Biological and Acoustic Sex Differences in Rat Ultrasonic Vocalization
Abstract
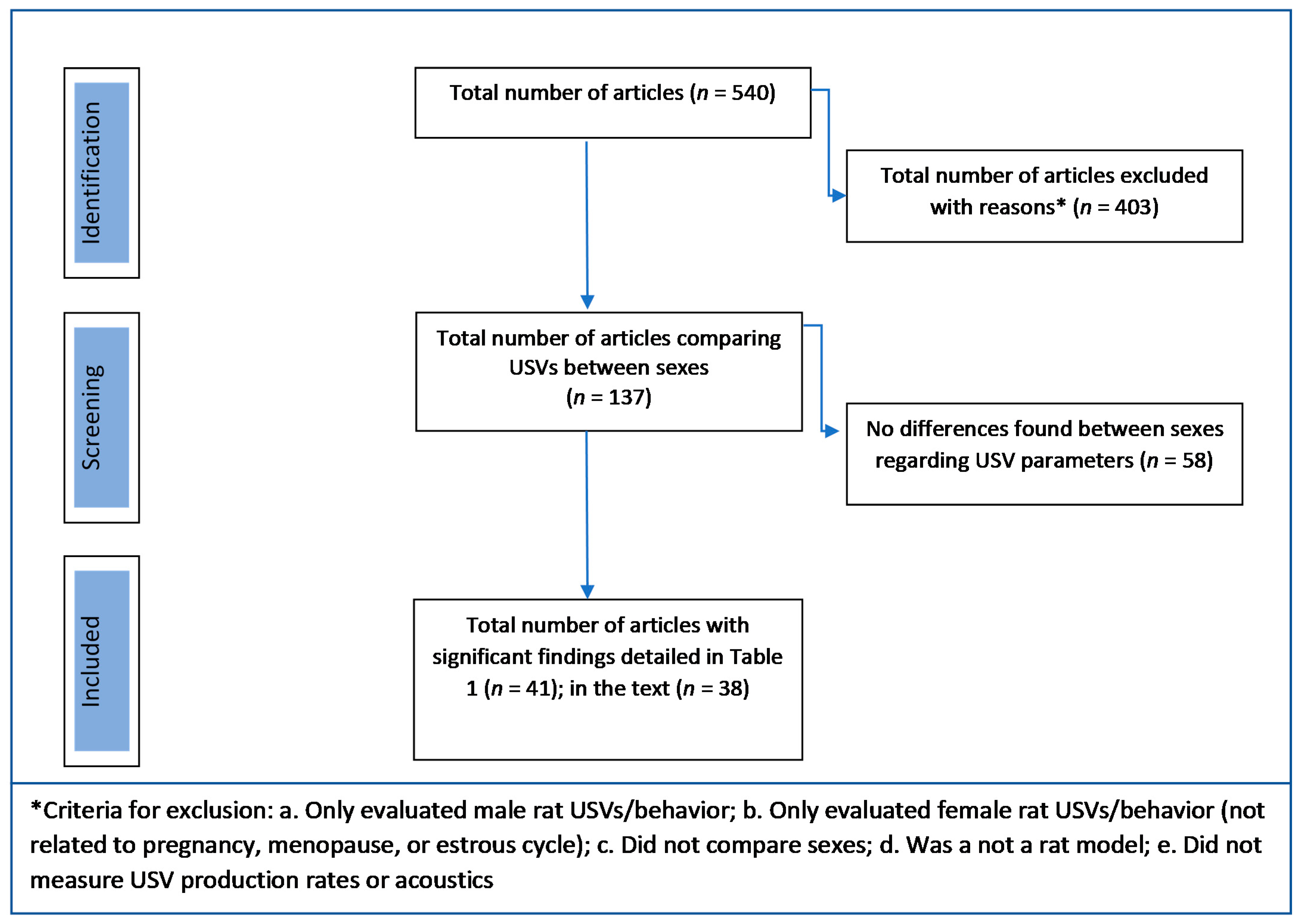
1. Introduction
2. Review of Sex Differences
2.1. Sexual Dimorphism of the Vocal Fold
2.1.1. Sex Differences in Intrinsic Laryngeal Muscles
2.1.2. Sex Differences in Vocal Fold Mucosa
2.2. Sex Differences in USV Production in the Main USV Categories
2.2.1. Alarm 22 kHz USVs
2.2.2. Pup Distress USVs
2.2.3. Adult 50 kHz USVs
Rough and Tumble Play
Mating
Housing Environment and Aging
2.3. Sex Differences in USV Acoustics
2.3.1. Alarm 22 kHz USV Acoustics
2.3.2. Pup Distress USV Acoustics
2.3.3. Adult 50 kHz USV Acoustics
2.3.4. Estrous Cycle, Pregnancy, Menopause, and Estropause Effects on USV Acoustics
Female Rat Hormone Cycle
Estrous Cycle, Pregnancy, and USVs
Alarm USVs
Adult 50 kHz USVs
Ovarian Hormone Summary
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wöhr, M.; Schwarting, R.K. Affective communication in rodents: Ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013, 354, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Sewell, G.D. Ultrasonic communication in rodents. Nature 1970, 227, 410. [Google Scholar] [CrossRef] [PubMed]
- Simola, N.; Granon, S. Ultrasonic vocalizations as a tool in studying emotional states in rodent models of social behavior and brain disease. Neuropharmacology 2019, 159, 107420. [Google Scholar] [CrossRef] [PubMed]
- Seffer, D.; Schwarting, R.K.; Wöhr, M. Pro-social ultrasonic communication in rats: Insights from playback studies. J. Neurosci. Methods 2014, 234, 73–81. [Google Scholar] [CrossRef]
- Wöhr, M.; Engelhardt, K.A.; Seffer, D.; Sungur, A.Ö.; Schwarting, R.K. Acoustic Communication in Rats: Effects of Social Experiences on Ultrasonic Vocalizations as Socio-Affective Signals. In Social Behavior from Rodents to Humans; Springer: Berlin/Heidelberg, Germany, 2015; pp. 67–89. [Google Scholar]
- Brudzynski, S.M. Ethotransmission: Communication of emotional states through ultrasonic vocalization in rats. Curr. Opin. Neurobiol. 2013, 23, 310–317. [Google Scholar] [CrossRef]
- Burgdorf, J.; Kroes, R.A.; Moskal, J.R.; Pfaus, J.G.; Brudzynski, S.M.; Panksepp, J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J. Comp. Psychol. 2008, 122, 357–367. [Google Scholar] [CrossRef]
- Portfors, C.V. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 28–34. [Google Scholar]
- Schwarting, R.; Wöhr, M. On the relationships between ultrasonic calling and anxiety-related behavior in rats. Braz. J. Med. Biol. Res. 2012, 45, 337–348. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Pharmacology of ultrasonic vocalizations in adult rats: Significance, call classification and neural substrate. Curr. Neuropharmacol. 2015, 13, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.A.; Shair, H.N. Ultrasonic vocalization, laryngeal braking, and thermogenesis in rat pups: A reappraisal. Behav. Neurosci. 1993, 107, 354–362. [Google Scholar] [CrossRef]
- Fernandez-Vargas, M.; Johnston, R.E. Ultrasonic vocalizations in golden hamsters (Mesocricetus auratus) reveal modest sex differences and nonlinear signals of sexual motivation. PLoS ONE 2015, 10, 0116789. [Google Scholar] [CrossRef]
- Warren, M.R.; Spurrier, M.S.; Roth, E.D.; Neunuebel, J.P. Sex differences in vocal communication of freely interacting adult mice depend upon behavioral context. PLoS ONE 2018, 13, 0204527. [Google Scholar] [CrossRef]
- Wright, S.L.; Brown, R.E. Sex differences in ultrasonic vocalizations and coordinated movement in the California mouse (Peromyscus californicus). Behav. Processes 2004, 65, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zala, S.M.; Reitschmidt, D.; Noll, A.; Balazs, P.; Penn, D.J. Sex-dependent modulation of ultrasonic vocalizations in house mice (Mus musculus musculus). PLoS ONE 2017, 12, 0188647. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.J.; Proville, R.; Heckman, G.J.; Azarfar, A.; Celikel, T.; Englitz, B. High-precision spatial localization of mouse vocalizations during social interaction. Sci. Rep. 2017, 7, 3017. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.M.; Perez-Pouchoulen, M.; Edwards, N.S.; McCarthy, M.M. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J. Neurosci. 2013, 33, 3276–3283. [Google Scholar] [CrossRef]
- Bowers, J.M.; Perez-Pouchoulen, M.; Roby, C.R.; Ryan, T.E.; McCarthy, M.M. Androgen modulation of Foxp1 and Foxp2 in the developing rat brain: Impact on sex specific vocalization. Endocrinology 2014, 155, 4881–4894. [Google Scholar] [CrossRef]
- Frohlich, H.; Rafiullah, R.; Schmitt, N.; Abele, S.; Rappold, G.A. Foxp1 expression is essential for sex-specific murine neonatal ultrasonic vocalization. Hum. Mol. Genet. 2017, 26, 1511–1521. [Google Scholar] [CrossRef]
- Wöhr, M.; Houx, B.; Schwarting, R.K.; Spruijt, B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol. Behav. 2008, 93, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Himmler, B.T.; Kisko, T.M.; Euston, D.R.; Kolb, B.; Pellis, S.M. Are 50-kHz calls used as play signals in the playful interactions of rats? I. Evidence from the timing and context of their use. Behav. Processes 2014, 106, 60–66. [Google Scholar] [CrossRef]
- Himmler, B.T.; Pellis, V.C.; Pellis, S.M. Peering into the dynamics of social interactions: Measuring play fighting in rats. JoVE J. Vis. Exp. 2013, 4288. [Google Scholar] [CrossRef]
- Thomas, D.A.; Takahashi, L.K.; Barfield, R.J. Analysis of ultrasonic vocalizations emitted by intruders during aggressive encounters among rats (Rattus norvegicus). J. Comp. Psychol. 1983, 97, 201. [Google Scholar] [CrossRef] [PubMed]
- Ziemka-Nalecz, M.; Jaworska, J.; Sypecka, J.; Polowy, R.; Filipkowski, R.K.; Zalewska, T. Sodium Butyrate, a Histone Deacetylase Inhibitor, Exhibits Neuroprotective/Neurogenic Effects in a Rat Model of Neonatal Hypoxia-Ischemia. Mol. Neurobiol. 2017, 54, 5300–5318. [Google Scholar] [CrossRef] [PubMed]
- Saucier, D.M.; Ehresman, C.A.; Keller, A.J.; Armstrong, E.; Elderkin, A.; Yager, J.Y. Hypoxia ischemia affects ultrasonic vocalization in the neonatal rat. Behav. Brain Res. 2008, 190, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.A.; Kelm-Nelson, C.A.; Ciucci, M.R. Changes to Ventilation, Vocalization, and Thermal Nociception in the Pink1-/- Rat Model of Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 489–504. [Google Scholar] [CrossRef]
- Kelm-Nelson, C.A.; Brauer, A.F.; Ciucci, M.R. Vocal training, levodopa, and environment effects on ultrasonic vocalizations in a rat neurotoxin model of Parkinson disease. Behav. Brain Res. 2016, 307, 54–64. [Google Scholar] [CrossRef]
- Ciucci, M.R.; Vinney, L.; Wahoske, E.J.; Connor, N.P. A translational approach to vocalization deficits and neural recovery after behavioral treatment in Parkinson disease. J. Commun. Disord. 2010, 43, 319–326. [Google Scholar] [CrossRef]
- Ciucci, M.R.; Ahrens, A.M.; Ma, S.T.; Kane, J.R.; Windham, E.B.; Woodlee, M.T.; Schallert, T. Reduction of dopamine synaptic activity: Degradation of 50-kHz ultrasonic vocalization in rats. Behav. Neurosci. 2009, 123, 328–336. [Google Scholar] [CrossRef]
- Johnson, A.M.; Grant, L.M.; Schallert, T.; Ciucci, M.R. Changes in Rat 50-kHz Ultrasonic Vocalizations During Dopamine Denervation and Aging: Relevance to Neurodegeneration. Curr. Neuropharmacol. 2015, 13, 211–219. [Google Scholar] [CrossRef]
- Johnson, A.M.; Ciucci, M.R.; Connor, N.P. Vocal training mitigates age-related changes within the vocal mechanism in old rats. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1458–1468. [Google Scholar] [CrossRef]
- Johnson, A.M.; Doll, E.J.; Grant, L.M.; Ringel, L.; Shier, J.N.; Ciucci, M.R. Targeted training of ultrasonic vocalizations in aged and Parkinsonian rats. J. Vis. Exp. 2011, 54, e2835. [Google Scholar] [CrossRef]
- Lenell, C.; Kelm-Nelson, C.A.; Ciucci, M.R.; Johnson, A.M. Chapter 36–Changes in Ultrasonic Vocalizations in Senescent Rats. In Handbook of Behavioral Neuroscience; Brudzynski, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 383–386. [Google Scholar]
- Basken, J.N.; Connor, N.P.; Ciucci, M.R. Effect of aging on ultrasonic vocalizations and laryngeal sensorimotor neurons in rats. Exp. Brain Res. 2012, 219, 351–361. [Google Scholar] [CrossRef]
- Peterson, J.R.; Watts, C.R.; Morris, J.A.; Shelton, J.M.; Cooper, B.G. Laryngeal aging and acoustic changes in male rat ultrasonic vocalizations. Dev. Psychobiol. 2013, 55, 818–828. [Google Scholar] [CrossRef]
- Simola, N. Rat Ultrasonic Vocalizations and Behavioral Neuropharmacology: From the Screening of Drugs to the Study of Disease. Curr. Neuropharmacol. 2015, 13, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M.; Gourdon, J.C.; Clarke, P.B. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: Effects of amphetamine and social context. Psychopharmacology (Berlin) 2010, 211, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Simmons, S.J.; West, M.O. Ultrasonic vocalizations as a measure of affect in preclinical models of drug abuse: A review of current findings. Curr. Neuropharmacol. 2015, 13, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, R.A.; Guthrie, D.H.; Varty, G.B. Duration of ultrasonic vocalizations in the isolated rat pup as a behavioral measure: Sensitivity to anxiolytic and antidepressant drugs. Pharmacol. Biochem. Behav. 2008, 88, 341–348. [Google Scholar] [CrossRef]
- Mu, P.; Fuchs, T.; Saal, D.B.; Sorg, B.A.; Dong, Y.; Panksepp, J. Repeated cocaine exposure induces sensitization of ultrasonic vocalization in rats. Neurosci. Lett. 2009, 453, 31–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naito, H.; Okumara, T.; Inoue, M.; Suzuki, Y. Ultrasonic vocalization response elicited in adjuvant-induced arthritic rats as a useful method for evaluating analgesic drugs. Exp. Anim. 2006, 55, 125–129. [Google Scholar] [CrossRef]
- Naito, H.; Nakamura, A.; Inoue, M.; Suzuki, Y. Effect of anxiolytic drugs on air-puff-elicited ultrasonic vocalization in adult rats. Exp. Anim. 2003, 52, 409–414. [Google Scholar] [CrossRef][Green Version]
- Wintink, A.J.; Brudzynski, S.M. The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacol. Biochem. Behav. 2001, 70, 317–323. [Google Scholar] [CrossRef]
- Simola, N.; Fenu, S.; Costa, G.; Pinna, A.; Plumitallo, A.; Morelli, M. Pharmacological characterization of 50-kHz ultrasonic vocalizations in rats: Comparison of the effects of different psychoactive drugs and relevance in drug-induced reward. Neuropharmacology 2012, 63, 224–234. [Google Scholar] [CrossRef]
- Riede, T. Peripheral Vocal Motor Dynamics and Combinatory Call Complexity of Ultrasonic Vocal Production in Rats. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 45–60. [Google Scholar]
- Riede, T. Stereotypic laryngeal and respiratory motor patterns generate different call types in rat ultrasound vocalization. J. Exp. Zool. A Ecol. Genet. Physiol. 2013, 319, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Riede, T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J. Neurophysiol. 2011, 106, 2580–2592. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, J.; Jiang, W.; Xue, Q.; Zheng, X.; Ding, M.; Agarwal, A.A.; Elemans, C.P.H. Aerodynamics and motor control of ultrasonic vocalizations for social communication in mice and rats. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kobler, J.B.; Datta, S.; Goyal, R.K.; Benecchi, E.J. Innervation of the larynx, pharynx, and upper esophageal sphincter of the rat. J. Comp. Neurol. 1994, 349, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Font, A.; Hernández-Morato, I.; McHanwell, S.; Vázquez, T.; Maranillo, E.; Sañudo, J.; Valderrama-Canales, F.J. The central projections of the laryngeal nerves in the rat. J. Anat. 2011, 219, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Van Daele, D.J.; Cassell, M.D. Multiple forebrain systems converge on motor neurons innervating the thyroarytenoid muscle. Neuroscience 2009, 162, 501–524. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, D.M.; Kelley, D.B.; Campbell, B.A. Central control of ultrasonic vocalizations in neonatal rats: I. Brain stem motor nuclei. J. Comp. Physiol. Psychol. 1980, 94, 596. [Google Scholar] [CrossRef] [PubMed]
- Kelm-Nelson, C.A.; Lenell, C.; Johnson, A.M.; Ciucci, M.R. Laryngeal Activity for Production of Ultrasonic Vocalizations in Rats. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 37–43. [Google Scholar]
- Johnson, A.M.; Ciucci, M.R.; Russell, J.A.; Hammer, M.J.; Connor, N.P. Ultrasonic output from the excised rat larynx. J. Acoust. Soc. Am. 2010, 128, 75–79. [Google Scholar] [CrossRef]
- Riede, T. Rat ultrasonic vocalization shows features of a modular behavior. J. Neurosci. 2014, 34, 6874–6878. [Google Scholar] [CrossRef] [PubMed]
- Riede, T.; Borgard, H.L.; Pasch, B. Laryngeal airway reconstruction indicates that rodent ultrasonic vocalizations are produced by an edge-tone mechanism. R. Soc. Open Sci. 2017, 4, 170976. [Google Scholar] [CrossRef] [PubMed]
- Inagi, K.; Schultz, E.; Ford, C.N. An anatomic study of the rat larynx: Establishing the rat model for neuromuscular function. Otolaryngol. Head Neck Surg. 1998, 118, 74–81. [Google Scholar] [CrossRef]
- Toya, Y.; Riabroy, N.; Davis, C.R.; Kishimoto, Y.; Tanumihardjo, S.A.; Bless, D.M.; Welham, N.V. Interspecies comparison of stellate cell-containing macula flavae and vitamin A storage in vocal fold mucosa. J. Anat. 2014, 225, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Fletcher, N.H. Rat Ultrasonic Vocalization: Short-Range Communication. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2010; Volume 19, pp. 69–76. [Google Scholar]
- Kaltwasser, M.-T. Acoustic signaling in the black rat (Rattus rattus). J. Comp. Psychol. 1990, 104, 227. [Google Scholar] [CrossRef]
- Lenell, C.; Johnson, A.M. Sexual dimorphism in laryngeal muscle fibers and ultrasonic vocalizations in the adult rat. Laryngoscope 2017, 127, 270–276. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, T.; Ralston, E.; Ludlow, C.L. Differences in neuromuscular junctions of laryngeal and limb muscles in rats. Laryngoscope 2012, 122, 1093–1098. [Google Scholar] [CrossRef]
- Lenell, C.; Johnson, A.M. The Effects of Menopause on Neuromuscular Parameters of the Rat Vocal Folds. Laryngoscope 2020. [Google Scholar] [CrossRef]
- Kim, J.M.; Shin, S.C.; Park, G.C.; Lee, J.C.; Jeon, Y.K.; Ahn, S.J.; Thibeault, S.; Lee, B.J. Effect of sex hormones on extracellular matrix of lamina propria in rat vocal fold. Laryngoscope 2020, 130, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, P.; Sepúlveda, A.; Valdivia, M.; Roa, I.; Cantín, M.; Trujillo, G.; Zavando, D.; Galdames, I.S. Variations of the Vocal Fold Epithelium in a Menopause Induced Model. Int. J. Morphol. 2011, 29, 377–381. [Google Scholar] [CrossRef]
- Tatlipinar, A.; Gunes, P.; Ozbeyli, D.; Cimen, B.; Gokceer, T. Effects of ovariectomy and estrogen replacement therapy on laryngeal tissue: A histopathological experimental animal study. Otolaryngol. Head Neck Surg. 2011, 145, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Ulkumen, B.; Ulkumen, B.A.; Batir, M.B.; Pala, H.G.; Vatansever, S.; Cam, S. Impact of pregnancy and glucocorticoid treatment on NF-κB and MUC5AC in mucosa of rat larynx. J. Voice 2019. [Google Scholar] [CrossRef] [PubMed]
- Şanal, S.K.; Biçer, Y.Ö.; Kükner, A.; Tezcan, E. Effect of pregnancy on vocal cord histology: An animal experiment. Balk. Med. J. 2016, 33, 448. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H. Chapter 32–Sex Differences in Ultrasonic Vocal Expression of Negative Emotional States in Rats. In Handbook of Behavioral Neuroscience; Brudzynski, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 337–344. [Google Scholar]
- Blanchard, R.J.; Agullana, R.; McGee, L.; Weiss, S.; Blanchard, D.C. Sex differences in the incidence and sonographic characteristics of antipredator ultrasonic cries in the laboratory rat (Rattus norvegicus). J. Comp. Psychol. 1992, 106, 270. [Google Scholar]
- Graham, L.K.; Yoon, T.; Lee, H.J.; Kim, J.J. Strain and sex differences in fear conditioning: 22 kHz ultrasonic vocalizations and freezing in rats. Psychol. Neurosci. 2009, 2, 219–225. [Google Scholar] [CrossRef]
- Willadsen, M.; Uengoer, M.; Schwarting, R.K.W.; Homberg, J.R.; Wöhr, M. Reduced emission of alarm 22-kHz ultrasonic vocalizations during fear conditioning in rats lacking the serotonin transporter. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Mallo, T.; Matrov, D.; Herm, L.; Koiv, K.; Eller, M.; Rinken, A.; Harro, J. Tickling-induced 50-kHz ultrasonic vocalization is individually stable and predicts behaviour in tests of anxiety and depression in rats. Behav. Brain Res. 2007, 184, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Mällo, T.; Matrov, D.; Kõiv, K.; Harro, J. Effect of chronic stress on behavior and cerebral oxidative metabolism in rats with high or low positive affect. Neuroscience 2009, 164, 963–974. [Google Scholar] [CrossRef]
- Kosten, T.A.; Lee, H.J.; Kim, J.J. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006, 1087, 142–150. [Google Scholar] [CrossRef]
- Dimatelis, J.J.; Stein, D.J.; Russell, V.A. Behavioral changes after maternal separation are reversed by chronic constant light treatment. Brain Res. 2012, 1480, 61–71. [Google Scholar] [CrossRef]
- Dimatelis, J.J.; Vermeulen, I.M.; Bugarith, K.; Stein, D.J.; Russell, V.A. Female rats are resistant to developing the depressive phenotype induced by maternal separation stress. Metab. Brain Dis. 2016, 31, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Willadsen, M.; Kisko, T.M.; Schwarting, R.K.; Fendt, M. Sex-dependent effects of Cacna1c haploinsufficiency on behavioral inhibition evoked by conspecific alarm signals in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109849. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Mori, Y. The emission of stress-induced 22-kHz calls in female rats is independent of testosterone levels. Horm. Behav. 2015, 69, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Mori, Y. Relationship between 22-kHz calls and testosterone in male rats. Horm. Behav. 2014, 65, 42–46. [Google Scholar] [CrossRef]
- Barfield, R.J.; Auerbach, P.; Geyer, L.A.; Mcintosh, T.K. Ultrasonic Vocalizations in Rat Sexual-Behavior. Am. Zool. 1979, 19, 469–480. [Google Scholar] [CrossRef]
- Barfield, R.J.; Geyer, L.A. The ultrasonic postejaculatory vocalization and the postejaculatory refractory period of the male rat. J. Comp. Physiol. Psychol. 1975, 88, 723–734. [Google Scholar] [CrossRef]
- Miczek, K.A.; Van Der Poel, A.M. Long ultrasonic calls in male rats following mating, defeat and aversive stimulation: Frequency modulation and bout structure. Behaviour 1991, 119, 127–142. [Google Scholar] [CrossRef]
- Barfield, R.J.; Geyer, L.A. Sexual behavior: Ultrasonic postejaculatory song of the male rat. Science 1972, 176, 1349–1350. [Google Scholar] [CrossRef]
- Delius, J.D. Irrelevant behaviour, information processing and arousal homeostasis. Psychol. Forsch. 1970, 33, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Kehoe, P.; Callahan, M. Sonographic structure of isolation-induced ultrasonic calls of rat pups. Dev. Psychobiol. 1999, 34, 195–204. [Google Scholar] [CrossRef]
- Stark, R.A.; Harker, A.; Salamanca, S.; Pellis, S.M.; Li, F.; Gibb, R.L. Development of ultrasonic calls in rat pups follows similar patterns regardless of isolation distress. Dev. Psychobiol. 2020, 62, 617–630. [Google Scholar] [CrossRef]
- Boulanger-Bertolus, J.; Rincón-Cortés, M.; Sullivan, R.M.; Mouly, A.-M. Understanding pup affective state through ethologically significant ultrasonic vocalization frequency. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Gardner, C.R. Distress vocalization in rat pups. A simple screening method for anxiolytic drugs. J. Pharmacol. Methods 1985, 14, 181–187. [Google Scholar] [CrossRef]
- Insel, T.R.; Hill, J.L.; Mayor, R.B. Rat pup ultrasonic isolation calls: Possible mediation by the benzodiazepine receptor complex. Pharm. Biochem. Behav. 1986, 24, 1263–1267. [Google Scholar] [CrossRef]
- Branchi, I.; Santucci, D.; Alleva, E. Ultrasonic vocalisation emitted by infant rodents: A tool for assessment of neurobehavioural development. Behav. Brain Res. 2001, 125, 49–56. [Google Scholar] [CrossRef]
- Brunelli, S.A. Selective breeding for an infant phenotype: Rat pup ultrasonic vocalization (USV). Behav. Genet. 2005, 35, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.A.; Shair, H.N.; Masmela, J.R.; Brunelli, S.A. Developmental effects of selective breeding for an infantile trait: The rat pup ultrasonic isolation call. Dev. Psychobiol. 2001, 39, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Tonoue, T. Sex difference in ultrasound distress call by rat pups. Behav. Brain Res. 1987, 25, 13–21. [Google Scholar] [CrossRef]
- Manduca, A.; Servadio, M.; Melancia, F.; Schiavi, S.; Manzoni, O.J.; Trezza, V. Sex-specific behavioural deficits induced at early life by prenatal exposure to the cannabinoid receptor agonist WIN55, 212-2 depend on mGlu5 receptor signalling. Br. J. Pharmacol. 2020, 177, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Shahrier, M.A.; Wada, H. Effects of prenatal ethanol exposure on acoustic characteristics of play fighting-induced ultrasonic vocalizations in juvenile rats. Neurotoxicology 2020, 79, 25–39. [Google Scholar] [CrossRef]
- Bird, C.W.; Barto, D.; Magcalas, C.M.; Rodriguez, C.I.; Donaldson, T.; Davies, S.; Savage, D.D.; Hamilton, D.A. Ifenprodil infusion in agranular insular cortex alters social behavior and vocalizations in rats exposed to moderate levels of ethanol during prenatal development. Behav. Brain Res. 2017, 320, 1–11. [Google Scholar] [CrossRef]
- Waddell, J.; Yang, T.; Ho, E.; Wellmann, K.A.; Mooney, S.M. Prenatal Ethanol Exposure and Whisker Clipping Disrupt Ultrasonic Vocalizations and Play Behavior in Adolescent Rats. Brain Sci. 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, K.; Lewis, B.; Barron, S. Agmatine reduces ultrasonic vocalization deficits in female rat pups exposed neonatally to ethanol. Neurotoxicol. Teratol. 2010, 32, 158–163. [Google Scholar] [CrossRef]
- Brasser, S.M.; Spear, N.E. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav. Neurosci. 2002, 116, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Thakore, N.; Bell, R.L.; Maddox, W.T.; Schallert, T.; Duvauchelle, C.L. Sex-specific ultrasonic vocalization patterns and alcohol consumption in high alcohol-drinking (HAD-1) rats. Physiol. Behav. 2019, 203, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Doncheck, E.M.; Liddiard, G.T.; Konrath, C.D.; Liu, X.; Yu, L.; Urbanik, L.A.; Herbst, M.R.; DeBaker, M.C.; Raddatz, N.; Van Newenhizen, E.C.; et al. Sex, stress, and prefrontal cortex: Influence of biological sex on stress-promoted cocaine seeking. Neuropsychopharmacology 2020, 45, 1974–1985. [Google Scholar] [CrossRef]
- Cox, E.T.; Hodge, C.W.; Sheikh, M.J.; Abramowitz, A.C.; Jones, G.F.; Jamieson-Drake, A.W.; Makam, P.R.; Zeskind, P.S.; Johns, J.M. Delayed developmental changes in neonatal vocalizations correlates with variations in ventral medial hypothalamus and central amygdala development in the rodent infant: Effects of prenatal cocaine. Behav. Brain Res. 2012, 235, 166–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campbell, J.O.; Fogarty, J.A.; Spear, L.P. Inhibition of nitric oxide synthesis with L-NAME suppresses isolation-induced ultrasounds in rat pups. Pharmacol. Biochem. Behav. 1999, 63, 45–53. [Google Scholar] [CrossRef]
- Shepherd, J.K.; Blanchard, D.C.; Weiss, S.M.; Rodgers, R.J.; Blanchard, R.J. Morphine attenuates antipredator ultrasonic vocalizations in mixed-sex rat colonies. Pharmacol. Biochem. Behav. 1992, 41, 551–558. [Google Scholar] [CrossRef]
- Vassoler, F.M.; Oranges, M.L.; Toorie, A.M.; Byrnes, E.M. Oxycodone self-administration during pregnancy disrupts the maternal-infant dyad and decreases midbrain OPRM1 expression during early postnatal development in rats. Pharmacol. Biochem. Behav. 2018, 173, 74–83. [Google Scholar] [CrossRef]
- Houwing, D.J.; Staal, L.; Swart, J.M.; Ramsteijn, A.S.; Wöhr, M.; de Boer, S.F.; Olivier, J.D.A. Subjecting Dams to Early Life Stress and Perinatal Fluoxetine Treatment Differentially Alters Social Behavior in Young and Adult Rat Offspring. Front. Neurosci. 2019, 13, 229. [Google Scholar] [CrossRef]
- Naito, H.; Inoue, M.; Makino, J. Ultrasonic isolation calls in genetically high- and low-emotional rat pups. Exp. Anim. 2000, 49, 289–294. [Google Scholar] [CrossRef][Green Version]
- Bekkedal, M.Y.; Rossi, J., 3rd; Panksepp, J. Fetal and neonatal exposure to trimethylolpropane phosphate alters rat social behavior and emotional responsivity. Neurotoxicol. Teratol. 1999, 21, 435–443. [Google Scholar] [CrossRef]
- Berg, E.L.; Copping, N.A.; Rivera, J.K.; Pride, M.C.; Careaga, M.; Bauman, M.D.; Berman, R.F.; Lein, P.J.; Harony-Nicolas, H.; Buxbaum, J.D.; et al. Developmental social communication deficits in the Shank3 rat model of phelan-mcdermid syndrome and autism spectrum disorder. Autism Res. 2018, 11, 587–601. [Google Scholar] [CrossRef]
- Umeda, T.; Takashima, N.; Nakagawa, R.; Maekawa, M.; Ikegami, S.; Yoshikawa, T.; Kobayashi, K.; Okanoya, K.; Inokuchi, K.; Osumi, N. Evaluation of Pax6 mutant rat as a model for autism. PLoS One 2010, 5, e15500. [Google Scholar] [CrossRef]
- Gzielo, K.; Potasiewicz, A.; Holuj, M.; Litwa, E.; Popik, P.; Nikiforuk, A. Valproic acid exposure impairs ultrasonic communication in infant, adolescent and adult rats. Eur. Neuropsychopharmacol. 2020, 41, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Morales-Navas, M.; Castano-Castano, S.; Perez-Fernandez, C.; Sanchez-Gil, A.; Teresa Colomina, M.; Leinekugel, X.; Sanchez-Santed, F. Similarities between the Effects of Prenatal Chlorpyrifos and Valproic Acid on Ultrasonic Vocalization in Infant Wistar Rats. Int. J. Environ. Res. Public Health 2020, 17, 6376. [Google Scholar] [CrossRef]
- Potasiewicz, A.; Gzielo, K.; Popik, P.; Nikiforuk, A. Effects of prenatal exposure to valproic acid or poly(I:C) on ultrasonic vocalizations in rat pups: The role of social cues. Physiol. Behav. 2020, 225, 113113. [Google Scholar] [CrossRef] [PubMed]
- Kisko, T.M.; Schwarting, R.K.W.; Wöhr, M. Sex differences in the acoustic features of social play-induced 50-kHz ultrasonic vocalizations: A detailed spectrographic analysis in wild-type Sprague-Dawley and Cacna1c haploinsufficient rats. Dev. Psychobiol. 2021, 63, 262–276. [Google Scholar] [CrossRef]
- Kisko, T.M.; Braun, M.D.; Michels, S.; Witt, S.H.; Rietschel, M.; Culmsee, C.; Schwarting, R.K.W.; Wöhr, M. Sex-dependent effects of Cacna1c haploinsufficiency on juvenile social play behavior and pro-social 50-kHz ultrasonic communication in rats. Genes Brain Behav. 2020, 19, 12552. [Google Scholar] [CrossRef]
- Potasiewicz, A.; Holuj, M.; Litwa, E.; Gzielo, K.; Socha, L.; Popik, P.; Nikiforuk, A. Social dysfunction in the neurodevelopmental model of schizophrenia in male and female rats: Behavioural and biochemical studies. Neuropharmacology 2020, 170, 108040. [Google Scholar] [CrossRef]
- Potasiewicz, A.; Holuj, M.; Piotrowska, D.; Zajda, K.; Wojcik, M.; Popik, P.; Nikiforuk, A. Evaluation of ultrasonic vocalizations in a neurodevelopmental model of schizophrenia during the early life stages of rats. Neuropharmacology 2019, 146, 28–38. [Google Scholar] [CrossRef]
- Paul, M.J.; Peters, N.V.; Holder, M.K.; Kim, A.M.; Whylings, J.; Terranova, J.I.; de Vries, G.J. Atypical Social Development in Vasopressin-Deficient Brattleboro Rats. eNeuro 2016, 3. [Google Scholar] [CrossRef]
- Schatz, K.C.; Kyne, R.F.; Parmeter, S.L.; Paul, M.J. Investigation of social, affective, and locomotor behavior of adolescent Brattleboro rats reveals a link between vasopressin’s actions on arousal and social behavior. Horm. Behav. 2018, 106, 1–9. [Google Scholar] [CrossRef]
- Marquis, J.M.; Lettenberger, S.E.; Kelm-Nelson, C.A. Early-onset Parkinsonian behaviors in female Pink1–/– rats. Behav. Brain Res. 2020, 377, 112175. [Google Scholar] [CrossRef]
- Lopez-Meraz, M.L.; Medel-Matus, J.S.; Morgado-Valle, C.; Beltran-Parrazal, L.; Perez-Estudillo, C.; Manzo, J. Effect of lithium-pilocarpine-induced status epilepticus on ultrasonic vocalizations in the infant rat pup. Epilepsy Behav. 2014, 31, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, T.B.; Chaves-Kirsten, G.P.; Chaible, L.M.; Silva, A.C.; Martins, D.O.; Britto, L.R.; Dagli, M.L.; Torrao, A.S.; Palermo-Neto, J.; Bernardi, M.M. Hypoactivity of the central dopaminergic system and autistic-like behavior induced by a single early prenatal exposure to lipopolysaccharide. J. Neurosci. Res. 2012, 90, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Mittal, N.; Thompson, L.M.; Rodriguez-Santiago, M.; Duvauchelle, C.L.; Crews, D.; Gore, A.C. Effects of the Endocrine-Disrupting Chemicals, Vinclozolin and Polychlorinated Biphenyls, on Physiological and Sociosexual Phenotypes in F2 Generation Sprague-Dawley Rats. Environ. Health Perspect. 2018, 126, 97005. [Google Scholar] [CrossRef] [PubMed]
- Topper, V.Y.; Reilly, M.P.; Wagner, L.M.; Thompson, L.M.; Gillette, R.; Crews, D.; Gore, A.C. Social and neuromolecular phenotypes are programmed by prenatal exposures to endocrine-disrupting chemicals. Mol. Cell. Endocrinol. 2019, 479, 133–146. [Google Scholar] [CrossRef]
- Bell, M.R.; Thompson, L.M.; Rodriguez, K.; Gore, A.C. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Horm. Behav. 2016, 78, 168–177. [Google Scholar] [CrossRef]
- Abuaish, S.; Tse, E.K.; McGowan, P.O. Perinatal high-fat diet impairs pup retrieval and induces sex-specific changes in ultrasonic vocalization characteristics of rat pups. Dev. Psychobiol. 2020, 62, 436–445. [Google Scholar] [CrossRef]
- Kosten, T.A.; Miserendino, M.J.; Bombace, J.C.; Lee, H.J.; Kim, J.J. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav. Brain Res. 2005, 157, 235–244. [Google Scholar] [CrossRef]
- Kalinichev, M.; Easterling, K.W.; Plotsky, P.M.; Holtzman, S.G. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol. Biochem. Behav. 2002, 73, 131–140. [Google Scholar] [CrossRef]
- Keller, A.; Saucier, D.; Sheerin, A.; Yager, J. Febrile convulsions affect ultrasonic vocalizations in the rat pup. Epilepsy Behav. 2004, 5, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, M.S.; Stolba, M.A. Thermogenesis, myoclonic twitching, and ultrasonic vocalization in neonatal rats during moderate and extreme cold exposure. Behav. Neurosci. 1996, 110, 305–314. [Google Scholar] [CrossRef]
- Williams, M.T.; Hennessy, M.B.; Davis, H.N. Stress during pregnancy alters rat offspring morphology and ultrasonic vocalizations. Physiol. Behav. 1998, 63, 337–343. [Google Scholar] [CrossRef]
- Williams, M.T.; Hennessy, M.B.; Davis, H.N. CRF administered to pregnant rats alters offspring behavior and morphology. Pharmacol. Biochem. Behav. 1995, 52, 161–167. [Google Scholar] [CrossRef]
- Knutson, B.; Burgdorf, J.; Panksepp, J. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 2002, 128, 961. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Holland, G. Acoustic characteristics of air puff-induced 22-kHz alarm calls in direct recordings. Neurosci. Biobehav. Rev. 2005, 29, 1169–1180. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Principles of rat communication: Quantitative parameters of ultrasonic calls in rats. Behav. Genet. 2005, 35, 85–92. [Google Scholar] [CrossRef]
- Simola, N.; Costa, G. Emission of categorized 50-kHz ultrasonic vocalizations in rats repeatedly treated with amphetamine or apomorphine: Possible relevance to drug-induced modifications in the emotional state. Behav. Brain Res. 2018, 347, 88–98. [Google Scholar] [CrossRef]
- Coffey, K.R.; Marx, R.G.; Neumaier, J.F. DeepSqueak: A deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 2019, 44, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Campolongo, P.; Palmery, M.; Vanderschuren, L.J.; Cuomo, V.; Trezza, V. Social play behavior, ultrasonic vocalizations and their modulation by morphine and amphetamine in Wistar and Sprague-Dawley rats. Psychopharmacology (Berlin) 2014, 231, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Knutson, B.; Burgdorf, J.; Panksepp, J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J. Comp. Psychol. 1998, 112, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, S.A.; Nie, R.; Whipple, C.; Winiger, V.; Hofer, M.A.; Zimmerberg, B. The effects of selective breeding for infant ultrasonic vocalizations on play behavior in juvenile rats. Physiol. Behav. 2006, 87, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Pellis, S.M.; Pellis, V.C. The prejuvenile onset of play fighting in laboratory rats (Rattus norvegicus). Dev. Psychobiol. 1997, 31, 193–205. [Google Scholar] [CrossRef]
- Kisko, T.M.; Euston, D.R.; Pellis, S.M. Are 50-khz calls used as play signals in the playful interactions of rats? III. The effects of devocalization on play with unfamiliar partners as juveniles and as adults. Behav. Processes 2015, 113, 113–121. [Google Scholar] [CrossRef]
- Willadsen, M.; Seffer, D.; Schwarting, R.K.; Wöhr, M. Rodent ultrasonic communication: Male prosocial 50-kHz ultrasonic vocalizations elicit social approach behavior in female rats (Rattus norvegicus). J. Comp. Psychol. 2014, 128, 56–64. [Google Scholar] [CrossRef]
- Barfield, R.J.; Thomas, D.A. The role of ultrasonic vocalizations in the regulation of reproduction in rats. Ann. N. Y. Acad. Sci. 1986, 474, 33–43. [Google Scholar] [CrossRef]
- Snoeren, E.M.; Agmo, A. The incentive value of males’ 50-kHz ultrasonic vocalizations for female rats (Rattus norvegicus). J. Comp. Psychol. 2014, 128, 40–55. [Google Scholar] [CrossRef]
- Gerson, C.A.; Mac Cionnaith, C.E.; Quintana, G.R.; Pfaus, J.G. Effects of ovarian hormones on the emission of 50-kHz ultrasonic vocalizations during distributed clitoral stimulation in the rat. Horm. Behav. 2019, 109, 1–9. [Google Scholar] [CrossRef]
- Snoeren, E.M.; Agmo, A. Female ultrasonic vocalizations have no incentive value for male rats. Behav. Neurosci. 2013, 127, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Agmo, A.; Snoeren, E.M. Silent or Vocalizing Rats Copulate in a Similar Manner. PLoS ONE 2015, 10, 0144164. [Google Scholar] [CrossRef] [PubMed]
- Snoeren, E.M.; Helander, L.R.; Iversen, E.E.; Agmo, A. On the role of individual differences in female odor and ultrasonic vocalizations for male’s choice of partner. Physiol. Behav. 2014, 132, 17–23. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, M.Y.; Vakulenko, M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiol. Behav. 2003, 80, 81–88. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Barfield, R.J.; Geyer, L.A. Ultrasonic vocalisations facilitate sexual behaviour of female rats. Nature 1978, 272, 163–164. [Google Scholar] [CrossRef]
- Lenell, C.; Johnson, A.M. The effects of the estrous cycle, menopause, and recording condition on female rat ultrasonic vocalizations. Physiol. Behav. 2021, 229, 113248. [Google Scholar] [CrossRef]
- Börner, A.; Hjemdahl, R.; Götz, T.; Brown, G.R. Ultrasonic vocalizations of female Norway rats (Rattus norvegicus) in response to social partners. J. Comp. Psychol. 2016, 130, 76. [Google Scholar] [CrossRef]
- Inagaki, H.; Kuwahara, M.; Tsubone, H.; Mori, Y. The effect of post-weaning individual housing on 50-kHz calls emitted from male rats to sexually receptive female rats. Physiol. Behav. 2013, 110–111, 30–33. [Google Scholar] [CrossRef]
- Johnson, A.M. Social isolation alters ultrasonic vocalizations but not thyroarytenoid neuromuscular junctions in old rats. Laryngoscope 2019, 129, 9–14. [Google Scholar] [CrossRef]
- Shembel, A.C.; Lenell, C.; Chen, S.; Johnson, A.M. Effects of Vocal Training on Thyroarytenoid Muscle Neuromuscular Junctions and Myofibers in Young and Older Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 244–252. [Google Scholar] [CrossRef]
- McMullen, C.A.; Andrade, F.H. Functional and morphological evidence of age-related denervation in rat laryngeal muscles. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Connor, N.P.; Suzuki, T.; Lee, K.; Sewall, G.K.; Heisey, D.M. Neuromuscular junction changes in aged rat thyroarytenoid muscle. Ann. Otol. Rhinol. Laryngol. 2002, 111, 579–586. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tanaka, S.; Tsubone, H.; Atoji, Y.; Suzuki, Y. Age-related changes in sensory and secretomotor nerve endings in the larynx of F344/N rat. Arch. Gerontol. Geriatr. 2003, 36, 173–183. [Google Scholar] [CrossRef]
- McMullen, C.A.; Andrade, F.H. Contractile dysfunction and altered metabolic profile of the aging rat thyroarytenoid muscle. J. Appl. Physiol. 2006, 100, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Taguchi, A.; Motoyoshi, K.; Hyodo, M.; Gyo, K.; Desaki, J. Age-related changes in rat intrinsic laryngeal muscles: Analysis of muscle fibers, muscle fiber proteins, and subneural apparatuses. Eur. Arch. Otorhinolaryngol. 2013, 270, 975–984. [Google Scholar] [CrossRef]
- Inagaki, H.; Sato, J. Air puff-induced 22-kHz calls in F344 rats. Physiol. Behav. 2016, 155, 237–241. [Google Scholar] [CrossRef]
- Haney, M.; Miczek, K.A. Ultrasounds during agonistic interactions between female rats (Rattus norvegicus). J. Comp. Psychol. 1993, 107, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Hoper, B.R.; Vargo, T.M.; Yen, S.S.C. Chronological changes in sex steroid, gonadotropin and prolactin secretion in aging female rats displaying diferent reproductive states. Biol. Reprod. 1979, 21, 193–203. [Google Scholar] [CrossRef]
- Long, J.A.; Evans, H.M. The Oestrous Cycle in the Rat and Its Associated Phenomena; University of California Press: Barkeley, CA, USA, 1922; p. 328. [Google Scholar]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef]
- Berkley, K.J.; McAllister, S.L.; Accius, B.E.; Winnard, K.P. Endometriosis-induced vaginal hyperalgesia in the rat: Effect of estropause, ovariectomy, and estradiol replacement. Pain 2007, 132 (Suppl. 1), 150–159. [Google Scholar] [CrossRef]
- Kalinichev, M.; Easterling, K.W.; Holtzman, S.G. Periodic postpartum separation from the offspring results in long-lasting changes in anxiety-related behaviors and sensitivity to morphine in Long-Evans mother rats. Psychopharmacology (Berlin) 2000, 152, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Mychasiuk, R.; Schmold, N.; Ilnytskyy, S.; Kovalchuk, O.; Kolb, B.; Gibb, R. Prenatal bystander stress alters brain, behavior, and the epigenome of developing rat offspring. Dev. Neurosci. 2011, 33, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Matochik, J.A.; White, N.R.; Barfield, R.J. Variations in scent marking and ultrasonic vocalizations by Long-Evans rats across the estrous cycle. Physiol. Behav. 1992, 51, 783–786. [Google Scholar] [CrossRef]
- Thomas, D.A.; Barfield, R.J. Ultrasonic vocalization of the female rat (Rattus norvegicus) during mating. Anim. Behav. 1985, 33, 720–725. [Google Scholar] [CrossRef]
- Matochik, J.A.; Barfield, R.J.; Nyby, J. Regulation of sociosexual communication in female Long-Evans rats by ovarian hormones. Horm. Behav. 1992, 26, 545–555. [Google Scholar] [CrossRef]
- Garcia, A.N.; Bezner, K.; Depena, C.; Yin, W.; Gore, A.C. The effects of long-term estradiol treatment on social behavior and gene expression in adult female rats. Horm. Behav. 2017, 87, 145–154. [Google Scholar] [CrossRef]


| Model | Sub Model | Age | Strain | Recording Duration | Major Sex Difference(s) in USV Acoustics |
|---|---|---|---|---|---|
| Drug exposure | Prenatal cannabis | P10 | Wistar | 15 s | Male pups produced fewer distress USVs during isolation, whereas females did not [95]. |
| Prenatal alcohol | P40–42 | Wistar | 10 min | For males, high ethanol exposure resulted in more 22 kHz and fewer 50 kHz USVs during play, whereas ethanol exposure did not affect female USV production during play [96]. | |
| ~P38–P48 | LE | 12 min | Prenatal exposure to alcohol decreased the mean frequency and total duration of 50 kHz USVs during same-sex social interaction for male rats, but not female rats [97]. | ||
| P28 P42 | LE | 10 min | At P28, during play female control whisker clipped rats produced more 22 kHz USVs than other female groups. At P42, during play male rats overall had more 50 kHz USVs than female rats [98]. | ||
| Postnatal alcohol | P15 | SD | 6 min | Neonatal alcohol exposure significantly reduced distress USV rate for both sexes and significantly increased USV latency in female pups. Agmatine reduced these deficits, in female but not male pups [99]. | |
| P25, P35, P110–P120 | SD | 45 min | Adult alcohol-treated males produced more 22 kHz USVs following initial handling which was suppressed with the startle stimulus than male rats receiving water. Alcohol did not affect female 22 kHz USV rate. Overall, male rats had a greater number of 22 kHz USVs in response to startle stimulus [100]. | ||
| ~2–5 mo. | NS | 4 h | Female rats produced more 50 kHz USVs than male rats during experimental conditions. EtOH males produce 50 kHz USVs with a higher mean frequency and greater power than EtOH females. EtOH males produced 22 kHz USVs with a lower mean frequency, reduced bandwidth, and longer duration than EtOH females [101]. | ||
| Cocaine | P90 | SD | 15 min | During foot shock procedure males had a dramatic increase in 22 kHz USVs and decrease in 50 kHz USVs. Male rats also had longer duration of 22 kHz USVs [102]. | |
| P1, P14, P21 | SD | 5 min | At P1, both male and female pups with prenatal cocaine exposure (PCE) produced fewer distress USVs than saline or untreated pups and male pups with PCE produced fewer USVs with at least one observable harmonic than male saline or untreated pups. At P21, male PCE rats produced more USVs with longer overall total duration of USVs than female PCE rats [103]. | ||
| P10, P11 | SD | 5 min | Male pups produced more distress USVs than female pups during the final 2 min of a 5 min isolation test [104]. | ||
| Morphine | P130–P288 | LE | 45 min | In the presence of a cat, both male and female rats produced fewer 22 kHz USVs when exposed to morphine. Additionally, both control and morphine females produced significantly more 22 kHz USVs with longer total duration than male counterparts [105]. | |
| Oxycodone | P3 P6 P9 P12 | SD | 3 min | Isolation distress USVs peaked in production rate at P9 for males and P6 and P9 for females [106]. | |
| Fluoxetine | P6 | SERT | 3 min | Fluoxetine reduced the total duration of distress USVs for male pups but did not affect female USV total duration [107]. | |
| Diazepam | P3–P18 | Wistar | 3 min | Overall, male pups in all experimental conditions produced more distress USVs than females [108]. | |
| Trimethylolpropane phosphate (TMPP) | P8, P14 | LE | 1 min | Males with prenatal TMPP treatment produced more distress USVs than control males, control females, and TMPP females [109]. | |
| Neurological disorder models | Shank3 deficiency | P7 | Shank3 | 3 min | Fewer distress USVs were observed in male Shank3 −/− pups but not females [110]. |
| Pax6 | P7 | rSey2/+ | 5 min | Female rSey2/+ rat pups produce fewer distress USV from wild-type female pups, which was not observed in male rat pups [111]. | |
| Valproic acid | P9 P31–P32 P65–P70 | SD | 5 min 10 min | In general, female rats had shorter duration of 50 kHz USVs during isolation, same-sex play, and same-sex social interaction than male rats. Female rats also had fewer 50 kHz USVs in same-sex social interaction [112]. | |
| Valproic acid, chlorpyrifos | P7 | Wistar | 3 min | In isolation, male pups produced more distress USVs [113]. | |
| Valproic acid, poly(I:C) | P6 | SD | 3 min | In the poly(I:C) condition, male pups produced more distress USVs than females [114]. | |
| Cacna1c | P32–P34 | Cacna1c | 5 min | For control animals, female rats produced fewer overall 50 kHz USVs during same-sex play, with fewer step USVs and more trill USVs, than males. Female rat USVs also had a higher peak frequency. For experimental animals, female rats produced a similar rate of 50 kHz USVs during play as male control animals, whereas experimental male animals had reduced 50 kHz USV production during play [115,116]. | |
| MAM | P60 | SD | 10 min | During same-sex social interaction, MAM exposure decreased the total number of 50 kHz USVs and increased the percentage of short USVs and decreased the percentage of frequency-modulated USVs for both sexes. However, control females had fewer frequency modulated USVs than control males, whereas it was opposite for MAM groups [117]. | |
| P8 P30 P31–P32 | SD | 3 min 3 min 10 min | At P8, males pups produced distress USVs with a lower frequency and reduced bandwidth than females. At P30, males produced tickle-induced 50 kHz USVs with a higher center frequency than females. At P31-P32, during same-sex play, males produced more USVs with greater bandwidth than females [118]. | ||
| AVP | P34 P44 | Brattleboro | 10 min | Males produced more trill 50 kHz USVs during same-sex play than females [119]. | |
| P34–37 | Brattleboro | 10 min | Males produced more 50 kHz USVs than females during same-sex play [120]. | ||
| PD | 2–8 mo | Pink1-/- | 90 s | Pink-/- female rats did not have as many 50 kHz USV deficits as Pink1-/- male rats in a mating context [121]. | |
| SE | P15 P16 P21 | Wistar | 5 min | SE male pups had a decrease in USV latency than control pups, which was not observed in female pups [122]. | |
| Liposaccharide (LPS) | P11 | Wistar | 5 min | Prenatal LPS exposure caused male pups to produce fewer distress USVs, but this was not observed with female pups [123]. | |
| Ischemic brain injury | P12 | Wistar | 3 min | Overall, ischemic pups produced fewer distress USVs than control pups with male ischemic pups experiencing greater reductions in USV call subcategories than female ischemic pups [25]. | |
| Endocrine disruption | A1221 VIN | P80–P100 d | SD | 5 min | In a mating paradigm, VIN males produced fewer 50 kHz USVs than control males. A1221 produced 50 kHz USVs with reduced power, bandwidth, and lower frequency. Experimental female USVs were unaffected [124]. |
| A1221 estradiol | P60 | SD | 10 min | For female rats, estradiol treatment decreased the number of step 50 kHz USVs following opposite-sex exposure. For male rats, A1221 treatment increased the number of rise and step 50 kHz USVs following opposite-sex exposure [125]. | |
| A1221 | P30–39 | SD | 5 min–4 h | PBCs affected the number of 50 kHz USVs for female rats but not male rats during same-sex play [126]. | |
| Diet and environmental stressors | High-fat diet | P7 P13 | LE | 10 min | Female pups on the high-fat diet produced more 1-sweep distress USVs, whereas male pups on the high-fat diet produced more 2-sweep distress USVs when compared to sex-matched controls [127]. |
| Maternal separation | P60 | SD | 15 min, 3 h | Brief maternal separation in pups resulted in changes in 22 kHz USVs in adulthood with fewer 22 kHz USVs in females but not in males, when compared to controls [75]. | |
| P70–P90 | SD | NS | After prenatal isolation, adult male rats produced 22 kHz USVs with greater duration compared to female rats [128]. | ||
| P120 | LE | 17 min | Maternal separation resulted in males producing more 22 kHz USVs in response to startle stimulus but did not affect female startle-induced 22 kHz USVs [129]. | ||
| Heat-induced convulsions | P10, P12 | LE | 2 min | Males produced more distress USVs (more category 5 and 6 USVs) compared to females [130]. | |
| Moderate and extreme cold | P7–8 | SD | 70 min | Male pups produced more distress USVs than female pups; both sexes increased distress USVs in the presence of extreme cold temperature [131]. | |
| Heat, light, and restraint stressors | P1 P6 P10 P14 | SD | 6 min | At P6, males produced more distress USVs than females during the first 2 min following maternal separation [132]. | |
| corticotropin-releasing factor (CRF) | P6 P10 P14 | SD | 6 min | Overall, male pups produced more distress USVs than females [133]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenell, C.; Broadfoot, C.K.; Schaen-Heacock, N.E.; Ciucci, M.R. Biological and Acoustic Sex Differences in Rat Ultrasonic Vocalization. Brain Sci. 2021, 11, 459. https://doi.org/10.3390/brainsci11040459
Lenell C, Broadfoot CK, Schaen-Heacock NE, Ciucci MR. Biological and Acoustic Sex Differences in Rat Ultrasonic Vocalization. Brain Sciences. 2021; 11(4):459. https://doi.org/10.3390/brainsci11040459
Chicago/Turabian StyleLenell, Charles, Courtney K. Broadfoot, Nicole E. Schaen-Heacock, and Michelle R. Ciucci. 2021. "Biological and Acoustic Sex Differences in Rat Ultrasonic Vocalization" Brain Sciences 11, no. 4: 459. https://doi.org/10.3390/brainsci11040459
APA StyleLenell, C., Broadfoot, C. K., Schaen-Heacock, N. E., & Ciucci, M. R. (2021). Biological and Acoustic Sex Differences in Rat Ultrasonic Vocalization. Brain Sciences, 11(4), 459. https://doi.org/10.3390/brainsci11040459






