Morphometric Analysis of Brain in Newborn with Congenital Diaphragmatic Hernia
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Magnetic Resonance Imaging (MRI) Acquisition
2.3. Image Processing
2.4. Statistical Analysis
3. Results
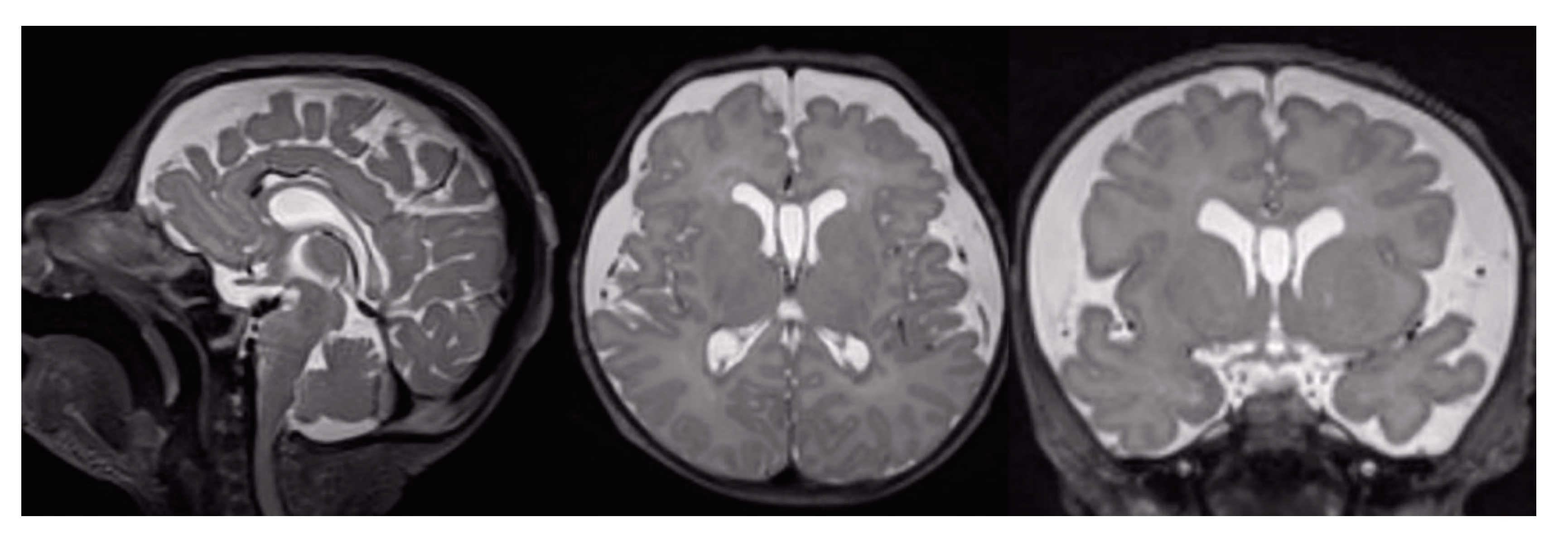
3.1. Conventional MRI Findings
3.2. Cortical Thickness Results
3.3. Gyrification Results
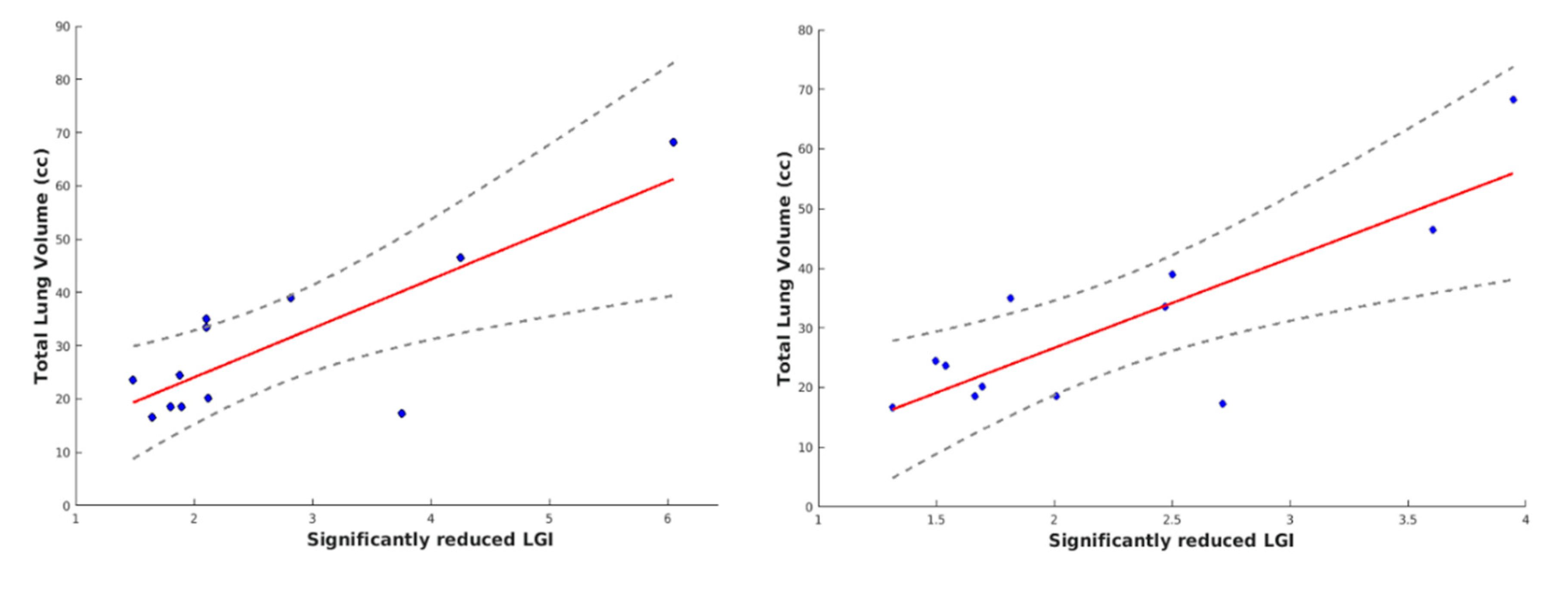
3.4. Correlation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, R.H.; Hardin, W.D.; Hirschl, R.B.; Jaksic, T.; Lally, K.P.; Langham, M.R.; Wilson, J.M. Current surgical management of congenital diaphragmatic hernia: A report from the congenital diaphragmatic hernia study group. J. Pediatr. Surg. 1998, 33, 1004–1009. [Google Scholar] [CrossRef]
- Morini, F.; Valfrè, L.; Bagolan, P. Long-term morbidity of congenital diaphragmatic hernia: A plea for standardization. Semin. Pediatr. Surg. 2017, 26, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Long, A.M.; Bunch, K.J.; Knight, M.; Kurinczuk, J.J.; Losty, P.D. Early population-based outcomes of infants born with congenital diaphragmatic hernia. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F517–F522. [Google Scholar] [CrossRef]
- Danzer, E.; Gerdes, M.; D’Agostino, J.A.; Bernbaum, J.; Hoffman, C.; Herkert, L.; Rintoul, N.E.; Peranteau, W.H.; Flake, A.W.; Adzick, N.S.; et al. Neurodevelopmental outcome at one year of age in congenital diaphragmatic hernia infants not treated with extracorporeal membrane oxygenation. J. Pediatr. Surg. 2015, 50, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Crankson, S.J.; Al Jadaan, S.A.; Namshan, M.A.; Al-Rabeeah, A.A.; Oda, O. The immediate and long-term outcomes of newborns with congenital diaphragmatic hernia. Pediatr. Surg. Int. 2006, 22, 335–340. [Google Scholar] [CrossRef]
- Bagolan, P.; Morini, F. Long-term follow-up of congenital diaphragmatic hernia. Semin. Pediatr. Surg. 2007, 16, 134–144. [Google Scholar] [CrossRef]
- Danzer, E.; Kim, S.S. Neurodevelopmental outcome in congenital diaphragmatic hernia: Evaluation, predictors and outcome. World J. Clin. Pediatr. 2014, 3, 30. [Google Scholar] [CrossRef]
- Hollinger, L.E.; Harting, M.T.; Lally, K.P. Long-term follow-up of congenital diaphragmatic hernia. Semin. Pediatr. Surg. 2017, 26, 178–184. [Google Scholar] [CrossRef]
- Antiel, R.M.; Lin, N.; Licht, D.J.; Hoffman, C.; Waqar, L.; Xiao, R.; Monos, S.; D’Agostino, J.A.; Bernbaum, J.; Herkert, L.M.; et al. Growth trajectory and neurodevelopmental outcome in infants with congenital diaphragmatic hernia. J. Pediatr. Surg. 2017, 52, 1944–1948. [Google Scholar] [CrossRef]
- Montalva, L.; Raffler, G.; Riccio, A.; Lauriti, G.; Zani, A. Neurodevelopmental impairment in children with congenital diaphragmatic hernia: Not an uncommon complication for survivors. J. Pediatr. Surg. 2019, 55, 625–634. [Google Scholar] [CrossRef]
- Bevilacqua, F.; Morini, F.; Zaccara, A.; Valfrè, L.; Capolupo, I.; Bagolan, P.; Aite, L. Neurodevelopmental outcome in congenital diaphragmatic hernia survivors: Role of ventilatory time. J. Pediatr. Surg. 2015, 50, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Danzer, E.; Zarnow, D.; Gerdes, M.; D’Agostino, J.A.; Siegle, J.; Bebbington, M.W.; Flake, A.W.; Adzick, N.S.; Hedrick, H.L. Abnormal brain development and maturation on magnetic resonance imaging in survivors of severe congenital diaphragmatic hernia. J. Pediatr. Surg. 2012, 47, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.W.; Kean, M.J.; Stewart, M.J.; Inder, T.E. Patterns of Cerebral Injury in a Series of Infants with Congenital Diaphragmatic Hernia Utilizing Magnetic Resonance Imaging. J. Pediatr. Surg. 2004, 39, 31–36. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Merhar, S.; Su, W.; Zhang, B.; Burns, P.; Lim, F.; Kline-Fath, B. Prenatal factors associated with postnatal brain injury in infants with congenital diaphragmatic hernia. Am. J. Neuroradiol. 2018, 39, 558–562. [Google Scholar] [CrossRef]
- Tracy, S.; Estroff, J.; Valim, C.; Friedman, S.; Chen, C. Abnormal neuroimaging and neurodevelopmental findings in a cohort of antenatally diagnosed congenital diaphragmatic hernia survivors. J. Pediatr. Surg. 2010, 45, 958–965. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Merhar, S.; Burns, P.; Zhang, B.; Lim, F.; Kline-Fath, B. Fetal brain morphometry on prenatal magnetic resonance imaging in congenital diaphragmatic hernia. Pediatr. Radiol. 2018. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Shi, F.; Lyall, A.E. Cortical thickness and surface area in neonates at high risk for schizophrenia. Brain Struct. Funct. 2014. [Google Scholar] [CrossRef]
- Li, G.; Lin, W.; Gilmore, J.H.; Shen, X.D. Spatial Patterns, Longitudinal Development, and Hemispheric Asymmetries of Cortical Thickness in Infants from Birth to 2 Years of Age. J. Neurosci. 2015, 35, 9150–9162. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Lerch, J.; Greenstein, D.; Sharp, W.; Clasen, L.; Evans, A.; Giedd, J.; Castellanos, F.X.; Rapoport, J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2006, 63, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kabani, N.J.; Lerch, J.P.; Eckstrand, K.; Lenroot, R.; Gogtay, N.; Greenstein, D.; Clasen, L.; Evans, A.; Rapoport, J.L.; et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008, 28, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- von Economo, C. The Cytoarchitectonics of the Human Cerebral Cortex. South. Med. J. 1929, 22, 1048. [Google Scholar] [CrossRef]
- Zielinski, B.A.; Prigge, M.B.D.; Nielsen, J.A.; Froehlich, A.L.; Abildskov, T.J.; Anderson, J.S.; Fletcher, P.T.; Zygmunt, K.M.; Travers, B.G.; Lange, N.; et al. Longitudinal changes in cortical thickness in autism and typical development. Brain 2014, 137, 1799–1812. [Google Scholar] [CrossRef] [PubMed]
- Rimol, L.M.; Nesvåg, R.; Hagler, D.J.; Bergmann, Ø.; Fennema-Notestine, C.; Hartberg, C.B.; Haukvik, U.K.; Lange, E.; Pung, C.J.; Server, A.; et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol. Psychiatry 2012, 71, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.D.; Kippenhan, J.S.; Dickinson, D.; Carrasco, J.; Mattay, V.S.; Weinberger, D.R.; Berman, K.F. Regional Variations in Brain Gyrification Are Associated with General Cognitive Ability in Humans. Curr Biol. 2016, 26, 1301–1305. [Google Scholar] [CrossRef]
- Cao, B.; Mwangi, B.; Passos, I.C.; Wu, M.J.; Keser, Z.; Zunta-Soares, G.B.; Xu, D.; Hasan, K.M.; Soares, J.C. Lifespan Gyrification Trajectories of Human Brain in Healthy Individuals and Patients with Major Psychiatric Disorders. Sci. Rep. 2017, 7, 511. [Google Scholar] [CrossRef]
- Hogstrom, L.J.; Westlye, L.T.; Walhovd, K.B.; Fjell, A.M. The structure of the cerebral cortex across adult life: Age-related patterns of surface area, thickness, and gyrification. Cereb. Cortex 2013, 23, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Sury, M.R.J.; Harker, H.; Begent, J.; Chong, W.K. The management of infants and children for painless imaging. Clin. Radiol. 2005, 60, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.E.; Thompson, C.; Gunny, R.; Jones, R.; Cox, T.; Chong, W.K. Magnetic resonance imaging protocols for paediatric neuroradiology. Pediatr. Radiol. 2007, 37, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Savelli, S.; Bascetta, S.; Carducci, C.; Carnevale, E.; Caforio, L.; Romiti, A.; Tomà, P. Fetal MRI assessment of mediastinal shift angle in isolated left congenital diaphragmatic hernia: A new postnatal survival predictive tool? Prenat. Diagn. 2020, 40, 136–141. [Google Scholar] [CrossRef]
- Prastawa, M.; Gilmore, J.H.; Lin, W.; Gerig, G. Automatic segmentation of MR images of the developing newborn brain. Med. Image Anal. 2005, 9, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Makropoulos, A.; Robinson, E.C.; Schuh, A. The Developing Human Connectome Project: A Minimal Processing Pipeline for Neonatal Cortical Surface Reconstruction. Neuroimage 2018, 173, 88–112. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Merhar, S.; Meinzen-Derr, J.; Haberman, B.; Lim, F.; Burns, P.; Zorn, E. Correlation of MRI brain injury findings with neonatal clinical factors in infants with congenital diaphragmatic hernia. Am. J. Neuroradiol. 2016, 37, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Dahnke, R.; Yotter, R.A.; Gaser, C. NeuroImage Cortical thickness and central surface estimation. Neuroimage 2013, 65, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Lyu, I.; Kim, S.H.; Girault, J.B.; Gilmore, J.H.; Styner, M.A. A cortical shape-adaptive approach to local gyrification index. Med. Image Anal. 2018, 48, 244–258. [Google Scholar] [CrossRef]
- Płonka, O.; Krześniak, A.; Adamczyk, P. Analysis of local gyrification index using a novel shape-adaptive kernel and the standard FreeSurfer spherical kernel—Evidence from chronic schizophrenia outpatients. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Mensen, A.; Khatami, R. Advanced EEG analysis using threshold-free cluster-enhancement and non-parametric statistics. Neuroimage 2013, 67, 111–118. [Google Scholar] [CrossRef]
- Brossard-Racine, M.; Du Plessis, A.; Vezina, G.; Robertson, R.; Bulas, D.; Evangelou, I.; Donofrio, M.; Freeman, D. Prevalence and spectrum of in utero structural brain abnormalities in fetuses with complex congenital heart disease. Am. J. Neuroradiol. 2014, 35, 1593–1599. [Google Scholar] [CrossRef]
- Benkarim, O.M.; Hahner, N.; Piella, G.; Gratacos, E.; González Ballester, M.A.; Eixarch, E.; Sanroma, G. Cortical folding alterations in fetuses with isolated non-severe ventriculomegaly. NeuroImage Clin. 2018, 18, 103–114. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Dierker, D.; Snyder, A.Z.; Raichle, M.E.; Reiss, A.L.; Korenberg, J. Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. J. Neurosci. 2006, 26, 5470–5483. [Google Scholar] [CrossRef] [PubMed]
- Nordahl, C.W.; Dierker, D.; Mostafavi, I.; Schumann, C.M.; Rivera, S.M.; Amaral, D.G.; Van Essen, D.C. Cortical folding abnormalities in autism revealed by surface-based morphometry. J. Neurosci. 2007, 27, 11725–11735. [Google Scholar] [CrossRef] [PubMed]
- Sripada, K.; Løhaugen, G.C.; Eikenes, L.; Bjørlykke, K.M.; Håberg, A.K.; Skranes, J.; Rimol, L.M. Visual-motor deficits relate to altered gray and white matter in young adults born preterm with very low birth weight. Neuroimage 2015, 109, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Benders, M.; Borradori-Tolsa, C.; Cachia, A.; Lazeyras, F.; Ha-Vinh Leuchter, R.; Sizonenko, S.V.; Warfield, S.K.; Mangin, J.F.; Hüppi, P.S. Primary cortical folding in the human newborn: An early marker of later functional development. Brain 2008, 131, 2028–2041. [Google Scholar] [CrossRef] [PubMed]
- Palaniyappan, L.; Crow, T.J.; Hough, M.; Voets, N.L.; Liddle, P.F.; James, S.; Winmill, L.; James, A.C. Gyrification of Broca’s region is anomalously lateralized at onset of schizophrenia in adolescence and regresses at 2year follow-up. Schizophr. Res. 2013, 147, 39–45. [Google Scholar] [CrossRef]
- Ohi, K.; Matsuda, Y.; Shimada, T.; Yasuyama, T.; Oshima, K.; Sawai, K.; Kihara, H.; Nitta, Y.; Okubo, H.; Uehara, T.; et al. Structural alterations of the superior temporal gyrus in schizophrenia: Detailed subregional differences. Eur. Psychiatry 2016, 35, 25–31. [Google Scholar] [CrossRef]
- Thibeault, D.W.; Haney, B. Lung volume, pulmonary vasculature, and factors affecting survival in congenital diaphragmatic hernia. Pediatrics 1998, 101, 289–295. [Google Scholar] [CrossRef]
- Walsh, D.S.; Hubbard, A.M.; Olutoye, O.O.; Howell, L.J.; Crombleholme, T.M.; Flake, A.W.; Johnson, M.P.; Adzick, N.S. Assessment of fetal lung volumes and liver herniation with magnetic resonance imaging in congenital diaphragmatic hernia. Am. J. Obstet. Gynecol. 2000, 183, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Gorincour, G.; Bouvenot, J.; Mourot, M.G.; Sonigo, P.; Chaumoitre, K.; Garel, C.; Guibaud, L.; Rypens, F.; Avni, F.; Cassart, M.; et al. Prenatal prognosis of congenital diaphragmatic hernia using magnetic resonance imaging measurement of fetal lung volume. Ultrasound Obstet. Gynecol. 2005, 26, 738–744. [Google Scholar] [CrossRef]
- Alfaraj, M.A.; Shah, P.S.; Bohn, D.; Pantazi, S.; O’Brien, K.; Chiu, P.P.; Gaiteiro, R.; Ryan, G. Congenital diaphragmatic hernia: Lung-to-head ratio and lung volume for prediction of outcome. Am. J. Obstet. Gynecol. 2011, 205, 43.e1–43.e8. [Google Scholar] [CrossRef]
- Papini, C.; Palaniyappan, L.; Kroll, J.; Froudist-Walsh, S.; Murray, R.M.; Nosarti, C. Altered Cortical Gyrification in Adults Who Were Born Very Preterm and Its Associations With Cognition and Mental Health. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 640–650. [Google Scholar] [CrossRef]
- Cordina, R.; Grieve, S.; Barnett, M.; Lagopoulos, J.; Malitz, N.; Celermajer, D.S. Brain volumetric, regional cortical thickness and radiographic findings in adults with cyanotic congenital heart disease. NeuroImage Clin. 2014, 4, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Mürner-Lavanchy, I.; Steinlin, M.; Nelle, M.; Rummel, C.; Perrig, W.J.; Schroth, G.; Everts, R. Delay of cortical thinning in very preterm born children. Early Hum. Dev. 2014, 90, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Barnes-Davis, M.E.; Williamson, B.J.; Merhar, S.L.; Holland, S.K.; Kadis, D.S. Extremely preterm children exhibit altered cortical thickness in language areas. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Claessens, N.H.P.; Moeskops, P.; Buchmann, A.; Latal, B.; Knirsch, W.; Scheer, I.; Išgum, I.; De Vries, L.S.; Benders, M.J.N.L.; Von Rhein, M. Delayed cortical gray matter development in neonates with severe congenital heart disease. Pediatr. Res. 2016, 80, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Rees, S.; Stringer, M.; Just, Y.; Hooper, S.B.; Harding, R. The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Dev. Brain Res. 1997, 103, 103–118. [Google Scholar] [CrossRef]
- Dean, J.M.; McClendon, E.; Hansen, K.; Azimi-Zonooz, A.; Chen, K.; Riddle, A.; Gong, X.; Sharifnia, E.; Hagen, M.; Ahmad, T.; et al. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef]
- Kelly, C.J.; Makropoulos, A.; Cordero-Grande, L.; Hutter, J.; Price, A.; Hughes, E.; Murgasova, M.; Teixeira, R.P.A.G.; Steinweg, J.K.; Kulkarni, S.; et al. Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Clouchoux, C.; du Plessis, A.J.; Bouyssi-Kobar, M.; Tworetzky, W.; McElhinney, D.B.; Brown, D.W.; Gholipour, A.; Kudelski, D.; Warfield, S.K.; McCarter, R.J.; et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex 2013, 23, 2932–2943. [Google Scholar] [CrossRef]
- Glauser, T.A.; Rorke, L.B.; Weinberg, P.M.; Clancy, R.R. Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics 1990, 85, 984–990. [Google Scholar]
- Peterson, B.S.; Anderson, A.W.; Ehrenkranz, R.; Staib, L.H.; Tageldin, M.; Colson, E.; Gore, J.C.; Duncan, C.C.; Makuch, R.; Ment, L.R. Regional Brain Volumes and Their Later Neurodevelopmental Correlates in Term and Preterm Infants. Pediatrics 2004, 111, 939–948. [Google Scholar] [CrossRef]
- Padilla, N.; Alexandrou, G.; Blennow, M.; Lagercrantz, H.; Ådén, U. Brain Growth Gains and Losses in Extremely Preterm Infants at Term. Cereb. Cortex 2015, 25, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Inder, T.E.; Huppi, P.S.; Warfield, S.; Kikinis, R.; Zientara, G.P.; Barnes, P.D.; Jolesz, F.A.; Volpe, J.J. Periventricular White Matter Injury in the Premature Infant Is Associated with a Reduction in Cerebral Cortical Gray Matter Volume at Term. Pediatr. Res. 1999, 45, 343. [Google Scholar] [CrossRef][Green Version]
- Moeskops, P.; Benders, M.J.N.L.; Kersbergen, K.J.; Groenendaal, F.; De Vries, L.S.; Viergever, M.A.; Išgum, I. Development of cortical morphology evaluated with longitudinal MR brain images of preterm infants. PLoS ONE 2015, 10, e0131552. [Google Scholar] [CrossRef] [PubMed]
- Bjuland, K.J.; Løhaugen, G.C.C.; Martinussen, M.; Skranes, J. Cortical thickness and cognition in very-low-birth-weight late teenagers. Early Hum. Dev. 2013, 89, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Vohr, B.; Schneider, K.C.; Katz, K.H.; Makuch, R.W.; Reiss, A.L.; Ment, L.R. Increased temporal lobe gyrification in preterm children. Neuropsychologia 2006, 44, 445–453. [Google Scholar] [CrossRef] [PubMed]

| CDH | HC | |
|---|---|---|
| Age | ||
| Mean | 5.52 | 5.41 |
| SD | 1.80 | 1.94 |
| Range | 3.1–9.4 | 3.0–9.0 |
| Gender | ||
| Female (%) | 9 (56%) | 8 (61%) |
| Male (%) | 7 (44%) | 5 (39%) |
| CDH | Gender | Herniation Side | Surgery (Day after Birth) | Days of Ventilation | Structural Heart Defect | # Sepsis | Units of Blood Transfused |
|---|---|---|---|---|---|---|---|
| sub-01 | M | L | 3 | 8 | N | 0 | 0 |
| sub-02 | F | L | 2 | 9 | N | 0 | 0 |
| sub-03 | F | L | 6 | 11 | PDA | 2 | 4 |
| sub-04 | F | L | 2 | 7 | PDA | 0 | 0 |
| sub-05 | F | L | 4 | 11 | N | 1 | 1 |
| sub-06 | M | L | 2 | 10 | ASD+PDA | 0 | 0 |
| sub-07 | F | L | 3 | 9 | PDA+PFO | 0 | 1 |
| sub-08 | M | L | 125 | 18 | ASD | 0 | 2 |
| sub-09 | M | L | 4 | 7 | PDA | 0 | 0 |
| sub-10 | F | L | 40 | 48 | ASD+PFO | 1 | 1 |
| sub-11 | F | L | 54 | 11 | ASD+PDA | 1 | 1 |
| sub-12 | M | L | 2 | 13 | PDA | 0 | 1 |
| sub-13 | F | L | 2 | 19 | PDA | 0 | 1 |
| sub-14 | M | L | 3 | 24 | ASD+PDA | 0 | 1 |
| sub-15 | F | R | 3 | 11 | PDA | 0 | 1 |
| sub-16 | M | L | 6 | 19 | PDA+PH | 0 | 3 |
| CDH | Gestational Age at Fetal MRI (weeks) | Age at Postnatal MRI (weeks) | Ipsi-Lateral Lung Vol (cc) | Contra-Lateral Lung Vol (cc) | Total Lung Volume (cc) |
|---|---|---|---|---|---|
| sub-01 | 31 | 4.4 | 6.0 | 33.0 | 39.0 |
| sub-02 | 30 | 7.3 | N.D. | 16.6 | 16.6 |
| sub-03 | 31 | 6 | N.D. | 17.3 | 17.3 |
| sub-04 | 31 | 7.4 | N.D. | 20.1 | 20.1 |
| sub-05 | 28 | 3.3 | 1.5 | 17.0 | 18.5 |
| sub-06 | -- | 4.1 | -- | -- | -- |
| sub-07 | 32 | 4.3 | 9.9 | 36.6 | 46.5 |
| sub-08 | -- | 4.7 | -- | -- | -- |
| sub-09 | -- | 5 | -- | -- | -- |
| sub-10 | 29 | 6.6 | N.D. | 33.5 | 33.5 |
| sub-11 | 32 | 9.4 | 1.5 | 17.0 | 18.5 |
| sub-12 | 37 | 3.4 | N.D. | 24.5 | 24.5 |
| sub-13 | 29 | 4.9 | 7.0 | 28.0 | 35.5 |
| sub-14 | 33 | 8.1 | 16.3 | 52.0 | 68.3 |
| sub-15 | -- | 6.4 | -- | -- | -- |
| sub-16 | 30 | 3.1 | N.D. | 23.6 | 23.6 |
| Brain Damage | CDH Patients at Postnatal MRI |
|---|---|
| Ventriculomegaly | 4 (24.8%) |
| SAS enlargement | 6 (37.5%) |
| Intraventricular hemorrhage | 3 (18.7%) |
| Intraparenchymal hemorrhage | 3 (18.7%) |
| WM damage | 1 (6.2%) |
| GM damage | 1 (6.2%) |
| Basal ganglia damage | 2 (12.5%) |
| HC Mean (sd) | CDH Mean (sd) | p-Value | Main Involved Structures (Ordered by Cluster Extent) | |
|---|---|---|---|---|
| Left Brain | ||||
| Frontal lobe | 3.29 (0.75) | 2.13 (0.34) | 0.007 | Pars Opercularis, Lateral Orbito Frontal, Pars Triangularis, Pre-central |
| Parietal lobe | 4.62 (0.52) | 2.94 (0.22) | 0.009 | Supramarginal, Post-central, Inferior Parietal |
| Temporal lobe | 3.75 (0.63) | 2.30 (0.38) | 0.008 | Insula, Superior Temporal, Transverse Temporal, Banks-sts, Middle Temporal, Temporal Pole |
| Right Brain | ||||
| Frontal lobe | 4.25 (0.32) | 2.88 (0.22) | 0.003 | Lateral Orbito Frontal, Pars Triangularis, Pre-central, Pars Orbitalis |
| Parietal lobe | 6.16 (0.68) | 3.87 (0.46) | 0.002 | Supramarginal, Post-central |
| Temporal lobe | 5.56 (1.17) | 3.28 (0.74) | 0.001 | Insula, Superior Temporal, Transverse Temporal |
| LGI CDH | LGI HC (p-Value) | Correlation (p-Value) | Main Involved Structures | |
|---|---|---|---|---|
| Left Brain | ||||
| Frontal lobe | 1.91 | 2.75 (0.006) | 0.72 (0.04) | Pars Opercularis, Pars Triangularis |
| Temporal lobe | 2.54 | 3.99 (0.007) | 0.73 (0.03) | Superior Temporal, Transverse Temporal |
| Right Brain | ||||
| Temporal lobe | 2.26 | 4.27 (0.001) | 0.76 (0.03) | Insula, Superior Temporal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucignani, M.; Longo, D.; Fontana, E.; Rossi-Espagnet, M.C.; Lucignani, G.; Savelli, S.; Bascetta, S.; Sgrò, S.; Morini, F.; Giliberti, P.; et al. Morphometric Analysis of Brain in Newborn with Congenital Diaphragmatic Hernia. Brain Sci. 2021, 11, 455. https://doi.org/10.3390/brainsci11040455
Lucignani M, Longo D, Fontana E, Rossi-Espagnet MC, Lucignani G, Savelli S, Bascetta S, Sgrò S, Morini F, Giliberti P, et al. Morphometric Analysis of Brain in Newborn with Congenital Diaphragmatic Hernia. Brain Sciences. 2021; 11(4):455. https://doi.org/10.3390/brainsci11040455
Chicago/Turabian StyleLucignani, Martina, Daniela Longo, Elena Fontana, Maria Camilla Rossi-Espagnet, Giulia Lucignani, Sara Savelli, Stefano Bascetta, Stefania Sgrò, Francesco Morini, Paola Giliberti, and et al. 2021. "Morphometric Analysis of Brain in Newborn with Congenital Diaphragmatic Hernia" Brain Sciences 11, no. 4: 455. https://doi.org/10.3390/brainsci11040455
APA StyleLucignani, M., Longo, D., Fontana, E., Rossi-Espagnet, M. C., Lucignani, G., Savelli, S., Bascetta, S., Sgrò, S., Morini, F., Giliberti, P., & Napolitano, A. (2021). Morphometric Analysis of Brain in Newborn with Congenital Diaphragmatic Hernia. Brain Sciences, 11(4), 455. https://doi.org/10.3390/brainsci11040455






