Gender Differences in Complete Blood Count and Inflammatory Ratios among Patients with Bipolar Disorder
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. Assessments and Procedures
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Sample
3.2. Differences in Complete Blood Count and Inflammatory Ratios According to the Phase of Bipolar Illness in Males and Females, Separately
3.3. Gender Differences in Cell Blood Count and Inflammatory Ratios According to the Bipolar Illness Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Carvalho, A.F.; Firth, J.; Vieta, E. Bipolar disorder. N. Engl. J. Med. 2020, 383, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Virani, S.; Saeed, H.; Nimmagadda, S.; Talukdar, J.; Youssef, N.A. Gender differences and comorbidities in US adults with bipolar disorder. Brain Sci. 2018, 8, 168. [Google Scholar] [CrossRef] [Green Version]
- Maina, G.; Rosso, G.; Aguglia, A.; Chiodelli, D.; Bogetto, F. Anxiety and bipolar disorders: Epidemiological and clinical aspects. J. Psychopathol. 2011, 17, 365–375. [Google Scholar]
- Acciavatti, T.; Lupi, M.; Santacroce, R.; Aguglia, A.; Attademo, L.; Bandini, L.; Ciambrone, P.; Lisi, G.; Migliarese, G.; Pinna, F.; et al. Novel psychoactive substance consumption is more represented in bipolar disorder than in psychotic disorders: A multicenter-observational study. Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2578. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Shariq, A.; Said, K.; Sharma, A.; Newport, D.J.; Salloum, I.M. Medical comorbidities in bipolar disorder. Curr. Psychiatry Rep. 2018, 20, 36. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Amerio, A.; Cimpoesu, P.; Natale, A.; Salvi, V.; Zappa, G.; Serafini, G.; Amore, M.; Aguglia, E.; Aguglia, A. Lipid and glycemic profiles in patients with bipolar disorder: Cholesterol levels are reduced in mania. Medicina 2021, 57, 28. [Google Scholar] [CrossRef] [PubMed]
- Maina, G.; D’Ambrosio, V.; Aguglia, A.; Paschetta, E.; Salvi, V.; Bogetto, F. Bipolar disorders and metabolic syndrome: A clinical study in 185 patients. Riv. Psichiatr. 2010, 45, 34–40. [Google Scholar] [PubMed]
- Salvi, V.; Aguglia, A.; Barone-Adesi, F.; Bianchi, D.; Donfrancesco, C.; Dragogna, F.; Palmieri, L.; Serafini, G.; Amore, M.; Mencacci, C. Cardiovascular risk in patients with severe mental illness in Italy. Eur. Psychiatry 2020, 63, e96. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Surace, T.; Vanella, A.; Meo, V.; Patania, F.; Furnari, R.; Signorelli, M.S.; Aguglia, E. The effect of adjunctive nutraceuticals in bipolar disorder: A systematic review of randomized placebo-controlled trials. J. Affect. Disord. 2019, 252, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Gonda, X.; Serafini, G.; Innamorati, M.; Sher, L.; Amore, M.; Rihmer, Z.; Girardi, P. Epidemiology of suicide in bipolar disorders: A systematic review of the literature. Bipolar Disord. 2013, 15, 457–490. [Google Scholar] [CrossRef]
- Plans, L.; Barrot, C.; Nieto, E.; Rios, J.; Schulze, T.; Papiol, S.; Mitjans, M.; Vieta, E.; Benabarre, A. Association between completed suicide and bipolar disorder: A systematic review of the literature. J. Affect. Disord. 2019, 242, 111–122. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Jin, R.; He, J.-P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey initiative. Arch. Gen. Psychiatry 2011, 68, 241–251. [Google Scholar] [CrossRef]
- Moreira, A.L.R.; Van Meter, A.; Genzlinger, J.; Youngstrom, E.A. Review and meta-analysis of epidemiologic studies of adult bipolar disorder. J. Clin. Psychiatry 2017, 78, 1259–1269. [Google Scholar] [CrossRef]
- Diflorio, A.; Jones, I. Is sex important? Gender differences in bipolar disorder. Int. Rev. Psychiatry 2010, 22, 437–452. [Google Scholar] [CrossRef]
- Baldassano, C.F.; Marangell, L.B.; Gyulai, L.; Ghaemi, S.N.; Joffe, H.; Kim, D.R.; Sagduyu, K.; Truman, C.J.; Wisniewski, S.R.; Sachs, G.S.; et al. Gender differences in bipolar disorder: Retrospective data from the first 500 STEP-BD participants. Bipolar Disord. 2005, 7, 465–470. [Google Scholar] [CrossRef]
- Schneck, C.D.; Miklowitz, D.J.; Miyahara, S.; Araga, M.; Wisniewski, S.; Gyulai, L.; Allen, M.H.; Thase, M.E.; Sachs, G.S. The prospective course of rapid-cycling bipolar disorder: Findings from the STEP-BD. Am. J. Psychiatry 2008, 165, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Cassano, G.B.; Akiskal, H.; Savino, M.; Musetti, L.; Perugi, G. Proposed subtypes of bipolar II and related disorders: With hypomanic episodes (or cyclothymia) and with hyperthymic temperament. J. Affect. Disord. 1992, 26, 127–140. [Google Scholar] [CrossRef]
- Tondo, L.; Baldessarini, R.J. Rapid cycling in women and men with bipolar manic-depressive disorders. Am. J. Psychiatry 1998, 155, 1434–1436. [Google Scholar] [CrossRef]
- Kupka, R.W.; Luckenbaugh, D.A.; Post, R.M.; Leverich, G.S.; Nolen, W.A. Rapid and non-rapid cycling bipolar disorder: A meta-analysis of clinical studies. J. Clin. Psychiatry 2003, 64, 1483–1494. [Google Scholar] [CrossRef]
- Coryell, W.; Endicott, J.; Keller, M. Rapidly cycling affective disorder: Demographics, diagnosis, family history, and course. Arch. Gen. Psychiatry 1992, 49, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, N.; Bauer, M.; Grof, P.; Gyulai, L.; Elman, S.; Glenn, T.; Whybrow, P.C. Sex-specific self-reported mood changes by patients with bipolar disorder. J. Psychiatr. Res. 2005, 39, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Benazzi, F. Frequency of bipolar spectrum in 111 private practice depression outpatients. Eur. Arch. Psychiatry Clin. Neurosci. 2003, 253, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kessing, L.V. Gender differences in the phenomenology of bipolar disorder. Bipolar Disord. 2004, 6, 421–425. [Google Scholar] [CrossRef]
- Grant, B.F.; Stinson, F.S.; Hasin, D.S.; Dawson, D.A.; Chou, S.P.; Ruan, W.; Huang, B. Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: Results from the national epidemiologic survey on alcohol and related conditions. J. Clin. Psychiatry 2005, 66, 1205–1215. [Google Scholar] [CrossRef]
- Kessing, L.V. The prevalence of mixed episodes during the course of illness in bipolar disorder. Acta Psychiatr. Scand. 2008, 117, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Suppes, T.; Mintz, J.; McElroy, S.L.; Altshuler, L.L.; Kupka, R.W.; Frye, M.A.; Keck, P.E.; Nolen, W.A.; Leverich, G.S.; Grunze, H.; et al. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley foundation bipolar treatment network: A sex-specific phenomenon. Arch. Gen. Psychiatry 2005, 62, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Altshuler, L.L.; Kupka, R.W.; Hellemann, G.; Frye, M.A.; Sugar, C.A.; McElroy, S.L.; Nolen, W.A.; Grunze, H.; Leverich, G.S.; Keck, P.E.; et al. Gender and depressive symptoms in 711 patients with bipolar disorder evaluated prospectively in the Stanley foundation bipolar treatment outcome network. Am. J. Psychiatry 2010, 167, 708–715. [Google Scholar] [CrossRef]
- Morgan, V.A.; Mitchell, P.B.; Jablensky, A.V. The epidemiology of bipolar disorder: Sociodemographic, disability and service utilization data from the Australian national study of low prevalence (psychotic) disorders. Bipolar Disord. 2005, 7, 326–337. [Google Scholar] [CrossRef]
- Suominen, K.; Mantere, O.; Valtonen, H.; Arvilommi, P.; Leppämäki, S.; Isometsä, E. Gender differences in bipolar disorder type I and II. Acta Psychiatr. Scand. 2009, 120, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Gałecka, M.; Bliźniewska-Kowalska, K.; Maes, M.; Su, K.P.; Gałecki, P. Update on the neurodevelopmental theory of depression: Is there any ‘unconscious code’? Pharm. Rep. 2020, 1–11. [Google Scholar] [CrossRef]
- Gałecka, M.; Bliźniewska-Kowalska, K.; Orzechowska, A.; Szemraj, J.; Maes, M.; Berk, M.; Su, K.P.; Gałecki, P. Inflammatory versus anti-inflammatory profiles in major depressive disorders-The role of IL-17, IL-21, IL-23, IL-35 and Foxp3. J. Personal. Med. 2021, 11, 66. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Bipolar disorder and immune dysfunction: Epidemiological findings, proposed pathophysiology and clinical implications. Brain Sci. 2017, 7, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetti, F.; Aggio, V.; Pratesi, M.L.; Greco, G.; Furlan, R. Neuroinflammation in bipolar depression. Front. Psychiatry 2020, 11, 71. [Google Scholar] [CrossRef] [Green Version]
- Rainville, J.R.; Hodes, G.E. Inflaming sex differences in mood disorders. Neuropsychopharmacology 2019, 44, 184–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misiak, B.; Bartoli, F.; Carrà, G.; Małecka, M.; Samochowiec, J.; Jarosz, K.; Banik, A.; Stańczykiewicz, B. Chemokine alterations in bipolar disorder: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 88, 870–877. [Google Scholar] [CrossRef]
- Zulfic, Z.; Weickert, C.S.; Weickert, T.W.; Liu, D.; Myles, N.; Galletly, C. Neutrophil–lymphocyte ratio–a simple, accessible measure of inflammation, morbidity and prognosis in psychiatric disorders? Australas. Psychiatry 2020, 28, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Lucchi, S.; Tringali, A.G.M.; Rossetti, A.; Botti, E.R.; Clerici, M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Tringali, A.G.M.; Rossetti, A.; Botti, R.E.; Clerici, M. Cross-sectional study of neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratios in mood disorders. Gen. Hosp. Psychiatry 2019, 58, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Natale, A.; Amerio, A.; Cimpoesu, P.; Grimaldi Filioli, P.; Aguglia, E.; Amore, M.; Serafini, G.; Aguglia, A. Neutrophil-to-lymphocyte, platelet-to-lymphocyte and monocyte-to-lymphocyte ratio in bipolar disorder. Brain Sci. 2021, 11, 58. [Google Scholar] [CrossRef]
- Özdin, S.; Usta, M.B. A comparison of inflammatory markers in manic and euthymic states of bipolar disorder. Nord. J. Psychiatry 2021, 75, 124–129. [Google Scholar] [CrossRef]
- Rubinow, D.R.; Schmidt, P.J. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 2019, 44, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, F.; Stallings, C.; Origoni, A.; Boronow, J.; Yolken, R. Elevated serum levels of c-reactive protein are associated with mania symptoms in outpatients with bipolar disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.I.; Collinger, K.A.; Lotrich, F.; Marsland, A.L.; Gill, M.-K.; Axelson, D.A.; Birmaher, B. Preliminary findings regarding proinflammatory markers and brain-derived neurotrophic factor among adolescents with bipolar spectrum disorders. J. Child Adolesc. Psychopharmacol. 2011, 21, 479–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; He, H.; Zhang, M.; Huang, X.; Fan, N. Altered serum levels of TNF-α, IL-6 and IL-18 in manic, depressive, mixed state of bipolar disorder patients. Psychiatry Res. 2016, 244, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, K.; Vinberg, M.; Kessing, L.V. Cytokines in bipolar disorder: A systematic review and meta-analysis. J. Affect. Disord. 2013, 144, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Köhler-Forsberg, O.; Sylvia, L.; Deckersbach, T.; Ostacher, M.J.; McInnis, M.; Iosifescu, D.; Bowden, C.; McElroy, S.; Calabrese, J.; Thase, M.; et al. Clinically relevant and simple immune system measure is related to symptom burden in bipolar disorder. Acta Neuropsychiatr. 2018, 30, 297–305. [Google Scholar] [CrossRef]
- Queissner, R.; Pilz, R.; Dalkner, N.; Birner, A.; Bengesser, S.A.; Platzer, M.; Fellendorf, F.T.; Kainzbauer, N.; Herzog-Eberhard, S.; Hamm, C. The relationship between inflammatory state and quantity of affective episodes in bipolar disorder. Psychoneuroendocrinology 2018, 90, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Åsberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Oliveira, J.; Silva, S.; Madeira, N.; Pereira, C.M.; Cruz, M.T. Inflammation in bipolar disorder (BD): Identification of new therapeutic targets. Pharmacol. Res. 2021, 163, 105325. [Google Scholar] [CrossRef]
- Fries, G.R.; Walss-Bass, C.; Bauer, M.E.; Teixeira, A.L. Revisiting inflammation in bipolar disorder. Pharmacol. Biochem. Behav. 2019, 177, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Özdin, S.; Sarisoy, G.; Böke, Ö. A comparison of the neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratios in schizophrenia and bipolar disorder patients–a retrospective file review. Nord. J. Psychiatry 2017, 71, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Inanli, I.; Aydin, M.; Çaliskan, A.M.; Eren, I. Neutrophil/lymphocyte ratio, monocyte/lymphocyte ratio, and mean platelet volume as systemic inflammatory markers in different states of bipolar disorder. Nord. J. Psychiatry 2019, 73, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.-W.; Chan, J.; Cembrowski, G.S.; Van Assendelft, O. Complete blood count reference interval diagrams derived from NHANES III: Stratification by age, sex, and race. Lab. Hematol. 2004, 10, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Adeli, K.; Raizman, J.E.; Chen, Y.; Higgins, V.; Nieuwesteeg, M.; Abdelhaleem, M.; Wong, S.L.; Blais, D. Complex biological profile of hematologic markers across pediatric, adult, and geriatric ages: Establishment of robust pediatric and adult reference intervals on the basis of the Canadian health measures survey. Clin. Chem. 2015, 61, 1075–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gender (female), n (%) | 197 (53.1) |
| Age (years), mean ± SD | 51.91 ± 13.88 |
| Years of education, mean ± SD | 11.97 ± 2.18 |
| Marital status, n (%) | |
| Single | 175 (47.2) |
| Married | 101 (27.2) |
| Divorced | 65 (17.5) |
| Widowed | 30 (8.1) |
| Employed, n (%) | 130 (35.0) |
| Illness phase, n (%) | |
| (Hypo)manic episode | 143 (38.5) |
| Major depressive episode | 151 (40.7) |
| Euthymic phase | 77 (20.8) |
| Pharmacological treatment (n = 313) | |
| Antidepressants, n (%) | 137 (43.8) |
| Mood stabilizers, n (%) | |
| Valproate | 134 (42.8) |
| Lithium | 132 (42.2) |
| Others | 77 (24.6) |
| Antipsychotics, n (%) | 268 (85.6) |
| Typical | 53 (16.9) |
| Atypical | 246 (78.6) |
| Long-acting injection | 13 (4.2) |
| Benzodiazepines, n (%) | 231 (73.8) |
| Number of medications, mean ± SD | 3.90 ± 1.19 |
| Mean ± SD | Male Gender | F | p | ||
|---|---|---|---|---|---|
| (Hypo)manic Episode (n = 73) | Major Depressive Episode (n = 64) | Euthymic Phase (n = 37) | |||
| Neutrophils | 5.12 ± 1.84 | 4.44 ± 1.70 | 4.77 ± 1.44 | 2.693 | 0.071 |
| Lymphocytes | 2.22 ± 0.85 | 2.39 ± 0.71 | 2.33 ± 0.67 | 0.867 | 0.422 |
| Monocytes | 0.65 ± 0.44 | 0.57 ± 0.21 | 0.68 ± 0.26 | 1.299 | 0.276 |
| Eosinophils | 0.20 ± 0.15 | 0.23 ± 0.13 | 0.23 ± 0.16 | 0.980 | 0.378 |
| Basophils | 0.04 ± 0.02 | 0.04 ± 0.03 | 0.05 ± 0.04 | 1.146 | 0.321 |
| Platelets | 259.25 ± 65.94 | 239.45 ± 67.24 | 258.19 ± 57.57 | 1.826 | 0.164 |
| NLR | 2.68 ± 1.59 | 1.99 ± 1.04 | 2.16 ± 0.74 | 5.506 | 0.005 * |
| PLR | 132.11 ± 62.24 | 105.07 ± 31.54 | 119.16 ± 42.95 | 5.224 | 0.006 * |
| MLR | 0.31 ± 0.24 | 0.25 ± 0.11 | 0.23 ± 0.19 | 2.829 | 0.062 |
| Red blood cell | 4.91 ± 0.65 | 4.77 ± 0.55 | 4.90 ± 0.43 | 1.136 | 0.323 |
| Hemoglobin | 142.99 ± 17.46 | 142.94 ± 15.32 | 145.27 ± 13.34 | 0.307 | 0.736 |
| Hematocrit | 42.66 ± 4.54 | 42.91 ± 4.40 | 43.33 ± 4.19 | 0.277 | 0.758 |
| MCV | 87.72 ± 7.81 | 90.31 ± 5.51 | 88.55 ± 6.59 | 2.522 | 0.083 |
| MCH | 29.33 ± 3.01 | 30.08 ± 2.06 | 29.71 ± 2.35 | 1.457 | 0.236 |
| MCHC | 334.07 ± 11.32 | 333.02 ± 10.09 | 335.57 ± 8.47 | 0.719 | 0.489 |
| RDW CV | 13.83 ± 1.30 | 13.62 ± 1.02 | 13.42 ± 1.43 | 1.410 | 0.247 |
| Mean ± SD | Female Gender | F | p | ||
|---|---|---|---|---|---|
| (Hypo)manic Episode (n = 70) | Major Depressive Episode (n = 87) | Euthymic Phase (n = 40) | |||
| Neutrophils | 4.62 ± 1.92 | 4.33 ± 1.45 | 4.04 ± 1.34 | 1.671 | 0.191 |
| Lymphocytes | 2.19 ± 0.70 | 2.35 ± 0.69 | 2.34 ± 0.92 | 0.983 | 0.376 |
| Monocytes | 0.52 ± 0.15 | 0.49 ± 0.20 | 0.53 ± 0.19 | 0.626 | 0.536 |
| Eosinophils | 0.22 ± 0.14 | 0.19 ± 0.13 | 0.15 ± 0.07 | 3.455 | 0.034 * |
| Basophils | 0.03 ± 0.02 | 0.04 ± 0.03 | 0.03 ± 0.03 | 3.236 | 0.042 * |
| Platelets | 263.33 ± 72.08 | 231.25 ± 52.20 | 229.55 ± 57.44 | 6.463 | 0.002 * |
| NLR | 2.22 ± 0.96 | 2.01 ± 1.20 | 1.87 ± 0.73 | 1.626 | 0.199 |
| PLR | 128.07 ± 41.16 | 106.77 ± 40.38 | 105.07 ± 29.71 | 7.181 | 0.001 * |
| MLR | 0.24 ± 0.09 | 0.21 ± 0.12 | 0.20 ± 0.11 | 2.706 | 0.069 |
| Red blood cell | 4.44 ± 0.43 | 4.44 ± 0.49 | 4.53 ± 0.52 | 0.495 | 0.610 |
| Hemoglobin | 128.57 ± 14.22 | 128.47 ± 13.00 | 133.25 ± 13.59 | 1.943 | 0.146 |
| Hematocrit | 39.46 ± 3.95 | 39.11 ± 3.54 | 39.98 ± 3.71 | 0.756 | 0.471 |
| MCV | 88.98 ± 6.03 | 88.69 ± 8.83 | 88.92 ± 7.10 | 0.033 | 0.968 |
| MCH | 29.00 ± 2.41 | 29.12 ± 3.28 | 29.61 ± 2.64 | 0.601 | 0.549 |
| MCHC | 325.73 ± 11.56 | 328.10 ± 10.33 | 333.00 ± 10.24 | 5.826 | 0.003 * |
| RDW CV | 13.86 ± 1.34 | 14.32 ± 1.87 | 13.67 ± 0.95 | 3.054 | 0.049 * |
| (Hypo)manic Episode | Major Depressive Episode | Euthymic Phase | ||||
|---|---|---|---|---|---|---|
| t° | p | t° | p | t° | p | |
| Neutrophils | 1.589 | 0.114 | 0.418 | 0.676 | 2.304 | 0.024 * |
| Lymphocytes | 0.235 | 0.814 | 0.366 | 0.715 | –0.040 | 0.968 |
| Monocytes | 2.235 | 0.027 * | 2.236 | 0.027 * | 2.498 | 0.015 * |
| Eosinophils | –0.504 | 0.615 | 2.081 | 0.039 * | 2.834 | 0.006 * |
| Basophils | 1.380 | 0.170 | –0.794 | 0.429 | 1.775 | 0.081 |
| Platelets | –0.353 | 0.724 | 0.844 | 0.400 | 2.183 | 0.032 * |
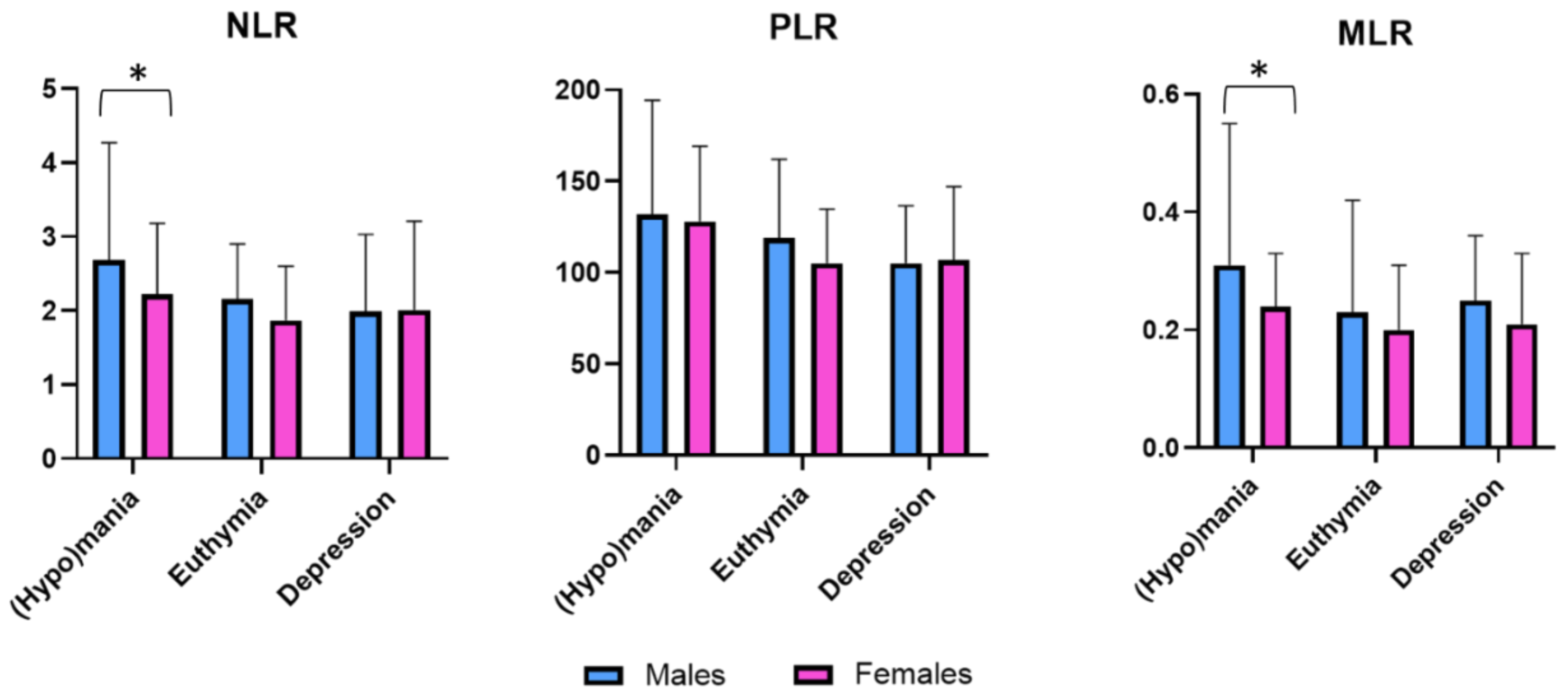
| NLR | 2.061 | 0.041 * | –0.132 | 0.895 | 1.728 | 0.088 |
| PLR | 0.455 | 0.649 | –0.281 | 0.779 | 1.685 | 0.096 |
| MLR | 2.027 | 0.045 * | 1.701 | 0.091 | 0.842 | 0.402 |
| Red blood cells | 5.065 | <0.001 * | 3.892 | <0.001 * | 3.416 | 0.001 * |
| Hemoglobin | 5.401 | <0.001 * | 6.263 | <0.001 * | 3.911 | <0.001 * |
| Hematocrit | 4.488 | <0.001 * | 5.884 | <0.001 * | 3.720 | <0.001 * |
| MCV | –1.076 | 0.284 | 1.297 | 0.197 | –0.237 | 0.813 |
| MCH | 0.725 | 0.469 | 2.051 | 0.042 * | 0.172 | 0.864 |
| MCHC | 4.358 | <0.001 * | 2.936 | 0.004 * | 1.194 | 0.236 |
| RDW CV | –0.154 | 0.878 | –2.695 | 0.008 * | –0.917 | 0.362 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusar-Poli, L.; Amerio, A.; Cimpoesu, P.; Grimaldi Filioli, P.; Natale, A.; Zappa, G.; Aguglia, E.; Amore, M.; Serafini, G.; Aguglia, A. Gender Differences in Complete Blood Count and Inflammatory Ratios among Patients with Bipolar Disorder. Brain Sci. 2021, 11, 363. https://doi.org/10.3390/brainsci11030363
Fusar-Poli L, Amerio A, Cimpoesu P, Grimaldi Filioli P, Natale A, Zappa G, Aguglia E, Amore M, Serafini G, Aguglia A. Gender Differences in Complete Blood Count and Inflammatory Ratios among Patients with Bipolar Disorder. Brain Sciences. 2021; 11(3):363. https://doi.org/10.3390/brainsci11030363
Chicago/Turabian StyleFusar-Poli, Laura, Andrea Amerio, Patriciu Cimpoesu, Pietro Grimaldi Filioli, Antimo Natale, Guendalina Zappa, Eugenio Aguglia, Mario Amore, Gianluca Serafini, and Andrea Aguglia. 2021. "Gender Differences in Complete Blood Count and Inflammatory Ratios among Patients with Bipolar Disorder" Brain Sciences 11, no. 3: 363. https://doi.org/10.3390/brainsci11030363








