Assessing Age-Related Gray Matter Differences in Young Adults with Voxel-Based Morphometry: The Effect of Field Strengths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. MRI Protocol
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
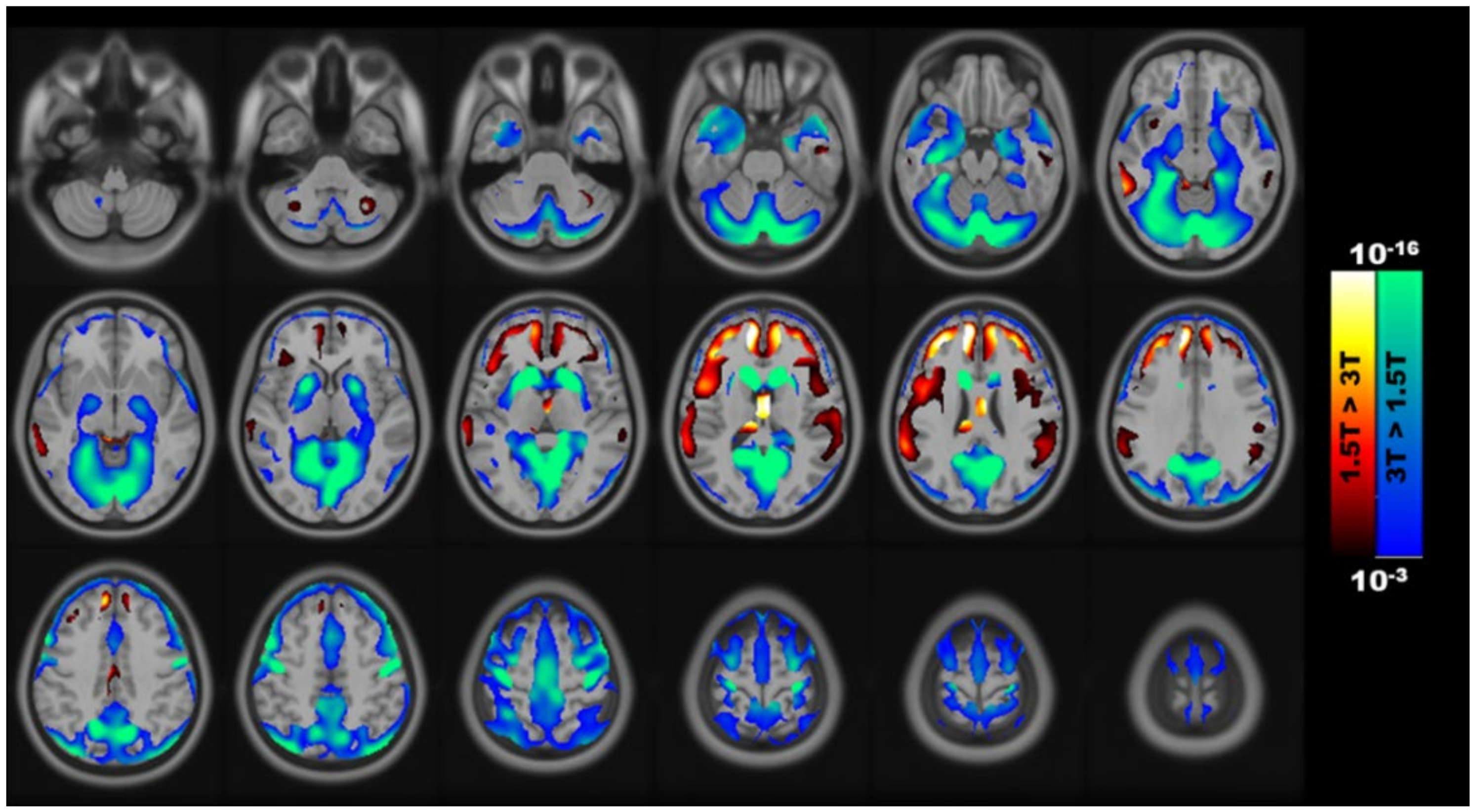
3.1. Voxel-Wise Differences in GM between Field Strengths
3.2. Age-Related Changes in the GM Volume between Field Strengths
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callaert, D.V.; Ribbens, A.; Maes, F.; Swinnen, S.P.; Wenderoth, N. Assessing age-related gray matter decline with voxel-based morphometry depends significantly on segmentation and normalization procedures. Front. Aging Neurosci. 2014, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Chebrolu, H.; Wekstein, D.R.; Schmitt, F.A.; Markesbery, W.R. Age and gender effects on human brain anatomy: A voxel-based morphometric study in healthy elderly. Neurobiol. Aging 2007, 28, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001, 14, 21–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolova, L.G.; Steiner, C.A.; Akopyan, G.G.; Dutton, R.A.; Hayashi, K.M.; Toga, A.W.; Cummings, J.L.; Thompson, P.M. Three-dimensional gray matter atrophy mapping in mild cognitive impairment and mild Alzheimer disease. Arch. Neurol. 2007, 64, 1489–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandanger, I.; Nygard, J.F.; Ingebrigtsen, G.; Sorensen, T.; Dalgard, O.S. Prevalence, incidence and age at onset of psychiatric disorders in Norway. Psychiatry Psychiatr. Epidemiol. 1999, 34, 570–579. [Google Scholar] [CrossRef]
- Rajji, T.K.; Ismail, Z.; Mulsant, B.H. Age at onset and cognition in schizophrenia: Meta-analysis. Br. J. Psychiatry 2009, 195, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Bourisly, A.K.; El-Beltagi, A.; Cherian, J.; Gejo, G.; Al-Jazzaf, A.; Ismail, M. A voxel-based morphometric magnetic resonance imaging study of the brain detects age-related gray matter volume changes in healthy subjects of 21–45 years old. Neuroradiol. J. 2015, 28, 450–459. [Google Scholar] [CrossRef]
- Seiger, R.; Hahn, A.; Hummer, A.; Kranz, G.S.; Ganger, S.; Kublbock, M.; Kraus, C.; Sladky, R.; Kasper, S.; Windischberger, C.; et al. Voxel-based morphometry at ultra-high fields. A comparison of 7T and 3 T MRI data. Neuroimage 2015, 113, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Tardif, C.L.; Collins, D.L.; Pike, G.B. Regional impact of field strength on voxel-based morphometry results. Hum. Brain Mapp. 2010, 31, 943–957. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishii, K.; Kakigi, T.; Yokoyama, K. Gender and age differences in normal adult human brain: Voxel-based morphometric study. Hum. Brain Mapp. 2011, 32, 1050–1058. [Google Scholar] [CrossRef]
- Bottomley, P.A.; Foster, T.H.; Argersinger, R.E.; Pfeifer, L.M. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1–100 MHz: Dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med. Phys. 1984, 11, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 2001, 14, 685–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taki, Y.; Goto, R.; Evans, A.; Zijdenbos, A.; Neelin, P.; Lerch, J.; Sato, K.; Ono, S.; Kinomura, S.; Nakagawa, M.; et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol. Aging 2004, 25, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; Erickson, K.I.; Rodrigue, K.M.; Voss, M.W.; Colcombe, S.J.; Kramer, A.F.; Acker, J.D.; Raz, N. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol. Aging 2009, 30, 1657–1676. [Google Scholar] [CrossRef] [Green Version]
- Aghaeifar, A.; Zhou, J.; Heule, R.; Tabibian, B.; Scholkopf, B.; Jia, F.; Zaitsev, M.; Scheffler, K. A 32-channel multi-coil setup optimized for human brain shimming at 9.4T. Magn. Reson. Med. 2020, 83, 749–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, A.S.; Malik, S.J.; O’Regan, D.P.; Hajnal, J.V. Impact of number of channels on RF shimming at 3 T. Magma 2013, 26, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Rowley, C.D.; Sehmbi, M.; Bazin, P.L.; Tardif, C.L.; Minuzzi, L.; Frey, B.N.; Bock, N.A. Age-related mapping of intracortical myelin from late adolescence to middle adulthood using T1 -weighted MRI. Hum. Brain Mapp. 2017, 38, 3691–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.J.; Duka, T.; Stimpson, C.D.; Schapiro, S.J.; Baze, W.B.; McArthur, M.J.; Fobbs, A.J.; Sousa, A.M.; Sestan, N.; Wildman, D.E.; et al. Prolonged myelination in human neocortical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 16480–16485. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, C.E.; Zilles, K.; Schleicher, A.; Rilling, J.K.; Gibson, K.R. Expansion of the neocerebellum in Hominoidea. J. Hum. Evol. 2003, 44, 401–429. [Google Scholar] [CrossRef]
- Buckner, R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Caligiore, D.; Pezzulo, G.; Baldassarre, G.; Bostan, A.C.; Strick, P.L.; Doya, K.; Helmich, R.C.; Dirkx, M.; Houk, J.; Jorntell, H.; et al. Consensus Paper: Towards a Systems-Level View of Cerebellar Function: The Interplay Between Cerebellum, Basal Ganglia, and Cortex. Cerebellum 2017, 16, 203–229. [Google Scholar] [CrossRef] [Green Version]
- Tiemeier, H.; Lenroot, R.K.; Greenstein, D.K.; Tran, L.; Pierson, R.; Giedd, J.N. Cerebellum development during childhood and adolescence: A longitudinal morphometric MRI study. Neuroimage 2010, 49, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, H.; Amado, I.; Mouchet-Mages, S.; Olie, J.P.; Krebs, M.O. The role of the cerebellum in schizophrenia: An update of clinical, cognitive, and functional evidences. Schizophr. Bull. 2008, 34, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Amianto, F.; Caroppo, P.; D’Agata, F.; Spalatro, A.; Lavagnino, L.; Caglio, M.; Righi, D.; Bergui, M.; Abbate-Daga, G.; Rigardetto, R.; et al. Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: A voxel-based morphometry study. Psychiatry Res. 2013, 213, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Domin, M.; Gerlach, F.H.; Gaser, C.; Lueders, E.; Schmidt, C.O.; Neumann, N. Novel findings from 2838 Adult Brains on Sex Differences in Gray Matter Brain Volume. Sci. Rep. 2019, 9, 1671. [Google Scholar] [CrossRef]
- Mishra, S.; Beheshti, I.; Khanna, P. A statistical region selection and randomized volumetric features selection framework for early detection of Alzheimer’s disease. Int. J. Imag. Syst. Tech. 2018, 28, 302–314. [Google Scholar] [CrossRef]
- Singh, S.; Tench, C.R.; Tanasescu, R.; Constantinescu, C.S. Localised Grey Matter Atrophy in Multiple Sclerosis and Clinically Isolated Syndrome-A Coordinate-Based Meta-Analysis, Meta-Analysis of Networks, and Meta-Regression of Voxel-Based Morphometry Studies. Brain Sci. 2020, 10, 798. [Google Scholar] [CrossRef]
- Jovicich, J.; Czanner, S.; Han, X.; Salat, D.; van der Kouwe, A.; Quinn, B.; Pacheco, J.; Albert, M.; Killiany, R.; Blacker, D.; et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 2009, 46, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, M.D.; Cunningham, W.A. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009, 4, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Farokhian, F.; Yang, C.; Beheshti, I.; Matsuda, H.; Wu, S. Age-Related Gray and White Matter Changes in Normal Adult Brains. Aging Dis. 2017, 8, 899–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farokhian, F.; Beheshti, I.; Sone, D.; Matsuda, H. Comparing CAT12 and VBM8 for Detecting Brain Morphological Abnormalities in Temporal Lobe Epilepsy. Front. Neurol. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, F.-Y.; Chen, J.-R.; Chen, C.-M.; Huang, Y.-C.; Peng, S.-L. Assessing Age-Related Gray Matter Differences in Young Adults with Voxel-Based Morphometry: The Effect of Field Strengths. Brain Sci. 2021, 11, 447. https://doi.org/10.3390/brainsci11040447
Su F-Y, Chen J-R, Chen C-M, Huang Y-C, Peng S-L. Assessing Age-Related Gray Matter Differences in Young Adults with Voxel-Based Morphometry: The Effect of Field Strengths. Brain Sciences. 2021; 11(4):447. https://doi.org/10.3390/brainsci11040447
Chicago/Turabian StyleSu, Feng-Yi, Jyun-Ru Chen, Chun-Ming Chen, Yen-Chih Huang, and Shin-Lei Peng. 2021. "Assessing Age-Related Gray Matter Differences in Young Adults with Voxel-Based Morphometry: The Effect of Field Strengths" Brain Sciences 11, no. 4: 447. https://doi.org/10.3390/brainsci11040447





