The Effects of Functionally Guided, Connectivity-Based rTMS on Amygdala Activation
Abstract
1. Introduction
2. Materials and Methods
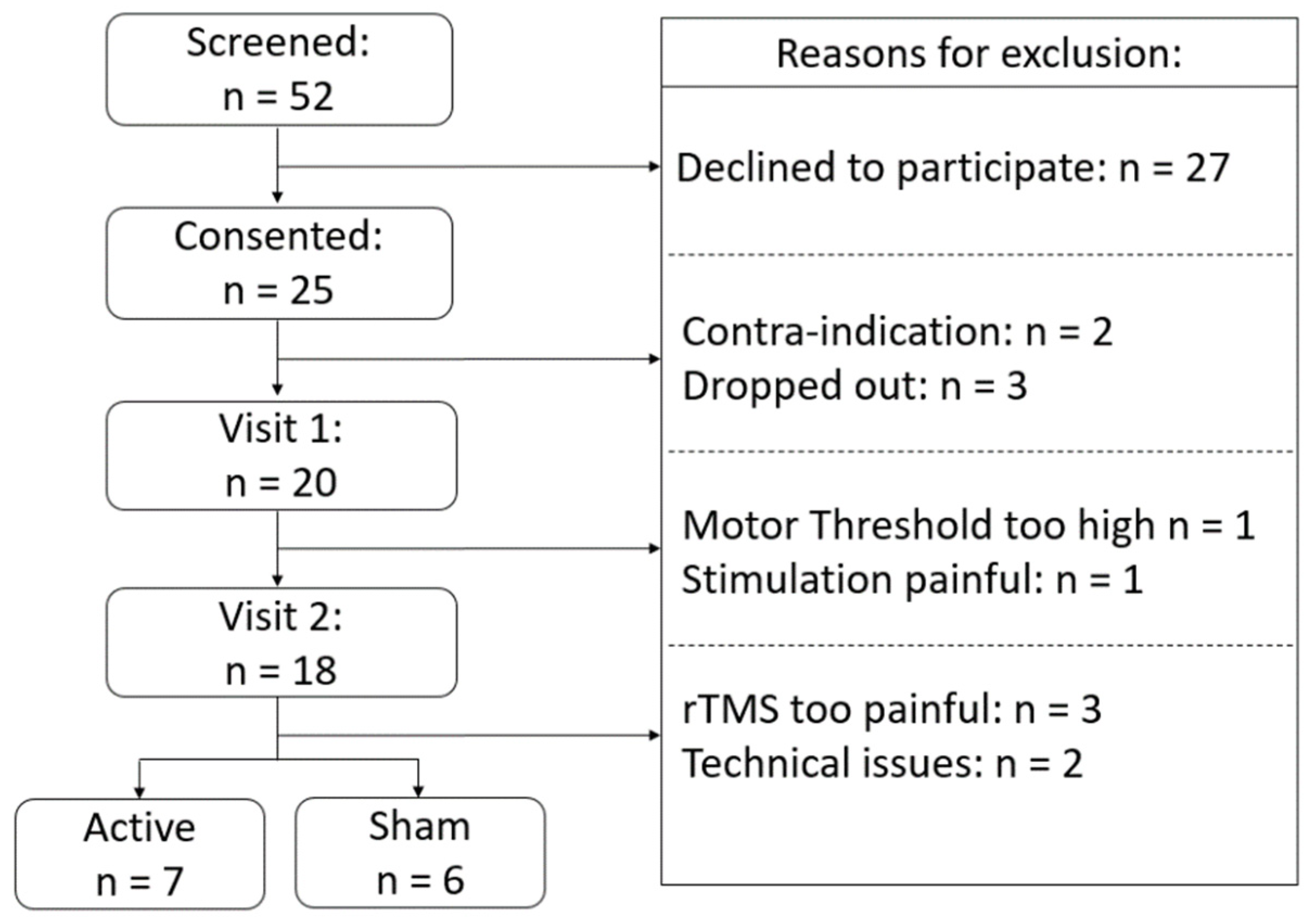
2.1. Participants
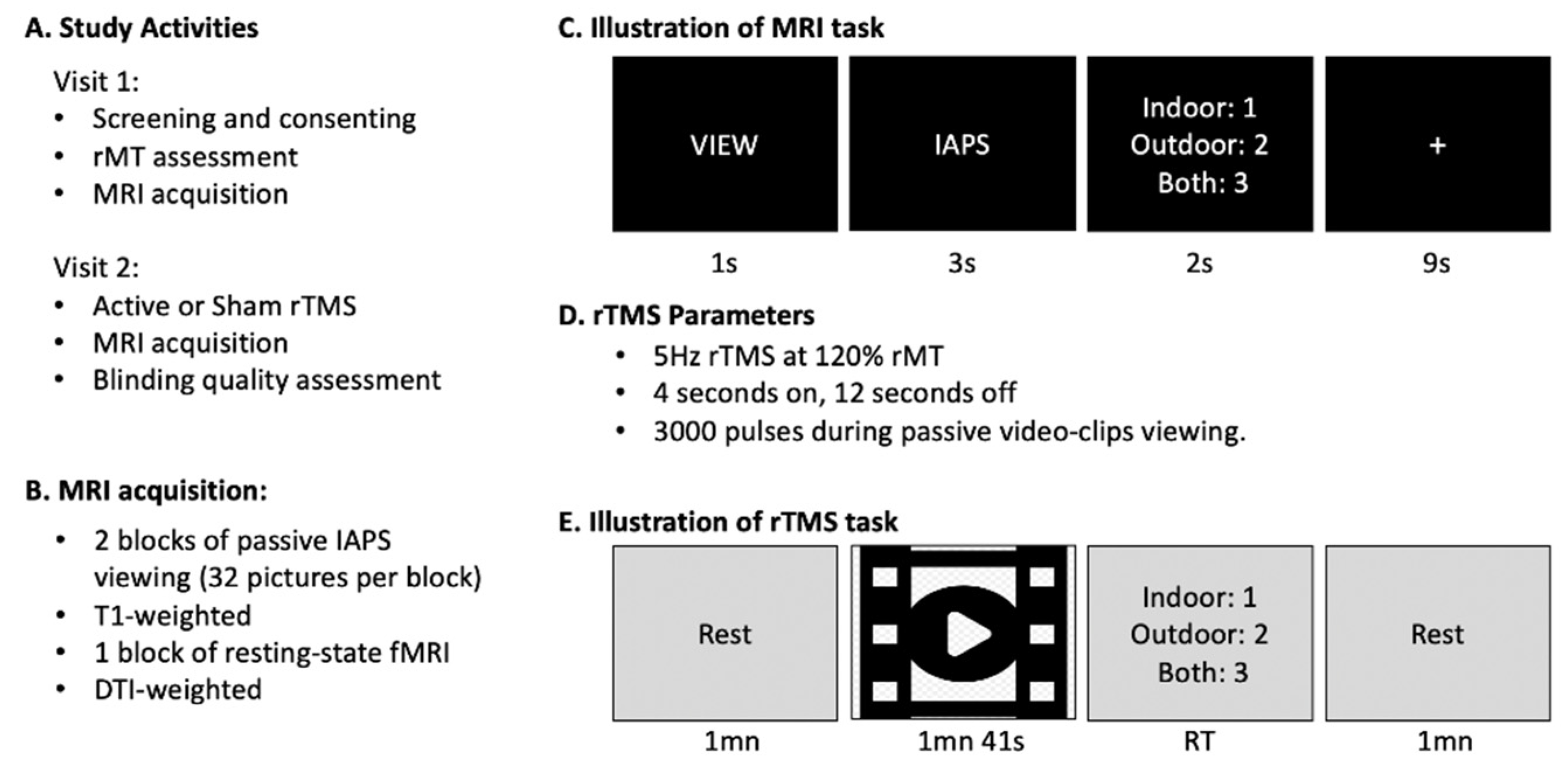
2.2. Experimental Procedure
2.2.1. Resting Motor Threshold (rMT)
2.2.2. MRI Acquisition
2.2.3. MRI Processing for Targeting Approach
2.2.4. Online rTMS Procedure
2.2.5. MRI Processing for Group Analysis to Assess rTMS Effect on Amygdala Activation and Connectivity Changes
2.2.6. Blinding Quality Assessment
2.2.7. ECG Processing
3. Results
3.1. Tolerability and Blinding
3.2. MRI and Electrophysiological Changes
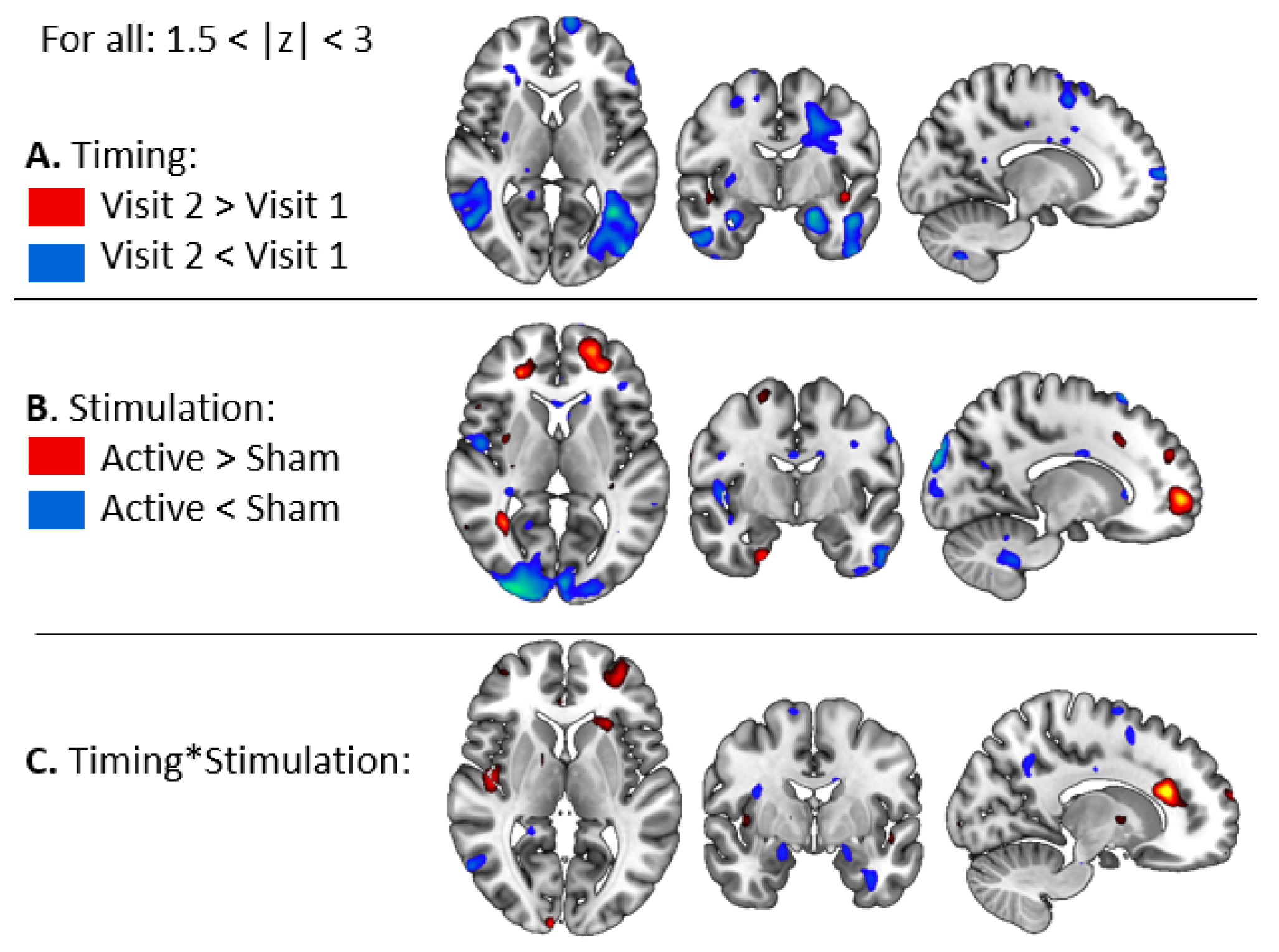
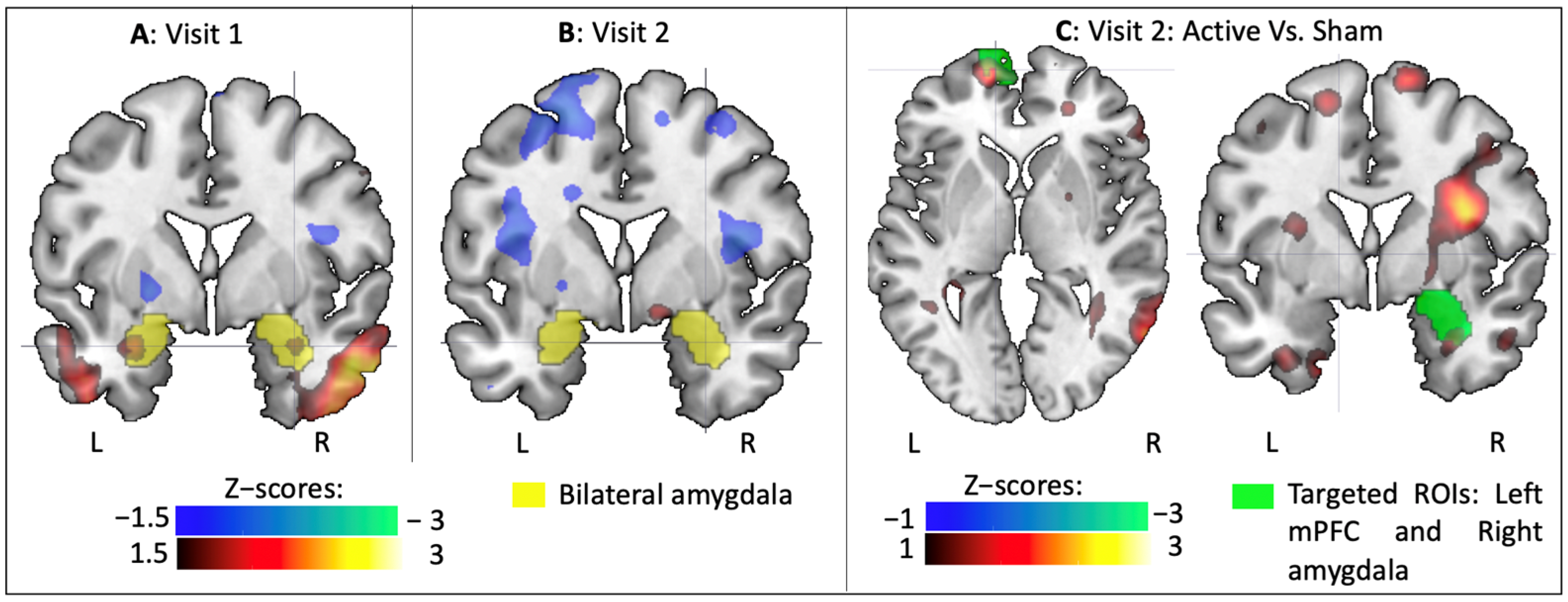
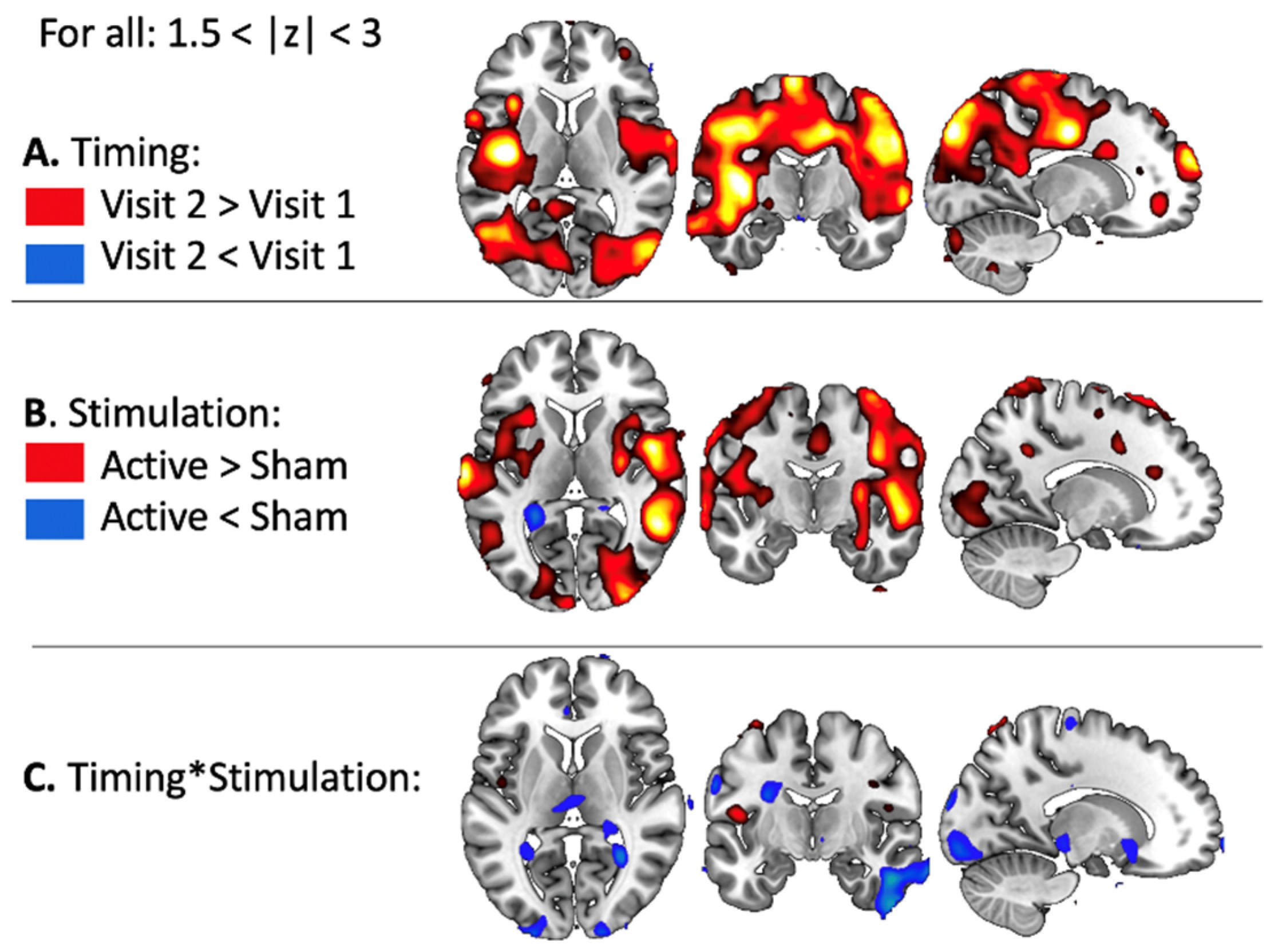
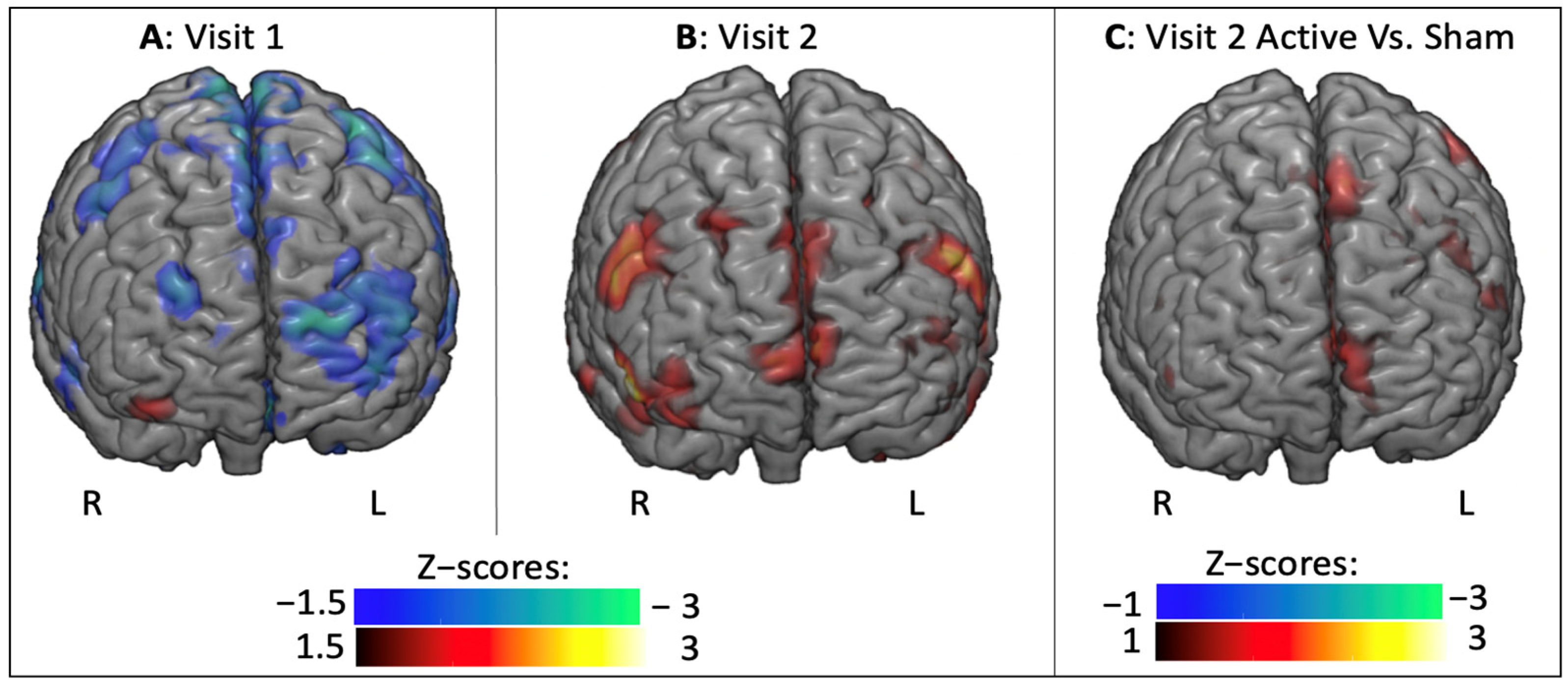
3.2.1. BOLD Changes in Fearful Versus Other Contrast
3.2.2. Task-Related Functional Connectivity Change
3.2.3. Resting-State Functional Connectivity Change
3.2.4. Beats per Minute and Heart Rate Variability during Movies and Resting Periods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beynel, L.; Appelbaum, L.G.; Kimbrel, N.A. Neurobiology and neuromodulation of emotion in PTSD. In Emotion in Posttraumatic Stress Disorder; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–210. [Google Scholar]
- Cohen, H.; Kaplan, Z.; Kotler, M.; Kouperman, I.; Moisa, R.; Grisaru, N. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: A double-blind, placebo-controlled study. Am. J. Psychiatry 2004, 161, 515–524. [Google Scholar] [CrossRef]
- Grisaru, N.; Amir, M.; Cohen, H.; Kaplan, Z. Effect of transcranial magnetic stimulation in posttraumatic stress disorder: A preliminary study. Biol. Psychiatry 1998, 44, 52–55. [Google Scholar] [CrossRef]
- Kozel, F.A.; Motes, M.A.; Didehbani, N.; DeLaRosa, B.; Bass, C.; Schraufnagel, C.D.; Jones, P.; Morgan, C.R.; Spence, J.S.; Kraut, M.A. Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: A randomized clinical trial. J. Affect. Disord. 2018, 229, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kozel, F.A.; Van Trees, K.; Larson, V.; Phillips, S.; Hashimie, J.; Gadbois, B.; Johnson, S.; Gallinati, J.; Barrett, B.; Toyinbo, P. One hertz versus ten hertz repetitive TMS treatment of PTSD: A randomized clinical trial. Psychiatry Res. 2019, 273, 153–162. [Google Scholar] [CrossRef] [PubMed]
- McCann, U.D.; Kimbrell, T.A.; Morgan, C.M.; Anderson, T.; Geraci, M.; Benson, B.E.; Wassermann, E.M.; Willis, M.W.; Post, R.M. Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch. Gen. Psychiatry 1998, 55, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-H.; Pae, C.-U.; Chae, J.-H. Low-frequency, repetitive transcranial magnetic stimulation for the treatment of patients with posttraumatic stress disorder: A double-blind, sham-controlled study. Clin. Psychopharmacol. Neurosci. 2013, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Osuch, E.A.; Benson, B.E.; Luckenbaugh, D.A.; Geraci, M.; Post, R.M.; McCann, U. Repetitive TMS combined with exposure therapy for PTSD: A preliminary study. J. Anxiety Disord. 2009, 23, 54–59. [Google Scholar] [CrossRef]
- Oznur, T.; Akarsu, S.; Celik, C.; Bolu, A.; Ozdemir, B.; Akcay, B.D.; Ozselek, S.; Bozkurt, A.; Ozmenler, K.N. Is transcranial magnetic stimulation effective in treatment-resistant combat related posttraumatic stress disorder? Neurosci. J. 2014, 19, 29–32. [Google Scholar]
- Rosenberg, P.B.; Mehndiratta, R.B.; Mehndiratta, Y.P.; Wamer, A.; Rosse, R.B.; Balish, M. Repetitive transcranial magnetic stimulation treatment of comorbid posttraumatic stress disorder and major depression. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 270–276. [Google Scholar] [CrossRef]
- Watts, B.V.; Landon, B.; Groft, A.; Young-Xu, Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 2012, 5, 38–43. [Google Scholar] [CrossRef]
- Boggio, P.S.; Rocha, M.; Oliveira, M.O.; Fecteau, S.; Cohen, R.B.; Campanhã, C.; Ferreira-Santos, E.; Meleiro, A.; Corchs, F.; Zaghi, S. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J. Clin. Psychiatry 2009, 71, 992–999. [Google Scholar] [CrossRef]
- Isserles, M.; Shalev, A.Y.; Roth, Y.; Peri, T.; Kutz, I.; Zlotnick, E.; Zangen, A. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder–a pilot study. Brain Stimul. 2013, 6, 377–383. [Google Scholar] [CrossRef]
- Philip, N.S.; Ridout, S.J.; Albright, S.E.; Sanchez, G.; Carpenter, L.L. 5-Hz transcranial magnetic stimulation for comorbid posttraumatic stress disorder and major depression. J. Trauma. Stress 2016, 29, 93–96. [Google Scholar] [CrossRef]
- Wilkes, S.; Ona, C.; Yang, M.; Liu, P.; Benton, A.; Lustik, M.; Coleman, J. Impacts of rTMS on refractory depression and comorbid PTSD symptoms at a military treatment facility. Mil. Med. 2020, 185, e1420–e1427. [Google Scholar] [CrossRef]
- Garland, J.S.; Gbade-Alabi, O.; Taylor, J.A.; Atwal, N.; Pasquina, P.F. A study of bilateral prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat the symptoms of mild TBI (mTBI) and PTSD: Preliminary tolerability and effectiveness. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 2020, 13, 1848. [Google Scholar]
- Deng, Z.-D.; Lisanby, S.H.; Peterchev, A.V. Electric field depth–focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimul. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Bestmann, S.; Baudewig, J.; Siebner, H.R.; Rothwell, J.C.; Frahm, J. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage 2003, 20, 1685–1696. [Google Scholar] [CrossRef]
- Vink, J.J.T.; Mandija, S.; Petrov, P.I.; van den Berg, C.A.T.; Sommer, I.E.C.; Neggers, S.F.W. A novel concurrent TMS-fMRI method to reveal propagation patterns of prefrontal magnetic brain stimulation. Hum. Brain Mapp. 2018, 39, 4580–4592. [Google Scholar] [CrossRef]
- Beynel, L.; Powers, J.P.; Appelbaum, L.G. Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: A systematic review. NeuroImage 2020, 211, 116596. [Google Scholar] [CrossRef]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef]
- Addicott, M.; Luber, B.; Nguyen, D.; Palmer, H.; Lisanby, S.; Appelbaum, L. Low and high frequency rTMS effects on resting-state functional connectivity between the postcentral gyrus and the insula. Brain Connect. 2019, 9, 322–328. [Google Scholar] [CrossRef]
- Li, X.; Du, L.; Sahlem, G.L.; Badran, B.W.; Henderson, S.; George, M.S. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex reduces resting-state insula activity and modulates functional connectivity of the orbitofrontal cortex in cigarette smokers. Drug Alcohol Depend. 2017, 174, 98–105. [Google Scholar] [CrossRef]
- Riedel, P.; Heil, M.; Bender, S.; Dippel, G.; Korb, F.M.; Smolka, M.N.; Marxen, M. Modulating functional connectivity between medial frontopolar cortex and amygdala by inhibitory and excitatory transcranial magnetic stimulation. Hum. Brain Mapp. 2019, 40, 4301–4315. [Google Scholar] [CrossRef]
- Luber, B.; Lisanby, S.H. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage 2014, 85, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B.; Fountain, S.; Daskalakis, Z.J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 2006, 117, 2584–2596. [Google Scholar] [CrossRef] [PubMed]
- Keel, J.C.; Smith, M.J.; Wassermann, E.M. A safety screening questionnaire for transcranial magnetic stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2001, 112, 720. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar]
- Available online: http://www.clinicalresearcher.org/software.htm (accessed on January 2019).
- Lang, P.; Bradley, M.M. The International Affective Picture System (IAPS) in the study of emotion and attention. In Handbook of Emotion Elicitation and Assessment; Oxford University Press: Oxford, UK, 2007; Volume 29, pp. 70–73. [Google Scholar]
- Barke, A.; Stahl, J.; Kröner-Herwig, B. Identifying a subset of fear-evoking pictures from the IAPS on the basis of dimensional and categorical ratings for a German sample. J. Behav. Ther. Exp. Psychiatry 2012, 43, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.K.; Veenstra, L.; van Harreveld, F.; Schwarz, N.; Koole, S.L. Let’s not be indifferent about neutrality: Neutral ratings in the International Affective Picture System (IAPS) mask mixed affective responses. Emotion 2016, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Patriat, R.; Molloy, E.K.; Meier, T.B.; Kirk, G.R.; Nair, V.A.; Meyerand, M.E.; Prabhakaran, V.; Birn, R.M. The effect of resting condition on resting-state fMRI reliability and consistency: A comparison between resting with eyes open, closed, and fixated. Neuroimage 2013, 78, 463–473. [Google Scholar] [CrossRef]
- Available online: https://fsl.fmrib.ox.ac.uk/fsl/fslwiki (accessed on January 2019).
- Available online: https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PPIHowToRun (accessed on January 2019).
- Available online: https://www.pmod.com/files/download/v35/doc/pneuro/6750.htm (accessed on January 2019).
- Philip, N.S.; Barredo, J.; van ’t Wout-Frank, M.; Tyrka, A.R.; Price, L.H.; Carpenter, L.L. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol. Psychiatry 2018, 83, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Silvanto, J.; Pascual-Leone, A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008, 21, 1. [Google Scholar] [CrossRef]
- Schaefer, A.; Nils, F.; Sanchez, X.; Philippot, P. Assessing the effectiveness of a large database of emotion-eliciting films: A new tool for emotion researchers. Cogn. Emot. 2010, 24, 1153–1172. [Google Scholar] [CrossRef]
- Breiter, H.C.; Etcoff, N.L.; Whalen, P.J.; Kennedy, W.A.; Rauch, S.L.; Buckner, R.L.; Strauss, M.M.; Hyman, S.E.; Rosen, B.R. Response and habituation of the human amygdala during visual processing of facial expression. Neuron 1996, 17, 875–887. [Google Scholar] [CrossRef]
- Kaur, M.; Michael, J.A.; Hoy, K.E.; Fitzgibbon, B.M.; Ross, M.S.; Iseger, T.A.; Arns, M.; Hudaib, A.-R.; Fitzgerald, P.B. Investigating high-and low-frequency neuro-cardiac-guided TMS for probing the frontal vagal pathway. Brain Stimul. 2020, 13, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Loggia, M.L.; Juneau, M.; Bushnell, M.C. Autonomic responses to heat pain: Heart rate, skin conductance, and their relation to verbal ratings and stimulus intensity. Pain 2011, 152, 592–598. [Google Scholar] [CrossRef]
- Krause-Utz, A.; Elzinga, B.M.; Oei, N.Y.L.; Paret, C.; Niedtfeld, I.; Spinhoven, P.; Bohus, M.; Schmahl, C. Amygdala and dorsal anterior cingulate connectivity during an emotional working memory task in borderline personality disorder patients with interpersonal trauma history. Front. Hum. Neurosci. 2014, 8, 848. [Google Scholar] [CrossRef]
- Koch, S.B.J.; van Zuiden, M.; Nawijn, L.; Frijling, J.L.; Veltman, D.J.; Olff, M. Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depress. Anxiety 2016, 33, 592–605. [Google Scholar] [CrossRef]

| Participants’ Guess | Confidence Level of Guess | ||||
|---|---|---|---|---|---|
| Active | Sham | Active | Sham | ||
| Actual Stimulation: | Active | 6 | 1 | 79.2 | 10.0 |
| Sham | 4 | 2 | 57.5 | 27.5 | |
| Movies | Rest | |||
|---|---|---|---|---|
| BPM | HRV | BPM | HRV | |
| Active rTMS | 65.19 ± 12.88 | 0.95 ± 0.19 | 65.39 ± 12.77 | 0.95 ± 0.19 |
| Sham rTMS | 68.31 ± 11.59 | 0.90 ± 0.18 | 66.17 ± 11.23 | 0.94 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beynel, L.; Campbell, E.; Naclerio, M.; Galla, J.T.; Ghosal, A.; Michael, A.M.; Kimbrel, N.A.; Davis, S.W.; Appelbaum, L.G. The Effects of Functionally Guided, Connectivity-Based rTMS on Amygdala Activation. Brain Sci. 2021, 11, 494. https://doi.org/10.3390/brainsci11040494
Beynel L, Campbell E, Naclerio M, Galla JT, Ghosal A, Michael AM, Kimbrel NA, Davis SW, Appelbaum LG. The Effects of Functionally Guided, Connectivity-Based rTMS on Amygdala Activation. Brain Sciences. 2021; 11(4):494. https://doi.org/10.3390/brainsci11040494
Chicago/Turabian StyleBeynel, Lysianne, Ethan Campbell, Maria Naclerio, Jeffrey T. Galla, Angikar Ghosal, Andrew M. Michael, Nathan A. Kimbrel, Simon W. Davis, and Lawrence G. Appelbaum. 2021. "The Effects of Functionally Guided, Connectivity-Based rTMS on Amygdala Activation" Brain Sciences 11, no. 4: 494. https://doi.org/10.3390/brainsci11040494
APA StyleBeynel, L., Campbell, E., Naclerio, M., Galla, J. T., Ghosal, A., Michael, A. M., Kimbrel, N. A., Davis, S. W., & Appelbaum, L. G. (2021). The Effects of Functionally Guided, Connectivity-Based rTMS on Amygdala Activation. Brain Sciences, 11(4), 494. https://doi.org/10.3390/brainsci11040494






