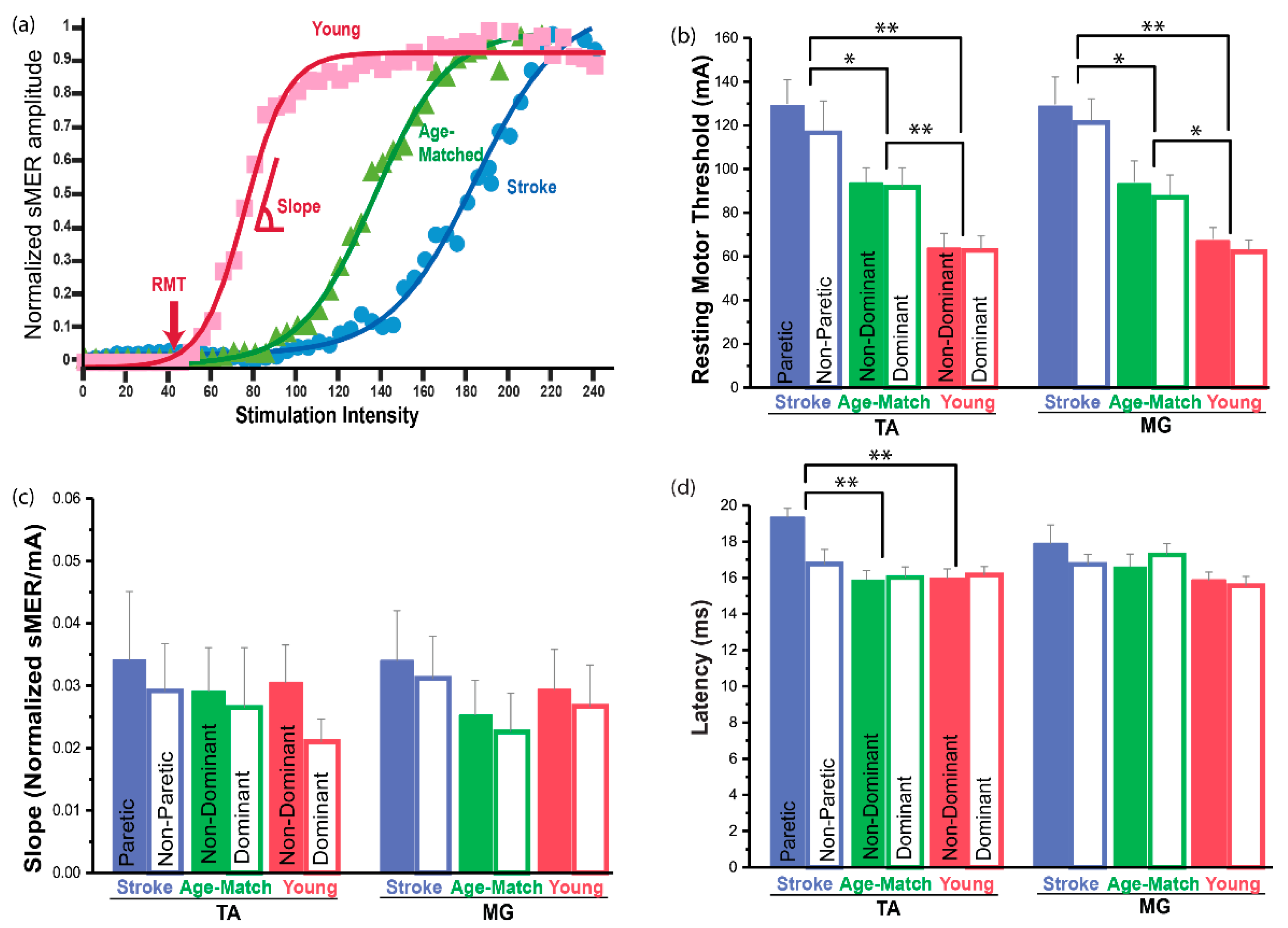
3.4. Resting Motor Threshold (RMT)
3.5. Slope of the sMER Recruitment Curve
3.6. Latency
4. Discussion
4.1. Changes in the Spinal Motor Responsiveness after Stroke
4.2. No Change in the Slope of the sMER Recruiment Curve after Stroke
4.3. Effects of Aging
4.4. Clinical Implication
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taccola, G.; Sayenko, D.; Gad, P.; Gerasimenko, Y.; Edgerton, V.R. And yet It Moves: Recovery of Volitional Control after Spinal Cord Injury. Prog. Neurobiol. 2008, 160, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.; Ruslan, G.; Tatiana, M.; Dimitry, S.; Parag, G.; Edgerton, V.R. Transcutaneous Electrical Spinal-Cord Stimulation in Humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.; Parag, G.; Dimitry, S.; Zach, M.; Ruslan, G.; Aleksandr, P.; Tatiana, M.; Aleksandr, S.; Victor, S.; Tatiana, S.; et al. Integration of Sensory, Spinal, and Volitional Descending Inputs in Regulation of Human Locomotion. J. Neurophysiol. 2016, 116, 98–105. [Google Scholar] [CrossRef]
- Bocci, T.; Sara, M.; Maurizio, V.; Valeria, C.; Filippo, C.; Ferdinando, S.; Alberto, P. Transcutaneous Spinal Direct Current Stimulation Modulates Human Corticospinal System Excitability. J. Neurophysiol. 2015, 114, 440–446. [Google Scholar] [CrossRef]
- Courtine, G.; Harkema, S.J.; Dy, C.J.; Gerasimenko, Y.P.; Dyhre-Poulsen, P. Modulation of Multisegmental Monosynaptic Responses in a Variety of Leg Muscles During Walking and Running in Humans. J. Physiol. 2007, 582, 1125–1139. [Google Scholar] [CrossRef]
- Minassian, K.; Persy, I.; Rattay, F.; Dimitrijevic, M.R.; Hofer, C.; Kern, H. Posterior Root—Muscle Reflexes Elicited by Transcutaneous Stimulation of the Human Lumbosacral Cord. Muscle Nerve 2007, 35, 327–336. [Google Scholar] [CrossRef]
- Murray, L.M.; Knikou, M. Transspinal Stimulation Increases Motoneuron Output of Multiple Segments in Human Spinal Cord Injury. PLoS ONE 2019, 14, e0213696. [Google Scholar] [CrossRef] [PubMed]
- Knikou, M. Neurophysiological Characterization of Transpinal Evoked Potentials in Human Leg Muscles. Bioelectromagnetics 2013, 34, 630–640. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Common Neural Structures Activated by Epidural and Transcutaneous Lumbar Spinal Cord Stimulation: Elicitation of Posterior Root-Muscle Reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef] [PubMed]
- Ladenbauer, J.; Minassian, K.; Hofstoetter, U.S.; Dimitrijevic, M.R.; Rattay, F. Stimulation of the Human Lumbar Spinal Cord with Implanted and Surface Electrodes: A Computer Simulation Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Kitano, K.; Koceja, D.M. Spinal Reflex in Human Lower Leg Muscles Evoked by Transcutaneous Spinal Cord Stimulation. J. Neurosci. Methods 2009, 180, 111–115. [Google Scholar] [CrossRef]
- Knikou, M. The H-Reflex as a Probe: Pathways and Pitfalls. J. Neurosci. Methods 2008, 171, 1–12. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Crone, C.; Hultborn, H. The Spinal Pathophysiology of Spasticity—From a Basic Science Point of View. Acta Physiol. 2007, 189, 171–180. [Google Scholar] [CrossRef]
- Danner, S.M.; Krenn, M.; Hofstoetter, U.S.; Toth, A.; Mayr, W.; Minassian, K. Body Position Influences Which Neural Structures Are Recruited by Lumbar Transcutaneous Spinal Cord Stimulation. PLoS ONE 2016, 11, e0147479. [Google Scholar] [CrossRef]
- Knikou, M.; Dixon, L.; Santora, D.; Ibrahim, M.M. Transspinal Constant-Current Long-Lasting Stimulation: A New Method to Induce Cortical and Corticospinal Plasticity. J. Neurophysiol. 2015, 114, 1486–1499. [Google Scholar] [CrossRef]
- Sabbahi, M.A.; Sengul, Y.S. Thoracolumbar Multisegmental Motor Responses in the Upper and Lower Limbs in Healthy Subjects. Spinal Cord 2011, 49, 741–748. [Google Scholar] [CrossRef][Green Version]
- Gerasimenko, Y.P.; Lu, D.C.; Modaber, M.; Zdunowski, S.; Gad, P.; Sayenko, D.G.; Morikawa, E.; Haakana, P.; Ferguson, A.R.; Roy, R.R.; et al. Noninvasive Reactivation of Motor Descending Control after Paralysis. J. Neurotrauma 2015, 32, 1968–1980. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.M.; Tahayori, B.; Knikou, M. Transspinal Direct Current Stimulation Produces Persistent Plasticity in Human Motor Pathways. Sci. Rep. 2018, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Benavides, F.D.; Jo, H.J.; Lundell, H.; Edgerton, V.R.; Gerasimenko, Y.; Perez, M.A. Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia. J. Neurosci. 2020, 40, 2633–2643. [Google Scholar] [CrossRef]
- Dang, G.; Chen, X.; Chen, Y.; Zhao, Y.; Ouyang, F.; Zeng, J. Dynamic Secondary Degeneration in the Spinal Cord and Ventral Root after a Focal Cerebral Infarction among Hypertensive Rats. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Karbasforoushan, H.; Cohen-Adad, J.; Dewald, J.P. Brainstem and Spinal Cord Mri Identifies Altered Sensorimotor Pathways Post-Stroke. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Phadke, C.P.; Robertson, C.T.; Condliffe, E.G.; Patten, C. Upper-Extremity H-Reflex Measurement Post-Stroke: Reliability and Inter-Limb Differences. Clin. Neurophysiol. 2012, 123, 1606–1615. [Google Scholar] [CrossRef]
- Higashi, T.; Funase, K.; Kusano, K.; Tabira, T.; Harada, N.; Sakakibara, A.; Yoshimura, T. Motoneuron Pool Excitability of Hemiplegic Patients: Assessing Recovery Stages by Using H-Reflex and M Response. Arch. Phys. Med. Rehabil. 2001, 82, 1604–1610. [Google Scholar] [CrossRef]
- Lamy, J.C.; Wargon, I.; Mazevet, D.; Ghanim, Z.; Pradat-Diehl, P.; Katz, R. Impaired Efficacy of Spinal Presynaptic Mechanisms in Spastic Stroke Patients. Brain 2008, 132, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Kido, A.; Tanaka, N.; Stein, R.B. Spinal Excitation and Inhibition Decrease as Humans Age. Can. J. Physiol. Pharmacol. 2004, 82, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Spinal Cord Maps of Spatiotemporal Alpha-Motoneuron Activation in Humans Walking at Different Speeds. J. Neurophysiol. 2006, 95, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Atkinson, D.A.; Floyd, T.C.; Gorodnichev, R.M.; Moshonkina, T.R.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Effects of Paired Transcutaneous Electrical Stimulation Delivered at Single and Dual Sites over Lumbosacral Spinal Cord. Neurosci. Lett. 2015, 609, 229–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grishin, A.A.; Moshonkina, T.R.; Solopova, I.A.; Gorodnichev, R.M.; Gerasimenko, Y.P. A Five-Channel Noninvasive Electrical Stimulator of the Spinal Cord for Rehabilitation of Patients with Severe Motor Disorders. Biomed. Eng. 2017, 50, 300–304. [Google Scholar] [CrossRef]
- Adams, R.W.; Gandevia, S.C.; Skuse, N.F. The Distribution of Muscle Weakness in Upper Motoneuron Lesions Affecting the Lower Limb. Brain 1990, 113, 1459–1476. [Google Scholar] [CrossRef]
- Ziemann, U.; Rothwell, J.C.; Ridding, M.C. Interaction between Intracortical Inhibition and Facilitation in Human Motor Cortex. J. Physiol. 1996, 496, 873–881. [Google Scholar] [CrossRef]
- Klimstra, M.; Zehr, E.P. A Sigmoid Function Is the Best Fit for the Ascending Limb of the Hoffmann Reflex Recruitment Curve. Exp. Brain Res. 2008, 186, 93–105. [Google Scholar] [CrossRef]
- Brinkworth, R.S.; Turker, K.S. A Method for Quantifying Reflex Responses from Intra-Muscular and Surface Electromyogram. J. Neurosci. Methods 2003, 122, 179–193. [Google Scholar] [CrossRef]
- Cohen, J. Chapter 8—F Tests on Means in the Analysis of Variance and Covariance. In Statistical Power Analysis for the Behavioral Sciences; Cohen, J., Ed.; Academic Press: Cambridege, MA, USA, 1977; pp. 273–406. [Google Scholar]
- Wheaton, L.A.; Villagra, F.; Hanley, D.F.; Macko, R.F.; Forrester, L.W. Reliability of Tms Motor Evoked Potentials in Quadriceps of Subjects with Chronic Hemiparesis after Stroke. J. Neurol. Sci. 2009, 276, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Cakar, E.; Akyuz, G.; Durmus, O.; Bayman, L.; Yagci, I.; Karadag-Saygi, E.; Gunduz, O.H. The Relationships of Motor-Evoked Potentials to Hand Dexterity, Motor Function, and Spasticity in Chronic Stroke Patients: A Transcranial Magnetic Stimulation Study. Acta Neurol. Belg. 2016, 116, 481–487. [Google Scholar] [CrossRef]
- Badawy, R.A.; Loetscher, T.; Macdonell, R.A.; Brodtmann, A. Cortical Excitability and Neurology: Insights into the Pathophysiology. Funct. Neurol. 2012, 27, 131–145. [Google Scholar] [PubMed]
- Hassanlouei, H.; Sundberg, C.W.; Smith, A.E.; Kuplic, A.; Hunter, S.K. Physical Activity Modulates Corticospinal Excitability of the Lower Limb in Young and Old Adults. J. Appl. Physiol. 2007, 123, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, B.; Ashby, P. Corticospinal Projections to Lower Limb Motoneurons in Man. Exp. Brain Res. 1992, 89, 649–654. [Google Scholar] [CrossRef]
- Marigold, D.S.; Eng, J.J.; Inglis, J.T. Modulation of Ankle Muscle Postural Reflexes in Stroke: Influence of Weight-Bearing Load. Clin. Neurophysiol. 2004, 115, 2789–2797. [Google Scholar] [CrossRef][Green Version]
- Vanderthommen, M.; Duteil, S.; Wary, C.; Raynaud, J.S.; Leroy-Willig, A.; Crielaard, J.M.; Carlier, P.G. A Comparison of Voluntary and Electrically Induced Contractions by Interleaved 1h- and 31p-Nmrs in Humans. J. Appl. Physiol. 2003, 94, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Vanderthommen, M.; Duchateau, J. Electrical Stimulation as a Modality to Improve Performance of the Neuromuscular System. Exerc. Sport Sci. Rev. 2007, 35, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, F.; Guerra, A.; Vollero, L.; Ponzo, D.; Maatta, S.; Mervaala, E.; Iannello, G.; Di Lazzaro, V. Age-Related Changes of Cortical Excitability and Connectivity in Healthy Humans: Non-Invasive Evaluation of Sensorimotor Network by Means of Tms-Eeg. Neuroscience 2017, 357, 255–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eisen, A.; Entezari-Taher, M.; Stewart, H. Cortical Projections to Spinal Motoneurons: Changes with Aging and Amyotrophic Lateral Sclerosis. Neurology 1996, 46, 1396. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; McKay, W.B.; Tansey, K.E.; Mayr, W.; Kern, H.; Minassian, K. Modification of Spasticity by Transcutaneous Spinal Cord Stimulation in Individuals with Incomplete Spinal Cord Injury. J. Spinal Cord Med. 2014, 37, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, J.B.; Ogston, K.M.; Miles, T.S. Age and Sex Differences in Human Motor Cortex Input-Output Characteristics. J. Physiol. 2003, 546, 605–613. [Google Scholar] [CrossRef] [PubMed]
| Outcome Measures | Definition | Physiological Significance of Change in Metric |
|---|---|---|
| Resting motor threshold (RMT) | Stimulation intensity required to activate the most excitable spinal motor pools without any voluntary muscle activation | An increased RMT indicates decreased ion channel conductivity and hence decreased membrane excitability of neurons in the spinal motor pools [30]. |
| Slope of sMER recruitment curve | Rate at which sMER size increases with increasing stimulus intensity | A decreased slope represents a decreased rate to recruit additional motor neurons with increasing stimulation intensity [2]. |
| Latency | Signal propagation time between stimulation of the spinal cord to the onset of muscle response | Increased latency indicates delayed conduction time in axons originating from the spinal cord [7]. |
| Stroke | Age-Matched | Younger | |
|---|---|---|---|
| n | 10 | 10 | 10 |
| Gender | 7 M: 3 F | 2 M: 8 F | 3 M: 7 F |
| Age (years) | 56.8 ± 2.3 | 55.5 ± 2.6 | 24.8 ± 0.9 |
| Height (cm) | 172.6 ± 1.9 | 166.3 ± 2.2 | 172.0 ± 3.1 |
| BMI (kg/m2) | 28.9 ± 1.8 | 25.3 ± 1.2 | 23.7 ± 1.4 |
| Handedness | 10 R: 0 L | 9 R: 1 L | 8 R: 2 L |
| Paretic side | 3 R: 7 L | -- | -- |
| Time since stroke (years) | 5.9 ± 1.1 | -- | -- |
| Type of stroke | 4 Hem: 6 Isc | ||
| FMA-LE | 22.2 ± 3.9 | ||
| MAS (dorsiflexor) | All scored 0 | ||
| MAS (plantar flexor) | 1.0 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.; Zuleger, T.; Lamberti, M.; Bansal, A.; Mummidisetty, C.K.; McKenzie, K.A.; Yingling, L.; Madhavan, S.; Roth, E.J.; Lieber, R.L.; et al. Characterization of Motor-Evoked Responses Obtained with Transcutaneous Electrical Spinal Stimulation from the Lower-Limb Muscles after Stroke. Brain Sci. 2021, 11, 289. https://doi.org/10.3390/brainsci11030289
Moon Y, Zuleger T, Lamberti M, Bansal A, Mummidisetty CK, McKenzie KA, Yingling L, Madhavan S, Roth EJ, Lieber RL, et al. Characterization of Motor-Evoked Responses Obtained with Transcutaneous Electrical Spinal Stimulation from the Lower-Limb Muscles after Stroke. Brain Sciences. 2021; 11(3):289. https://doi.org/10.3390/brainsci11030289
Chicago/Turabian StyleMoon, Yaejin, Taylor Zuleger, Martina Lamberti, Ashir Bansal, Chaithanya K. Mummidisetty, Kelly A. McKenzie, Lindsey Yingling, Sangeetha Madhavan, Elliot J. Roth, Richard L. Lieber, and et al. 2021. "Characterization of Motor-Evoked Responses Obtained with Transcutaneous Electrical Spinal Stimulation from the Lower-Limb Muscles after Stroke" Brain Sciences 11, no. 3: 289. https://doi.org/10.3390/brainsci11030289
APA StyleMoon, Y., Zuleger, T., Lamberti, M., Bansal, A., Mummidisetty, C. K., McKenzie, K. A., Yingling, L., Madhavan, S., Roth, E. J., Lieber, R. L., & Jayaraman, A. (2021). Characterization of Motor-Evoked Responses Obtained with Transcutaneous Electrical Spinal Stimulation from the Lower-Limb Muscles after Stroke. Brain Sciences, 11(3), 289. https://doi.org/10.3390/brainsci11030289









