The Effect of Maternal Immune Activation on Social Play-Induced Ultrasonic Vocalization in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Poly(I:C) Administration
2.3. Social Play Test (PND 32–35)
2.4. USV Recording
2.5. Locomotor and Repetitive/Stereotypic-Like Activity (PND 35–38)
2.6. Marble-Burying Test (PND 35–38)
2.7. Statistics
3. Results
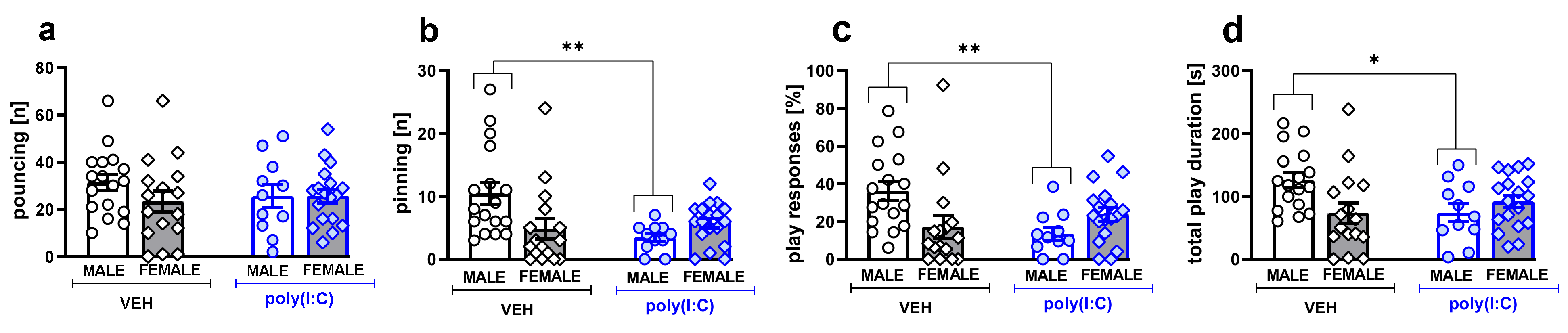
3.1. Social Play Behavior
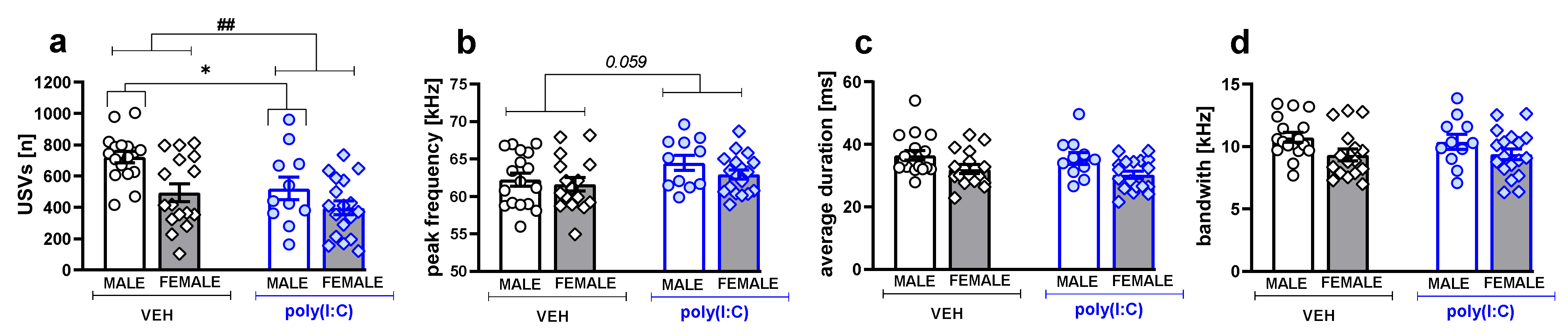
3.2. Ultrasonic Vocalizations
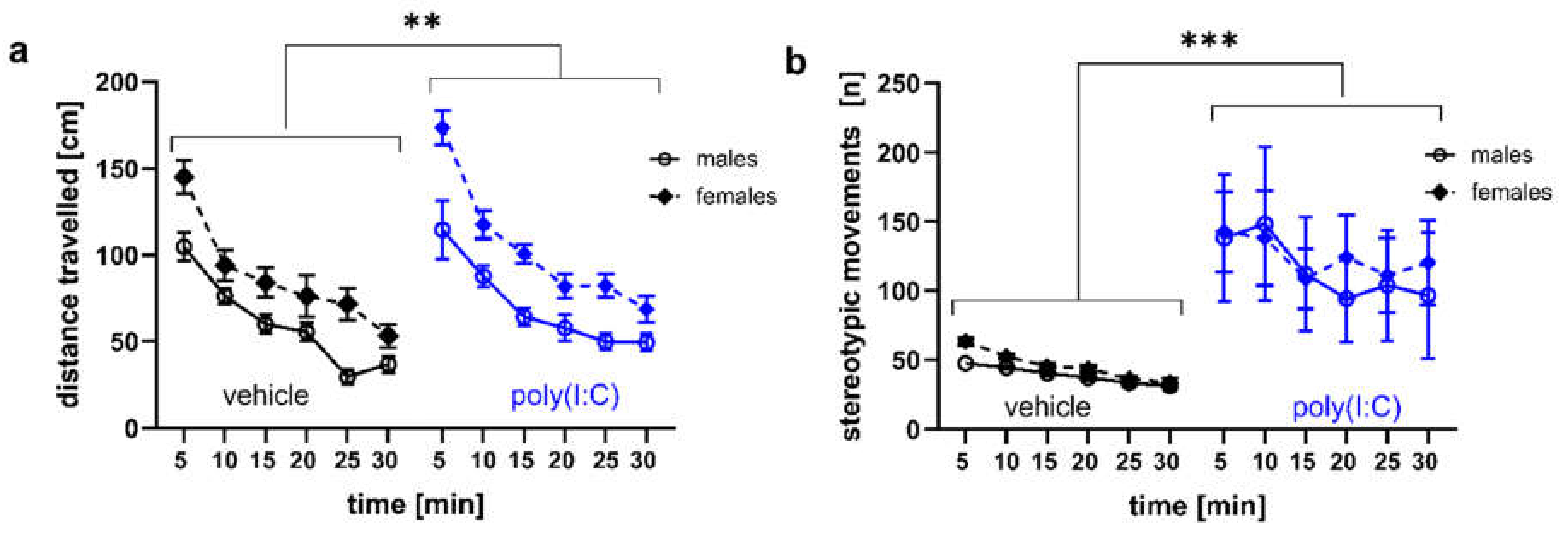
3.3. Locomotor Activity and Stereotypic-Like Movements
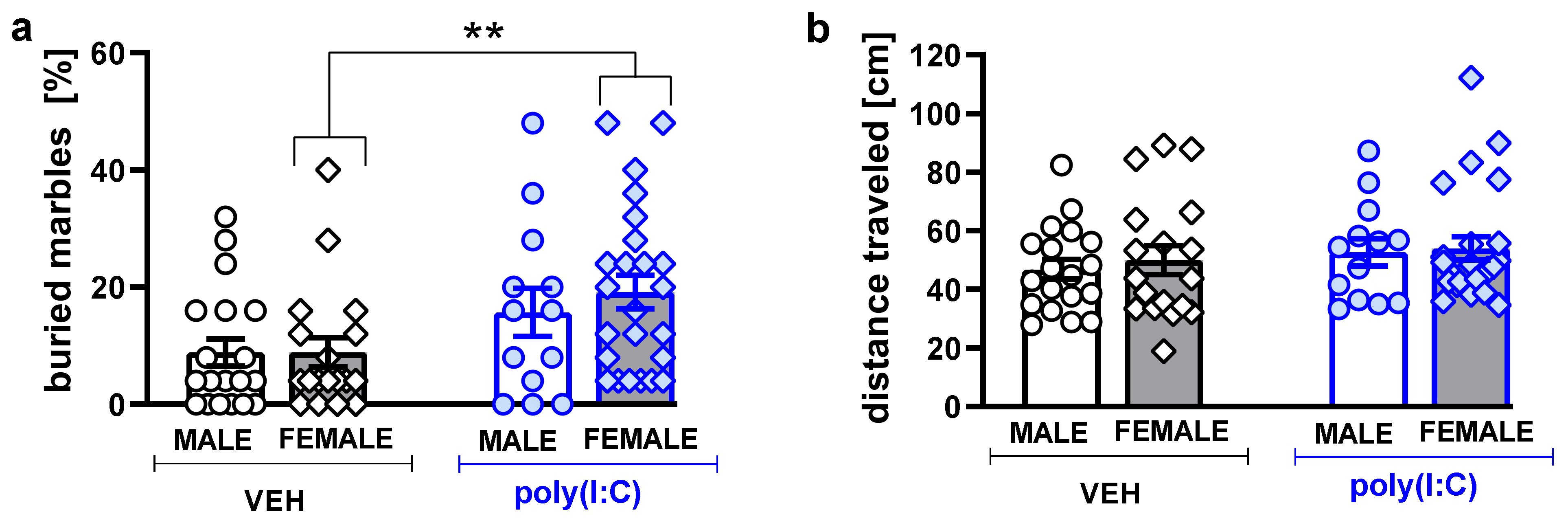
3.4. Marble-Burying
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Scola, G.; Duong, A. Prenatal maternal immune activation and brain development with relevance to psychiatric disorders. Neuroscience 2017, 346, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci. 2015, 16, 469–486. [Google Scholar] [CrossRef]
- Ji-Xu, A.; Vincent, A. Maternal Immunity in Autism Spectrum Disorders: Questions of Causality, Validity, and Specificity. J. Clin. Med. 2020, 9, 2590. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U. Prenatal Poly(I:C) Exposure and Other Developmental Immune Activation Models in Rodent Systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef]
- Haddad, F.L.; Patel, S.V.; Schmid, S. Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia. Neurosci. Biobehav. Rev. 2020, 113, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Primers 2020, 6, 5. [Google Scholar] [CrossRef]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Christensen, D.L.; Maenner, M.J.; Bilder, D.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; Pettygrove, S.D.; Robinson, C.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 4 Years—Early Autism and Developmental Disabilities Monitoring Network, Seven Sites, United States, 2010, 2012, and 2014. MMWR Surveill. Summ. 2019, 68, 1–19. [Google Scholar] [CrossRef]
- LeClerc, S.; Easley, D. Pharmacological therapies for autism spectrum disorder: A review. Pharm. Ther. 2015, 40, 389–397. [Google Scholar] [PubMed]
- Wohr, M.; Scattoni, M.L. Behavioural methods used in rodent models of autism spectrum disorders: Current standards and new developments. Behav. Brain Res. 2013, 251, 5–17. [Google Scholar] [CrossRef]
- Schweinfurth, M.K. The social life of Norway rats (Rattus norvegicus). eLife 2020, 9, e54020. [Google Scholar] [CrossRef]
- Panksepp, J. The ontogeny of play in rats. Dev. Psychobiol. 1981, 14, 327–332. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.M.J.; Trezza, V. What the Laboratory Rat has Taught us about Social Play Behavior: Role in Behavioral Development and Neural Mechanisms. In The Neurobiology of Childhood; Andersen, S.L., Pine, D.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 189–212. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.M.J.; Achterberg, E.J.M.; Trezza, V. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 2016, 70, 86–105. [Google Scholar] [CrossRef]
- Pellis, S.M.; Burke, C.J.; Kisko, T.M.; Euston, D.R. Chapter 11—50-kHz Vocalizations, Play and the Development of Social Competence. In Handbook of Behavioral Neuroscience; Brudzynski, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 117–126. [Google Scholar]
- Simola, N.; Brudzynski, S.M. Chapter 17—Repertoire and Biological Function of Ultrasonic Vocalizations in Adolescent and Adult Rats. In Handbook of Behavioral Neuroscience; Brudzynski, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 177–186. [Google Scholar]
- Wright, J.M.; Gourdon, J.C.; Clarke, P.B.S. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: Effects of amphetamine and social context. Psychopharmacology 2010, 211, 1–13. [Google Scholar] [CrossRef]
- Jouda, J.; Wohr, M.; Del Rey, A. Immunity and ultrasonic vocalization in rodents. Ann. N. Y. Acad. Sci. 2019, 1437, 68–82. [Google Scholar] [CrossRef]
- Raza, S.; Himmler, B.T.; Himmler, S.M.; Harker, A.; Kolb, B.; Pellis, S.M.; Gibb, R. Effects of prenatal exposure to valproic acid on the development of juvenile-typical social play in rats. Behav. Pharmacol. 2015, 26, 707–719. [Google Scholar] [CrossRef]
- Gzielo, K.; Potasiewicz, A.; Hołuj, M.; Litwa, E.; Popik, P.; Nikiforuk, A. Valproic acid exposure impairs ultrasonic communication in infant, adolescent and adult rats. Eur. Neuropsychopharmacol. 2020, 41, 52–62. [Google Scholar] [CrossRef]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad Child. Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Lai, M.-C.; Baron-Cohen, S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015, 2, 1013–1027. [Google Scholar] [CrossRef]
- Hull, L.; Lai, M.-C.; Baron-Cohen, S.; Allison, C.; Smith, P.; Petrides, K.V.; Mandy, W. Gender differences in self-reported camouflaging in autistic and non-autistic adults. Autism 2019, 24, 352–363. [Google Scholar] [CrossRef]
- Lins, B.R.; Marks, W.N.; Zabder, N.K.; Greba, Q.; Howland, J.G. Maternal Immune Activation during Pregnancy Alters the Behavior Profile of Female Offspring of Sprague Dawley Rats. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Schwarting, R.K.; Fuchs, E.; Wohr, M. Increased affective ultrasonic communication during fear learning in adult male rats exposed to maternal immune activation. J. Psychiatr. Res. 2012, 46, 1199–1205. [Google Scholar] [CrossRef]
- Potasiewicz, A.; Gzielo, K.; Popik, P.; Nikiforuk, A. Effects of prenatal exposure to valproic acid or poly(I:C) on ultrasonic vocalizations in rat pups: The role of social cues. Physiol. Behav. 2020, 225, 113113. [Google Scholar] [CrossRef]
- Potasiewicz, A.; Holuj, M.; Piotrowska, D.; Zajda, K.; Wojcik, M.; Popik, P.; Nikiforuk, A. Evaluation of ultrasonic vocalizations in a neurodevelopmental model of schizophrenia during the early life stages of rats. Neuropharmacology 2019, 146, 28–38. [Google Scholar] [CrossRef]
- Golebiowska, J.; Hołuj, M.; Potasiewicz, A.; Piotrowska, D.; Kuziak, A.; Popik, P.; Homberg, J.R.; Nikiforuk, A. Serotonin transporter deficiency alters socioemotional ultrasonic communication in rats. Sci. Rep. 2019, 9, 20283. [Google Scholar] [CrossRef]
- Vuillermot, S.; Luan, W.; Meyer, U.; Eyles, D. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol. Autism 2017, 8, 9. [Google Scholar] [CrossRef]
- Aavani, T.; Rana, S.A.; Hawkes, R.; Pittman, Q.J. Maternal immune activation produces cerebellar hyperplasia and alterations in motor and social behaviors in male and female mice. Cerebellum 2015, 14, 491–505. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef]
- Lins, B.R.; Hurtubise, J.L.; Roebuck, A.J.; Marks, W.N.; Zabder, N.K.; Scott, G.A.; Greba, Q.; Dawicki, W.; Zhang, X.; Rudulier, C.D.; et al. Prospective Analysis of the Effects of Maternal Immune Activation on Rat Cytokines during Pregnancy and Behavior of the Male Offspring Relevant to Schizophrenia. eNeuro 2018. [Google Scholar] [CrossRef]
- Vigli, D.; Palombelli, G.; Fanelli, S.; Calamandrei, G.; Canese, R.; Mosca, L.; Scattoni, M.L.; Ricceri, L. Maternal Immune Activation in Mice Only Partially Recapitulates the Autism Spectrum Disorders Symptomatology. Neuroscience 2020. [Google Scholar] [CrossRef]
- Gray, A.; Tattoli, R.; Dunn, A.; Hodgson, D.M.; Michie, P.T.; Harms, L. Maternal immune activation in mid-late gestation alters amphetamine sensitivity and object recognition, but not other schizophrenia-related behaviours in adult rats. Behav. Brain Res. 2019, 356, 358–364. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS ONE 2017, 12, e0171643. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Taricano, M.; Maiorka, P.C.; Palermo-Neto, J.; Bernardi, M.M. Prenatal lipopolysaccharide reduces social behavior in male offspring. Neuroimmunomodulation 2010, 17, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.V.; Veenema, A.H.; Paul, M.J.; Bredewold, R.; Isaacs, S.; de Vries, G.J. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol. Sex Differ. 2012, 3, 15. [Google Scholar] [CrossRef]
- Carlezon, W.A.; Kim, W.; Missig, G.; Finger, B.C.; Landino, S.M.; Alexander, A.J.; Mokler, E.L.; Robbins, J.O.; Li, Y.; Bolshakov, V.Y.; et al. Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Sci. Rep. 2019, 9, 16928. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Felice, D.; Golubeva, A.V.; Moloney, G.; Dinan, T.G.; Cryan, J.F. Strain differences in the susceptibility to the gut-brain axis and neurobehavioural alterations induced by maternal immune activation in mice. Behav. Pharmacol. 2018, 29, 181–198. [Google Scholar] [CrossRef]
- Cieslik, M.; Gassowska-Dobrowolska, M.; Jesko, H.; Czapski, G.A.; Wilkaniec, A.; Zawadzka, A.; Dominiak, A.; Polowy, R.; Filipkowski, R.K.; Boguszewski, P.M.; et al. Maternal Immune Activation Induces Neuroinflammation and Cortical Synaptic Deficits in the Adolescent Rat Offspring. Int. J. Mol. Sci. 2020, 21, 4097. [Google Scholar] [CrossRef]
- Knutson, B.; Burgdorf, J.; Panksepp, J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J. Comp. Psychol. 1998, 112, 65–73. [Google Scholar] [CrossRef]
- Himmler, B.T.; Kisko, T.M.; Euston, D.R.; Kolb, B.; Pellis, S.M. Are 50-kHz calls used as play signals in the playful interactions of rats? I. Evidence from the timing and context of their use. Behav. Process. 2014, 106, 60–66. [Google Scholar] [CrossRef]
- Burke, C.J.; Kisko, T.M.; Swiftwolfe, H.; Pellis, S.M.; Euston, D.R. Specific 50-kHz vocalizations are tightly linked to particular types of behavior in juvenile rats anticipating play. PLoS ONE 2017, 12, e0175841. [Google Scholar] [CrossRef]
- Mulvihill, K.G.; Brudzynski, S.M. Non-pharmacological induction of rat 50 kHz ultrasonic vocalization: Social and non-social contexts differentially induce 50 kHz call subtypes. Physiol. Behav. 2018, 196, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.H.; Stevenson, R.A.; Wallace, M.T. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog. Neurobiol. 2015, 134, 140–160. [Google Scholar] [CrossRef] [PubMed]
- Tschida, K.; Michael, V.; Takatoh, J.; Han, B.-X.; Zhao, S.; Sakurai, K.; Mooney, R.; Wang, F. A Specialized Neural Circuit Gates Social Vocalizations in the Mouse. Neuron 2019, 103, 459–472.e4. [Google Scholar] [CrossRef] [PubMed]
- Kelm-Nelson, C.A.; Gammie, S. Gene expression within the periaqueductal gray is linked to vocal behavior and early-onset parkinsonism in Pink1 knockout rats. BMC Genom. 2020, 21, 625. [Google Scholar] [CrossRef] [PubMed]
- Melancia, F.; Schiavi, S.; Servadio, M.; Cartocci, V.; Campolongo, P.; Palmery, M.; Pallottini, V.; Trezza, V. Sex-specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling. Br. J. Pharmacol. 2018, 175, 3699–3712. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gzielo, K.; Potasiewicz, A.; Litwa, E.; Piotrowska, D.; Popik, P.; Nikiforuk, A. The Effect of Maternal Immune Activation on Social Play-Induced Ultrasonic Vocalization in Rats. Brain Sci. 2021, 11, 344. https://doi.org/10.3390/brainsci11030344
Gzielo K, Potasiewicz A, Litwa E, Piotrowska D, Popik P, Nikiforuk A. The Effect of Maternal Immune Activation on Social Play-Induced Ultrasonic Vocalization in Rats. Brain Sciences. 2021; 11(3):344. https://doi.org/10.3390/brainsci11030344
Chicago/Turabian StyleGzielo, Kinga, Agnieszka Potasiewicz, Ewa Litwa, Diana Piotrowska, Piotr Popik, and Agnieszka Nikiforuk. 2021. "The Effect of Maternal Immune Activation on Social Play-Induced Ultrasonic Vocalization in Rats" Brain Sciences 11, no. 3: 344. https://doi.org/10.3390/brainsci11030344
APA StyleGzielo, K., Potasiewicz, A., Litwa, E., Piotrowska, D., Popik, P., & Nikiforuk, A. (2021). The Effect of Maternal Immune Activation on Social Play-Induced Ultrasonic Vocalization in Rats. Brain Sciences, 11(3), 344. https://doi.org/10.3390/brainsci11030344





