Clock/Sleep-Dependent Learning and Memory in Male 3xTg-AD Mice at Advanced Disease Stages and Extrinsic Effects of Huprine X and the Novel Multitarget Agent AVCRI104P3
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug Treatment
2.2. Side Effects and NPS-Like Behaviors
2.3. Behavioral Assessment
2.3.1. Exploratory Activity and Anxiety-Like Behaviors
2.3.2. Morris Water Maze (MWM)
2.4. Statistics
3. Results
3.1. Presence of AD-Phenotype before Treatments
3.2. Absence of Side Effects of AVCRIP104P3 and Huprine X
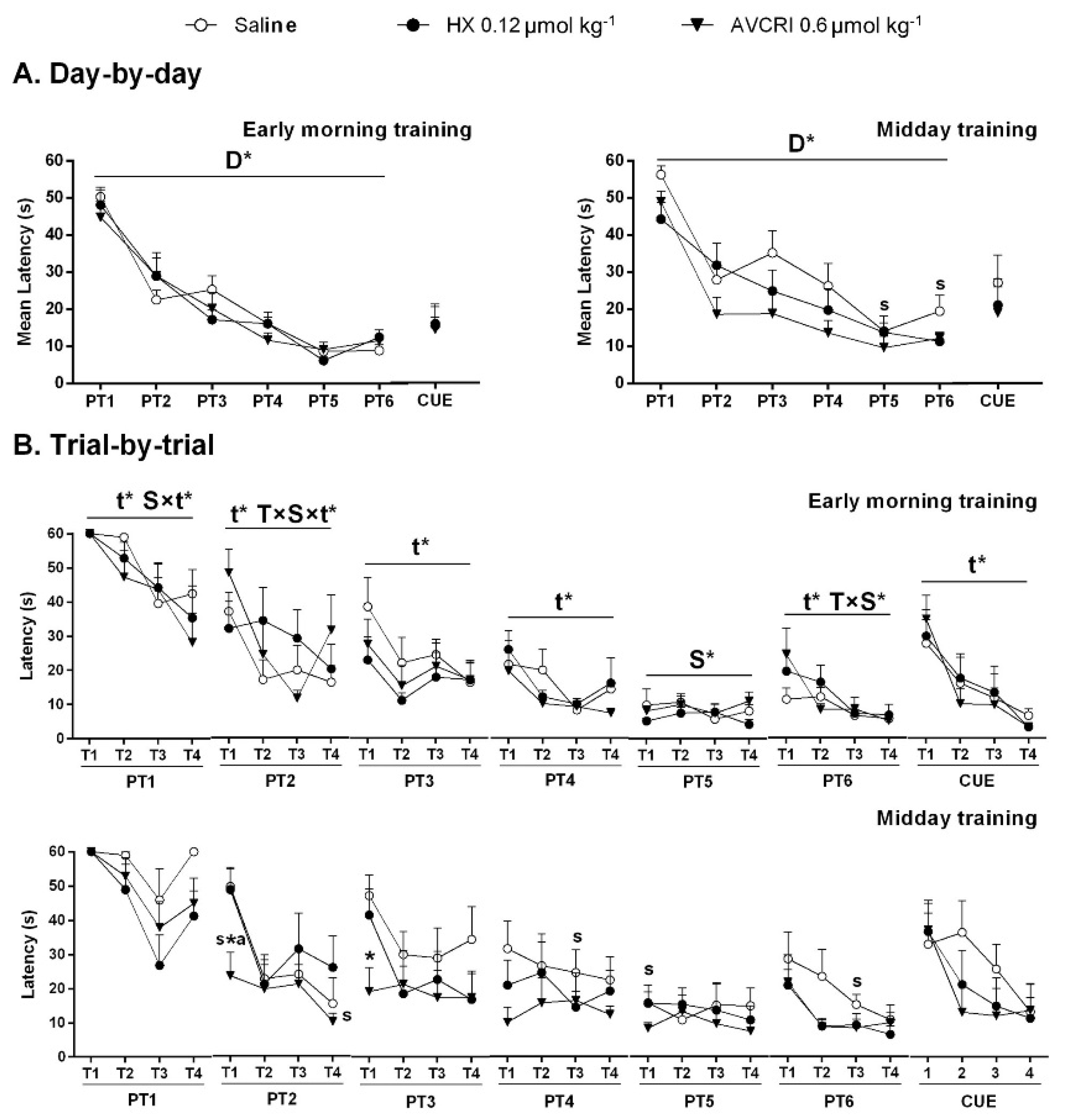
3.3. Training Schedule Affected Learning and Memory
3.4. Training Schedule Affected Memory Retrieval
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, M.P.; Brakefield, T.; Morgan, A.; Hobson, J.A.; Stickgold, R. Practice with Sleep Makes Perfect: Sleep-dependent Motor Skill Learning. Neuron 2002, 35, 205–211. [Google Scholar] [CrossRef]
- Walker, M.P.; Stickgold, R. Sleep, Memory, and Plasticity. Annu. Rev. Psychol. 2006, 57, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Stickgold, R.; James, L.; Hobson, J.A. Visual Discrimination Learning Requires Sleep after Training. Nat. Neurosci. 2000, 3, 1237–1238. [Google Scholar] [CrossRef] [PubMed]
- Gais, S.; Plihal, W.; Wagner, U.; Born, J. Early Sleep Triggers Memory for Early Visual Discrimination Skills. Nat. Neurosci. 2000, 3, 1335–1339. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J. The Memory Function of Sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Liu, A. Sleep Training. Pediatr. Ann. 2020, 49, e101–e105. [Google Scholar] [CrossRef]
- Ju, Y.E.; Lucey, B.P.; Holtzman, D.M. Sleep and Alzheimer Disease Pathology--a Bidirectional Relationship. Nat. Rev. Neurol. 2014, 10, 115–119. [Google Scholar] [CrossRef]
- Macedo, A.C.; Balouch, S.; Tabet, N. Is Sleep Disruption a Risk Factor for Alzheimer’s Disease? J. Alzheimers Dis. 2017, 58, 993–1002. [Google Scholar] [CrossRef]
- Cagnin, A.; Fragiacomo, F.; Camporese, G.; Turco, M.; Bussè, C.; Ermani, M.; Montagnese, S. Sleep-wake Profile in Dementia with Lewy Bodies, Alzheimer’s Disease, and Normal Aging. J. Alzheimers Dis. 2017, 55, 1529–1536. [Google Scholar] [CrossRef]
- Saeed, Y.; Abbott, S.M. Circadian Disruption Associated with Alzheimer’s Disease. Curr. Neurol. Neurosci. Rep. 2017, 17, 29. [Google Scholar] [CrossRef]
- Ju, Y.S.; Ooms, S.J.; Sutphen, C.; Macauley, S.L.; Zangrilli, M.A.; Jerome, G.; Fagan, A.M.; Mignot, E.; Zempel, J.M.; Claassen, J.A.H.R.; et al. Slow Wave Sleep Disruption Increases Cerebrospinal Fluid Amyloid-β Levels. Brain 2017, 140, 2104–2111. [Google Scholar] [CrossRef]
- Musiek, E.S.; Xiong, D.D.; Holtzman, D.M. Sleep, Circadian Rhythms, and the Pathogenesis of Alzheimer Disease. Exp. Mol. Med. 2015, 47, e148. [Google Scholar] [CrossRef] [PubMed]
- Holth, J.K.; Fritschi, S.K.; Wang, C.; Pedersen, N.P.; Cirrito, J.R.; Mahan, T.E.; Finn, M.B.; Manis, M.; Geerling, J.C.; Fuller, P.M.; et al. The Sleep-wake Cycle Regulates Brain Interstitial Fluid Tau in Mice and CSF Tau in Humans. Science 2019, 363, 880–884. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Parker, L.; Sinaee, R.; Fell, R.L.; Kripke, D.F. Sleep Fragmentation in Patients from a Nursing Home. J. Gerontol. 1989, 44, M18–M21. [Google Scholar] [CrossRef] [PubMed]
- OkawaOkawa, M.; Mishima, K.; Hishikawa, Y.; Hozumi, S.; Hori, H.; Takahashi, K. Circadian Rhythm Disorders in Sleep-waking and Body Temperature in Elderly Patients with Dementia and Their Treatment. Sleep 1991, 14, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Satlin, A.; Volicer, L.; Stopa, E.G.; Harper, D. Circadian Locomotor Activity and Core-body Temperature Rhythms in Alzheimer’s Disease. Neurobiol. Aging 1995, 16, 765–771. [Google Scholar] [CrossRef]
- Smith, C.T. Sleep States and Learning: A Review of the Animal Literature. Neurosci. Biobehav. Rev. 1985, 9, 157–168. [Google Scholar] [CrossRef]
- Cummings, J.L. The Neuropsychiatric Inventory: Assessing Psychopathology in Dementia Patients. Neurology 1997, 48 (Suppl. 6), S10–S16. [Google Scholar] [CrossRef]
- Oddo, S.; Cacamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Aβ and Synaptic Dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Blázquez, G.; Cañete, T.; Johansson, B.; Oddo, S.; Tobeña, A.; LaFerla, F.M.; Fernández-Teruel, A. Modeling Behavioural and Neuronal Symptoms of Alzheimer’s Disease in Mice: A Role for Intraneuronal Amyloid. Neurosci. Biobehav. Rev. 2007, 31, 125–147. [Google Scholar] [CrossRef]
- Sterniczuk, R.; Dyck, R.H.; Laferla, F.M.; Antle, M.C. Characterization of the 3xTg-AD Mouse Model of Alzheimer’s Disease: Part 1. Circadian Changes. Brain Res. 2010, 1348, 139–148. [Google Scholar] [CrossRef]
- Knight, E.M.; Brown, T.M.; Gümüsgöz, S.; Smith, J.C.; Waters, E.J.; Allan, S.M.; Lawrence, C.B. Age-related Changes in Core Body Temperature and Activity in Triple-transgenic Alzheimer’s Disease (3xTgAD) Mice. Dis. Model Mech. 2013, 6, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Cañete, T.; Blázquez, G.; Tobeña, A.; Giménez-Llort, L.; Fernández-Teruel, A. Cognitive and Emotional Alterations in Young Alzheimer’s Disease (3xTgAD) Mice: Effects of Neonatal Handling Stimulation and Sexual Dimorphism. Behav. Brain Res. 2015, 281, 156–171. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; Johansson, B.; Giménez-Llort, L. Long-term Treatment with Low-dose Caffeine Worsens BPSD-like Profile in 3xTg-AD Mice Model of Alzheimer’s Disease and Affects Mice with Normal Aging. Front. Pharmacol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.C.; Forner, S.; Trujillo-Estrada, L.; Baglietto-Vargas, D.; LaFerla, F.M. Past to Future: What Animal Models Have Taught Us About Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64 (Suppl. 1), S365–S378. [Google Scholar] [CrossRef] [PubMed]
- Rothman, S.M.; Herdener, N.; Frankola, K.A.; Mughal, M.R.; Mattson, M.P. Chronic Mild Sleep Restriction Accentuates Contextual Memory Impairments, and Accumulations of Cortical Aβ and pTau in a Mouse Model of Alzheimer’s Disease. Brain Res. 2013, 1529, 200–208. [Google Scholar] [CrossRef]
- Bellanti, F.; Iannelli, G.; Blonda, M.; Tamborra, R.; Villani, R.; Romano, A.; Calcagnini, S.; Mazzoccoli, G.; Vinciguerra, M.; Gaetani, S.; et al. Alterations of Clock Gene RNA Expression in Brain Regions of a Triple Transgenic Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 59, 615–631. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, F.; Cao, X.; Yang, J.; Bai, Y.; Yan, X.; Cao, J.; Qi, J. Abnormal Circadian Locomotor Rhythms and Per Gene Expression in Six-month-old Triple Transgenic Mice Model of Alzheimer’s Disease. Neurosci. Lett. 2018, 676, 13–18. [Google Scholar] [CrossRef]
- Castano-Prat, P.; Perez-Mendez, L.; Perez-Zabalza, M.; Sanfeliu, C.; Giménez-Llort, L.; Sánchez-Vives, M.V. Altered Slow (<1 Hz) and Fast (Beta and Gamma) Neocortical Oscillations in the 3xTg-AD Mouse Model of Alzheimer’s Disease under Anesthesia. Neurobiol. Aging 2019, 79, 142–151. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Ratia, M.; Pérez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Clos, M.V. AVCRI104P3, a Novel Multitarget Compound with Cognition-enhancing and Anxiolytic Activities: Studies in Cognitively Poor Middle-aged Mice. Behav. Brain Res. 2015, 286, 97–103. [Google Scholar] [CrossRef]
- Muñoz-Torrero, D. Acetylcholinesterase Inhibitors as Disease-modifying Therapies for Alzheimer’s Disease. Curr. Med. Chem. 2008, 15, 2433–2455. [Google Scholar] [CrossRef]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s Disease: Past, Present and Future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Camps, P.; Cusack, B.; Mallender, W.D.; El Achab, R.; Morral, J.; Muñoz-Torrero, D.; Rosenberry, T.L. Huprine X is a Novel High-affinity Inhibitor of Acetylcholinesterase That is of Interest for Treatment of Alzheimer’s Disease. Mol. Pharmacol. 2000, 57, 409–417. [Google Scholar] [PubMed]
- Román, S.; Badia, A.; Camps, P.; Clos, M.V. Potentiation Effects of (+/−) Huprine X, a New Anticholinesterasic Inhibitor, on Nicotinic Receptors in Rat Cortical Synaptosomes. Neuropharmacology 2004, 46, 95–102. [Google Scholar] [CrossRef]
- Román, S.; Vivas, N.M.; Badia, A.; Clos, M.V. Interaction of a New Potent Anticholinesterasic Compound (+/−) Huprine X with Muscarinic Receptors in Rat Brain. Neurosci. Lett. 2002, 325, 103–106. [Google Scholar] [CrossRef]
- Clos, M.V.; Pera, M.; Ratia, M.; Román, S.; Camps, P.; Muñoz-Torrero, D.; Colombo, L.; Salmona, M.; Badia, A. Effect of Acetylcholinesterase Inhibitors on AChE-induced PrP106-126 Aggregation. J. Mol. Neurosci. 2006, 30, 89–90. [Google Scholar] [CrossRef]
- Viayna, E.; Gómez, T.; Galdeano, C.; Ramírez, L.; Ratia, M.; Badia, A.; Clos, M.V.; Verdaguer, E.; Junyent, F.; Camins, A.; et al. Novel Huprine Derivatives with Inhibitory Activity toward β-amyloid Aggregation and Formation as Disease-modifying Anti-Alzheimer Drug Candidates. ChemMedChem 2010, 5, 1855–1870. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, M.M.; Clos, M.V.; Ratia, M.; Gonzalez, D.; Unger Lithner, C.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Giménez-Llort, L.; Nordberg, A. Effect of Huprine X on β-amyloid, Synaptophysin and α7 Neuronal Nicotinic Acetylcholine Receptors in the Brain of 3xTg-AD and APPswe Transgenic Mice. Neurodegener. Dis. 2010, 7, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Ratia, M.; Pérez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Clos, M.V. Behavioural Effects of Novel Multitarget Anticholinesterasic Derivatives in Alzheimer’s Disease. Behav. Pharmacol. 2015, 28, 124–131. [Google Scholar] [CrossRef]
- Relat, J.; Come, J.; Perez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Gimenez-Llort, L.; Clos, M.V. Neuroprotective Effects of the Multitarget Agent AVCRI104P3 in Brain of Middle-aged Mice. Int. J. Mol. Sci. 2018, 19, 2615. [Google Scholar] [CrossRef]
- Ratia, M.; Giménez-Llort, L.; Camps, P.; Muñoz-Torrero, D.; Clos, M.V.; Badia, A. Behavioural Effects and Regulation of PKCalpha and MAPK by Huprine X in Middle Aged Mice. Pharmacol. Biochem. Behav. 2010, 95, 485–493. [Google Scholar] [CrossRef]
- Ratia, M.; Giménez-Llort, L.; Camps, P.; Muñoz-Torrero, D.; Pérez, B.; Clos, M.V.; Badia, A. Huprine X and Huperzine A Improve Cognition and Regulate some Neurochemical Processes Related with Alzheimer’s Disease in Triple Transgenic Mice (3xTg-AD). Neurodegener. Dis. 2013, 11, 129–140. [Google Scholar] [CrossRef]
- Belfiore, R.; Rodin, A.; Ferreira, E.; Velazquez, R.; Branca, C.; Caccamo, A.; Oddo, S. Temporal and Regional Progression of Alzheimer’s Disease-like Pathology in 3xTg-AD Mice. Aging Cell. 2019, 18, e12873. [Google Scholar] [CrossRef] [PubMed]
- Janus, C. Search Strategies Used by APP Transgenic Mice during Navigation in the Morris Water Maze. Learn. Mem. 2004, 11, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Baeta-Corral, R.; Giménez-Llort, L. Persistent Hyperactivity and Distinctive Strategy Features in the Morris Water Maze in 3xTg-AD Mice at Advanced Stages of Disease. Behav. Neurosci. 2015, 129, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Fernández-Teruel, A.; Escorihuela, R.M.; Fredholm, B.B.; Tobeña, A.; Pekny, M.; Johansson, B. Mice Lacking the Adenosine A1 Receptor are Anxious and Aggressive, but are Normal Learners with Reduced Muscle Strength and Survival Rate. Eur. J. Neurosci. 2002, 16, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Sandi, C.; Loscertales, M.; Guaza, C. Experience-dependent Facilitating Effect of Corticosterone on Spatial Memory Formation in the Water Maze. Eur. J. Neurosci. 1997, 9, 637–642. [Google Scholar] [CrossRef]

| Saline (n = 14) | Huprine X 0.12 μmol kg−1 (n = 14) | AVCRI104P3 0.6 μmol kg−1 (n = 14) | |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| Weight | |||
| % vs. initial weight | 95.1 ± 1.7 | 92.8 ± 2.3 | 93.3 ± 1.5 |
| Open field (before treatment) | |||
| Latency to leave the center (s) | 10.79 ± 1.84 | 11.57 ± 2.03 | 13.07 ± 2.60 |
| Latency to enter into the periphery (s) | 36.50 ± 14.60 | 32.56 ± 6.48 | 26.93 ± 5.95 |
| Total number of crossings | 46.71 ± 8.62 | 49.50 ± 10.32 | 46.71 ± 10.30 |
| Total number of rearings | 10.57 ± 2.11 | 8.29 ± 2.14 | 9.50 ± 2.03 |
| Number of groomings | 2.29 ± 0.40 | 1.29 ± 0.19 | 0.71 ± 0.16 |
| Incidence of defecations | 13/14 | 12/14 | 13/14 |
| Number of defecations | 2.29 ± 0.30 | 2.07 ± 0.37 | 2.57 ± 0.45 |
| Incidence of urinations | 7/14 | 6/14 | 6/14 |
| Presence of urine | 0.57 ± 0.17 | 0.50 ± 0.17 | 0.57 ± 0.23 |
| Open field (after treatment) | |||
| Latency to leave the center (s) | 34.50 ± 13.05 | 13.00 ± 2.97 | 14.50 ± 1.92 |
| Latency to enter into the periphery (s) | 56.64 ± 23.50 | 39.79 ± 16.97 | 23.14 ± 4.77 |
| Total number of crossings | 32.29 ± 6.86 | 36.64 ± 8.68 a | 36.43 ± 8.29 |
| Total number of rearings | 4.43 ± 1.01 aa | 5.21 ± 1.43 | 6.07 ± 1.69 a |
| Number of groomings | 1.14 ± 0.21 | 1.07 ± 0.20 | 1.29 ± 0.24 a |
| Incidence of defecations | 13/14 | 14/14 | 12/14 |
| Number of defecations | 1.86 ± 0.35 | 2.07 ± 0.29 | 2.36 ± 0.52 |
| Incidence of urinations | 1/14 | 5/14 | 2/14 |
| Presence of urine | 0.7 ± 0.7 a | 0.36 ± 0.13 | 0.29 ± 0.22 |
| Dark/light box test | |||
| Latency to entry in the lit area (s) | 134.29 ± 34.63 | 140.21 ± 34.05 | 1.88.29 ± 33.81 |
| Time in the lit area (s) | 13.00 ± 3.55 | 18.79 ± 7.93 | 15.57 ± 6.28 |
| Number of entries | 1.50 ± 0.45 | 1.93 ± 0.69 | 1.36 ± 0.43 |
| Total number of risk assessment | 3.57 ± 0.84 | 2.21 ± 0.43 | 2.43 ± 0.49 |
| Total number of groomings | 1.29 ± 0.30 | 1.36 ± 0.23 | 2.57 ± 0.91 |
| Incidence of defecations | 12/14 | 11/14 | 13/14 |
| Number of defecations | 2.07 ± 0.37 | 1.50 ± 0.33 | 1.79 ± 0.32 |
| Incidence of urinations | 6/14 | 9/14 | 8/14 |
| Presence of urine | 0.43 ± 0.14 | 1.00 ± 0.43 | 0.57 ± 0.14 |
| Corner test | |||
| Number of visited corners | 5.71 ± 0.87 | 6.21 ± 0.91 | 6.36 ± 0.68 |
| Number of rearings | 1.64 ± 0.55 | 3.14 ± 0.64 | 3.14 ± 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-Llort, L.; Santana-Santana, M.; Ratia, M.; Pérez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Clos, M.V. Clock/Sleep-Dependent Learning and Memory in Male 3xTg-AD Mice at Advanced Disease Stages and Extrinsic Effects of Huprine X and the Novel Multitarget Agent AVCRI104P3. Brain Sci. 2021, 11, 426. https://doi.org/10.3390/brainsci11040426
Giménez-Llort L, Santana-Santana M, Ratia M, Pérez B, Camps P, Muñoz-Torrero D, Badia A, Clos MV. Clock/Sleep-Dependent Learning and Memory in Male 3xTg-AD Mice at Advanced Disease Stages and Extrinsic Effects of Huprine X and the Novel Multitarget Agent AVCRI104P3. Brain Sciences. 2021; 11(4):426. https://doi.org/10.3390/brainsci11040426
Chicago/Turabian StyleGiménez-Llort, Lydia, Mikel Santana-Santana, Míriam Ratia, Belén Pérez, Pelayo Camps, Diego Muñoz-Torrero, Albert Badia, and Maria Victòria Clos. 2021. "Clock/Sleep-Dependent Learning and Memory in Male 3xTg-AD Mice at Advanced Disease Stages and Extrinsic Effects of Huprine X and the Novel Multitarget Agent AVCRI104P3" Brain Sciences 11, no. 4: 426. https://doi.org/10.3390/brainsci11040426
APA StyleGiménez-Llort, L., Santana-Santana, M., Ratia, M., Pérez, B., Camps, P., Muñoz-Torrero, D., Badia, A., & Clos, M. V. (2021). Clock/Sleep-Dependent Learning and Memory in Male 3xTg-AD Mice at Advanced Disease Stages and Extrinsic Effects of Huprine X and the Novel Multitarget Agent AVCRI104P3. Brain Sciences, 11(4), 426. https://doi.org/10.3390/brainsci11040426








