Elevated and Slowed EEG Oscillations in Patients with Post-Concussive Syndrome and Chronic Pain Following a Motor Vehicle Collision
Abstract
1. Introduction
1.1. EEG in mTBI/PCS
1.2. EEG in Chronic Pain
1.3. Hypotheses for PCS and CP
2. Materials and Methods
2.1. Participants
2.2. EEG Data Acquisition
2.3. EEG Data Analysis
2.4. Statistical Analysis
2.5. Support Vector Analysis
3. Results
3.1. Statistical Analysis
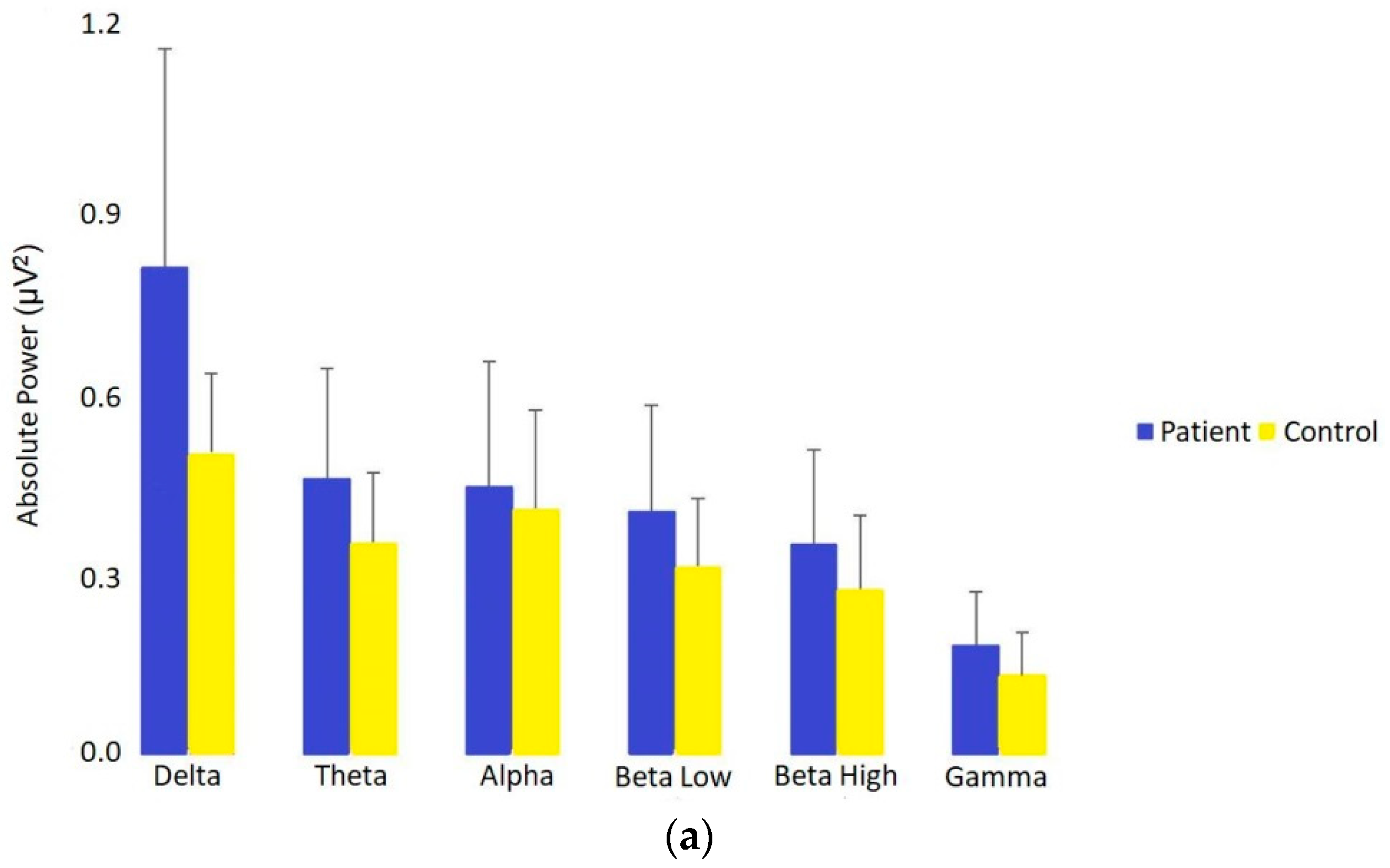
3.1.1. Absolute Power in Patients vs. Controls
3.1.2. Relative Power
3.1.3. Phase-Locking Connectivity
3.1.4. Correlation between Absolute Power and Duration of Symptoms
3.2. Support Vector Machines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC) grand rounds: Reducing severe traumatic brain injury in the United States. MMWR. Morb. Mortal. Wkly. Rep. 2013, 62, 549–552.
- The Centres for Disease Control and Prevention. Traumatic Brain Injury; Centres for Disease Control and Prevention: Atlanta, GA, USA, 2014. [Google Scholar]
- Statistics Canada. Activity-Limiting Brain Injuries 2009–2010; Statistics Canada: Ottawa, ON, Canada, 2011. [Google Scholar]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Ontario Neurotrauma Foundation. Guidelines for Concussion Mild/Traumatic Brain Injury and Prolonged Symptoms; Ontario, Canada, 2018. Available online: https://braininjuryguidelines.org/concussion/ (accessed on 20 March 2019).
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Schopflocher, D.; Taenzer, P.; Jovey, R. The prevalence of chronic pain in Canada. Pain Res. Manag. 2011, 16, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Nahin, R.L. Estimates of Pain Prevalence and Severity in Adults: United States, 2012. J. Pain 2015, 16, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Nampiaparampil, D.E. Prevalence of Chronic Pain after Traumatic Brain Injury. J. Am. Med. Assoc. 2008, 300, 711. [Google Scholar] [CrossRef]
- Lahz, S.; Bryant, R.A. Incidence of chronic pain following traumatic brain injury. Arch. Phys. Med. Rehabil. 1996, 77, 889–891. [Google Scholar] [CrossRef]
- Dimitriadis, S.I.; Zouridakis, G.; Rezaie, R.; Babajani-Feremi, A.; Papanicolaou, A.C. Functional connectivity changes detected with magnetoencephalography after mild traumatic brain injury. NeuroImage Clin. 2015, 9, 519–531. [Google Scholar] [CrossRef]
- Dunkley, B.T.; Urban, K.; Da Costa, L.; Wong, S.M.; Pang, E.W.; Taylor, M.J. Default Mode Network Oscillatory Coupling Is Increased Following Concussion. Front. Neurol. 2018, 9, 280. [Google Scholar] [CrossRef]
- Dunkley, B.T.; Da Costa, L.; Bethune, A.; Jetly, R.; Pang, E.W.; Taylor, M.J.; Doesburg, S.M. Low-frequency connectivity is associated with mild traumatic brain injury. NeuroImage Clin. 2015, 7, 611–621. [Google Scholar] [CrossRef]
- Khong, E.; Odenwald, N.; Hashim, E.; Cusimano, M.D. Diffusion tensor imaging findings in post-concussion syndrome patients after mild traumatic brain injury: A systematic review. Front. Neurol. 2016, 7, 156. [Google Scholar] [CrossRef]
- Munia, T.T.K.; Haider, A.; Schneider, C.; Romanick, M.; Fazel-Rezai, R. A Novel EEG Based Spectral Analysis of Persistent Brain Function Alteration in Athletes with Concussion History. Sci. Rep. 2017, 7, 17221. [Google Scholar] [CrossRef]
- Oehr, L.; Anderson, J. Diffusion-Tensor Imaging Findings and Cognitive Function Following Hospitalized Mixed-Mechanism Mild Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 2308–2319. [Google Scholar] [CrossRef]
- González-Roldán, A.M.; Cifre, I.; Sitges, C.; Montoya, P. Altered Dynamic of EEG Oscillations in Fibromyalgia Patients at Rest. Pain Med. 2016, 17, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kim, J.S.; Kim, D.J.; Chung, C.K. Increased Low- and High-Frequency Oscillatory Activity in the Prefrontal Cortex of Fibromyalgia Patients. Front. Hum. Neurosci. 2016, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- May, E.S.; Nickel, M.M.; Ta Dinh, S.; Tiemann, L.; Heitmann, H.; Voth, I.; Tölle, T.R.; Gross, J.; Ploner, M. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum. Brain Mapp. 2019, 40, 293–305. [Google Scholar] [CrossRef]
- Khoury, S.; Chouchou, F.; Amzica, F.; Giguère, J.-F.; Denis, R.; Rouleau, G.A.; Lavigne, G.J. Rapid EEG Activity during Sleep Dominates in Mild Traumatic Brain Injury Patients with Acute Pain. J. Neurotrauma 2013, 30, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.; Khoury, S.; Chauny, J.-M.; Desautels, A. Pain and sleep in post-concussion/mild traumatic brain injury. Pain 2015, 156, S75–S85. [Google Scholar] [CrossRef]
- Barth, J.T.; Freeman, J.R.; Broshek, D.K.; Varney, R.N. Acceleration-Deceleration Sport-Related Concussion: The Gravity of It All. J. Athl. Train. 2001, 36, 253–256. [Google Scholar]
- Meaney, D.F.; Smith, D.H. Biomechanics of Concussion. Clin. Sports Med. 2011, 30, 19–31. [Google Scholar] [CrossRef]
- Smith, D.H.; Meaney, D.F.; Shull, W.H. Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 2003, 18, 307–316. [Google Scholar] [CrossRef]
- Korn, A.; Golan, H.; Melamed, I.; Pascual-Marqui, R.; Friedman, A. Focal Cortical Dysfunction and Blood-Brain Barrier Disruption in Patients With Postconcussion Syndrome. J. Clin. Neurophysiol. 2005, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Narayana, P.A.; Yu, X.; Hasan, K.M.; Wilde, E.A.; Levin, H.S.; Hunter, J.V.; Miller, E.R.; Patel, V.K.S.; Robertson, C.S.; McCarthy, J.J. Multi-modal MRI of mild traumatic brain injury. NeuroImage Clin. 2015, 7, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.C.; Schaffer, C.E.; Young, J.A.; McNally, K.A.; Fischer, A.N.; Heyer, G.L. Utilization of conventional neuroimaging following youth concussion. Brain Inj. 2017, 31, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Gloor, P.; Ball, G.; Schaul, N. Brain lesions that produce delta waves in the EEG. Neurology 1977, 27, 326–333. [Google Scholar] [CrossRef]
- Tebano, M.T.; Cameroni, M.; Gallozzi, G.; Loizzo, A.; Palazzino, G.; Pezzini, G.; Ricci, G.F. EEG spectral analysis after minor head injury in man. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 185–189. [Google Scholar] [CrossRef]
- Thatcher, R.W.; Walker, R.A.; Gerson, I.; Geisler, F.H. EEG discriminant analyses of mild head trauma. Electroencephalogr. Clin. Neurophysiol. 1989, 73, 94–106. [Google Scholar] [CrossRef]
- Rapp, P.E.; Keyser, D.O.; Albano, A.; Hernandez, R.; Gibson, D.B.; Zambon, R.A.; Hairston, W.D.; Hughes, J.D.; Krystal, A.; Nichols, A.S. Traumatic brain injury detection using electrophysiological methods. Front. Hum. Neurosci. 2015, 9, 11. [Google Scholar] [CrossRef]
- Chen, A.C.N.; Dworkin, S.F.; Drangsholt, M.T. Cortical Power Spectral Analysis of Acute Pathophysiological Pain. Int. J. Neurosci. 1983, 18, 269–278. [Google Scholar] [CrossRef]
- Green, A.L.; Wang, S.; Stein, J.F.; Pereira, E.A.C.; Kringelbach, M.L.; Liu, X.; Brittain, J.-S.; Aziz, T.Z. Neural signatures in patients with neuropathic pain. Neurology 2009, 72, 569–571. [Google Scholar] [CrossRef]
- Boshra, R.; Dhindsa, K.; Boursalie, O.; Ruiter, K.I.; Sonnadara, R.; Samavi, R.; Doyle, T.E.; Reilly, J.P.; Connolly, J.F. From Group-Level Statistics to Single-Subject Prediction: Machine Learning Detection of Concussion in Retired Athletes. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1492–1501. [Google Scholar] [CrossRef]
- Boshra, R.; Ruiter, K.I.; DeMatteo, C.; Reilly, J.P.; Connolly, J.F. Neurophysiological Correlates of Concussion: Deep Learning for Clinical Assessment. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lewine, J.D.; Plis, S.; Ulloa, A.; Williams, C.; Spitz, M.; Foley, J.; Paulson, K.; Davis, J.; Bangera, N.; Snyder, T.; et al. Quantitative EEG Biomarkers for Mild Traumatic Brain Injury. J. Clin. Neurophysiol. 2019, 36, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Misra, G.; Wang, W.; Archer, D.B.; Roy, A.; Coombes, S.A. Automated classification of pain perception using high-density electroencephalography data. J. Neurophysiol. 2017, 117, 786–795. [Google Scholar] [CrossRef]
- Musall, S.; von Pföstl, V.; Rauch, A.; Logothetis, N.K.; Whittingstall, K. Effects of Neural Synchrony on Surface EEG. Cereb. Cortex 2014, 24, 1045–1053. [Google Scholar] [CrossRef]
- Keil, J.; Senkowski, D. Neural Oscillations Orchestrate Multisensory Processing. Neurosci. 2018, 24, 609–626. [Google Scholar] [CrossRef]
- Sweeney-Reed, C.M.; Zaehle, T.; Voges, J.; Schmitt, F.C.; Buentjen, L.; Kopitzki, K.; Esslinger, C.; Hinrichs, H.; Heinze, H.-J.; Knight, R.T.; et al. Corticothalamic phase synchrony and cross-frequency coupling predict human memory formation. Elife 2014, 3, e05352. [Google Scholar] [CrossRef] [PubMed]
- Calderone, D.J.; Lakatos, P.; Butler, P.D.; Castellanos, F.X. Entrainment of neural oscillations as a modifiable substrate of attention. Trends Cogn. Sci. 2014, 18, 300–309. [Google Scholar] [CrossRef]
- Gosselin, N.; Lassonde, M.; Petit, D.; Leclerc, S.; Mongrain, V.; Collie, A.; Montplaisir, J. Sleep following sport-related concussions. Sleep Med. 2009, 10, 35–46. [Google Scholar] [CrossRef]
- Gosselin, N.; Bottari, C.; Chen, J.K.; Huntgeburth, S.C.; De Beaumont, L.; Petrides, M.; Cheung, B.; Ptito, A. Evaluating the cognitive consequences of mild traumatic brain injury and concussion by using electrophysiology. Neurosurg. Focus 2012, 33, 1–7. [Google Scholar] [CrossRef]
- Meisel, C.; Olbrich, E.; Shriki, O.; Achermann, P. Fading signatures of critical brain dynamics during sustained wakefulness in humans. J. Neurosci. 2013, 33, 17363–17372. [Google Scholar] [CrossRef]
- Duclos, C.; Dumont, M.; Wiseman-Hakes, C.; Arbour, C.; Mongrain, V.; Gaudreault, P.O.; Khoury, S.; Lavigne, G.; Desautels, A.; Gosselin, N. Sleep and wake disturbances following traumatic brain injury. Pathol. Biol. 2014, 62, 252–261. [Google Scholar] [CrossRef]
- Balba, N.M.; Elliott, J.E.; Weymann, K.B.; Opel, R.A.; Duke, J.W.; Oken, B.S.; Morasco, B.J.; Heinricher, M.M.; Lim, M.M. Increased Sleep Disturbances and Pain in Veterans with Comorbid Traumatic Brain Injury and Posttraumatic Stress Disorder. J. Clin. Sleep Med. 2018, 14, 1865–1878. [Google Scholar] [CrossRef]
- Duff, J. The Usefulness of Quantitative EEG (QEEG) and Neurotherapy in the Assessment and Treatment of Post-Concussion Syndrome. Clin. EEG Neurosci. 2004, 35, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Roche, R.A.P.; Dockree, P.M.; Garavan, H.; Foxe, J.J.; Robertson, I.H.; O’Mara, S.M. EEG alpha power changes reflect response inhibition deficits after traumatic brain injury (TBI) in humans. Neurosci. Lett. 2004, 362, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Deiber, M.-P.; Sallard, E.; Ludwig, C.; Ghezzi, C.; Barral, J.; Ibañez, V. EEG alpha activity reflects motor preparation rather than the mode of action selection. Front. Integr. Neurosci. 2012, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, V.A.; Gurskaia, O.E.; Kropotov, I.D.; Artiushkova, L.V.; Muller, A. The comparison of clustering methods of EEG independent components in healthy subjects and patients with post concussion syndrome after traumatic brain injury. Fiziol. Cheloveka 2010, 36, 5–14. [Google Scholar] [PubMed]
- Dos Pinheiro, E.S.S.; de Queirós, F.C.; Montoya, P.; Santos, C.L.; do Nascimento, M.A.; Ito, C.H.; Silva, M.; Nunes Santos, D.B.; Benevides, S.; Miranda, J.G.V.; et al. Electroencephalographic Patterns in Chronic Pain: A Systematic Review of the Literature. PLoS ONE 2016, 11, e0149085. [Google Scholar] [CrossRef] [PubMed]
- Sarnthein, J.; Stern, J.; Aufenberg, C.; Rousson, V.; Jeanmonod, D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain 2006, 129, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Jeanmonod, D.; Sarnthein, J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage 2006, 31, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, A.; Hasan, M.A.; Fraser, M.; Conway, B.A.; Nasseroleslami, B.; Allan, D.B. Dynamic oscillatory signatures of central neuropathic pain in spinal cord injury. J. Pain 2014, 15, 645–655. [Google Scholar] [CrossRef]
- Bjørk, M.H.; Stovner, L.J.; Engstrøm, M.; Stjern, M.; Hagen, K.; Sand, T. Interictal quantitative EEG in migraine: A blinded controlled study. J. Headache Pain 2009, 10, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ploner, M.; Sorg, C.; Gross, J. Brain Rhythms of Pain. Trends Cogn. Sci. 2017, 21, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.R.; Ribary, U.; Jeanmonod, D.; Kronberg, E.; Mitra, P.P. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. USA 1999, 96, 15222–15227. [Google Scholar] [CrossRef] [PubMed]
- Vakorin, V.A.; Doesburg, S.M.; da Costa, L.; Jetly, R.; Pang, E.W.; Taylor, M.J. Detecting Mild Traumatic Brain Injury Using Resting State Magnetoencephalographic Connectivity. PLoS Comput. Biol. 2016, 12, e1004914. [Google Scholar] [CrossRef]
- Choe, M.K.; Lim, M.; Kim, J.S.; Lee, D.S.; Chung, C.K. Disrupted Resting State Network of Fibromyalgia in Theta frequency. Sci. Rep. 2018, 8, 2064. [Google Scholar] [CrossRef] [PubMed]
- Human Brain Institute. Human Brain Institute Database. Available online: https://www.hbimed.com/ (accessed on 25 July 2017).
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 52, 3–6. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; Mckeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef]
- Nolan, H.; Whelan, R.; Reilly, R.B. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J. Neurosci. Methods 2010, 192, 152–162. [Google Scholar] [CrossRef]
- Poil, S.; Simpraga, S.; Simon, J.; Houtman, K.L.-H. Neurophysiological Biomarker Toolbox. Amsterdam, The Netherlands.
- MATLAB and Statistics Toolbox Release 2018b; The MathWorks, Inc.: Natick, MA, USA.
- Olsen, T. Current Source Density (CSD). 2019. Available online: https://www.mathworks.com/matlabcentral/fileexchange/69399-current-source-density-csd (accessed on 20 March 2019).
- IBM Corp. IBM SPSS Statistics for Windows, Version 20.0.
- Mierswa, I.; Wurst, M.; Klinkenberg, R.; Scholz, M.; Euler, T. YALE: Rapid prototyping for complex data mining tasks. In Proceedings of the 12th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Philadelphia, PA, USA, 20–23 August 2006. [Google Scholar]
- Fenton, G.; McClelland, R.; Montgomery, A.; MacFlynn, G.; Rutherford, W. The postconcussional syndrome: Social antecedents and psychological sequelae. Br. J. Psychiatry 1993, 162, 493–497. [Google Scholar] [CrossRef]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef]
- Walker, A.E.; Kollros, J.J.; Case, T.J. The physiological basis of cerebral concussion. J. Neurosurg. 1944, 1, 103–116. [Google Scholar] [CrossRef]
- Patterson, Z.R.; Holahan, M.R. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front. Cell. Neurosci. 2012, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Lord, L.-D.; Expert, P.; Huckins, J.F.; Turkheimer, F.E. Cerebral energy metabolism and the brain’s functional network architecture: An integrative review. J. Cereb. Blood Flow Metab. 2013, 33, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Koski, L.; Kolivakis, T.; Yu, C.; Chen, J.-K.; Delaney, S.; Ptito, A. Noninvasive Brain Stimulation for Persistent Postconcussion Symptoms in Mild Traumatic Brain Injury. J. Neurotrauma 2015, 32, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ros, T.; Baars, B.J.; Lanius, R.A.; Vuilleumier, P. Tuning pathological brain oscillations with neurofeedback: A systems neuroscience framework. Front. Hum. Neurosci. 2014, 8, 1008. [Google Scholar] [CrossRef]



| Patient | Age | Sex | Direction of Impact | LOC | Location in Vehicle | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 38 | M | Front | No | Driver | CP, PCS, PTSD |
| 2 | 52 | F | Rear | No | Front passenger | CP, PCS, anxiety |
| 3 | 51 | M | Rear | No | Driver | CP, PCS, depression |
| 4 | 46 | M | Side | Yes | Driver | CP, PCS, PTSD |
| 5 | 60 | F | Front | Yes | Driver | CP, PCS, PTSD |
| 6 | 53 | F | Side | No | Driver | CP, PCS, PTSD |
| 7 | 43 | F | Vehicle rollover | No | Driver | CP, PCS |
| 8 | 42 | F | Rear | No | Front passenger | CP, PCS, PTSD |
| 9 | 23 | F | Side | Yes | Driver | CP, PCS, anxiety |
| 10 | 44 | M | Rear | No | Driver | CP, PCS |
| 11 | 55 | F | Pedestrian hit by car | Yes | Pedestrian (hit by car, walking) | CP, PCS, PTSD |
| 12 | 56 | F | Side | No | Driver | CP, PCS |
| 13 | 57 | F | Rear | No | Front passenger | CP, PCS |
| 14 | 48 | F | Rear | No | Driver | CP, PCS |
| 15 | 40 | M | Rear | No | Driver | CP, PCS, depression, PTSD |
| 16 | 48 | F | Rear | Yes | Driver | CP, PCS |
| 17 | 24 | M | Rear | No | Driver | CP, PCS |
| 18 | 52 | M | Pedestrian hit by car | Yes | Pedestrian (hit by car, walking) | CP, PCS, depression |
| 19 | 65 | M | Side | Yes | Driver | CP, PCS |
| 20 | 54 | F | Rear | No | Driver | CP, PCS, anxiety |
| 21 | 32 | M | Rear | Yes | Driver | CP, PCS |
| 22 | 24 | F | Rear ended then hit another car head on | Yes | Driver | CP, PCS, depression, anxiety |
| 23 | 21 | F | Rear | No | Driver | CP, PCS |
| 24 | 45 | M | Side | No | Front passenger | CP, PCS, anxiety |
| 25 | 52 | M | Rear | No | Driver | CP, PCS |
| 26 | 40 | F | Rear | Yes | Driver | CP, PCS, PTSD |
| 27 | 34 | F | Rear ended another vehicle | Yes | Front passenger | CP, PCS, anxiety |
| 28 | 55 | F | Side | Yes | Driver | CP, PCS |
| 29 | 58 | F | Side | No | Driver | CP, PCS, anxiety |
| 30 | 37 | M | Side | Yes | Driver | CP, PCS, PTSD, depression, anxiety |
| 31 | 45 | F | on bus | Yes | Front passenger | CP, PCS |
| 32 | 23 | F | Front | Yes | Driver | CP, PCS |
| 33 | 43 | F | Rear | No | Driver | CP, PCS |
| 34 | 40 | M | Side | Yes | Front passenger | CP, PCS, depression |
| 35 | 45 | F | Rear | Yes | Driver | CP, PCS |
| 36 | 48 | M | Front | No | Driver | CP, PCS |
| 37 | 52 | M | Front | No | Front passenger | CP, PCS, PTSD |
| 38 | 36 | F | Side | Yes | Driver | CP, PCS |
| 39 | 32 | F | Front | No | Driver | CP, PCS, PTSD |
| 40 | 45 | M | Side | No | Driver | CP, PCS, PTSD |
| 41 | 40 | F | Head on | No | Driver | CP, PCS, PTSD |
| 42 | 45 | F | Side | No | Moose hit driver | CP, PCS, anxiety |
| 43 | 12 | M | Side | No | Front passenger | CP, PCS, anxiety |
| 44 | 61 | F | Side | Yes | Driver | CP, PCS, PTSD |
| 45 | 49 | M | Front | NA | Driver | CP, PCS, PTSD |
| 46 | 49 | F | Front | Yes | Driver | CP, PCS |
| 47 | 48 | F | Rear | NA | Driver | CP, PCS |
| 48 | 52 | M | Front | Yes | Rear passenger | CP, PCS, anxiety |
| 49 | 52 | F | Rear | Yes | Driver | CP, PCS, PTSD |
| 50 | 53 | M | Side | Yes | Driver | CP, PCS |
| 51 | 38 | F | Front | Yes | Front passenger | CP, PCS, PTSD |
| 52 | 31 | F | Rear | Yes | Front passenger | CP, PCS, anxiety |
| 53 | 51 | F | Pedestrian hit by car | Yes | Pedestrian (hit by car, jogging) | CP, PCS |
| 54 | 56 | F | Rear | Yes | Driver | CP, PCS |
| 55 | 60 | F | Rear | Yes | Driver | CP, PCS |
| 56 | 45 | M | Side | Yes | Bicyclist (hit by car) | CP, PCS, anxiety |
| 57 | 42 | F | Rear | Yes | Driver | CP, PCS, PTSD |
| qEEG Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute Power µv2 | Relative Power | Phase-Locking | |||||||
| Frequency Bands | Patient Mdn (IQR) 25th 75th | Control Mdn (IQR) 25th 75th | p-value (r) | Patient Mdn (IQR) 25th 75th | Control Mdn (IQR) 25th 75th | p-value (r) | Patient Mdn (IQR) 25th 75th | Control Mdn (IQR) 25th 75th | p-value (r) |
| Delta (1–4 Hz) | 0.79 (0.58) (1.03) | 0.49 (0.44) (0.59) | 0.000000 0.6 | 0.30 (0.26) (0.34) | 0.25 (0.23) (0.28) | 0.000006 0.43 | 0.28 (0.28) (0.28) | 0.28 (0.28) (0.28) | 0.078 0.17 |
| Theta (4–7 Hz) | 0.45 (0.35) (0.59) | 0.35 (0.29) (0.39) | 0.00003 0.4 | 0.18 (0.16) (0.19) | 0.18 (0.16) (0.19) | 0.75 0.03 | 0.25 (0.25) (0.25) | 0.25 (0.25) (0.25) | 0.42 0.07 |
| Alpha (7–13 Hz) | 0.44 (0.36) (0.55) | 0.4 (0.28) (0.52) | 0.029 0.21 | 0.17 (0.15) (0.19) | 0.19 (0.17) (0.23) | 0.0005 0.33 | 0.19 (0.19) (0.20) | 0.207 (0.19) (0.21) | 0.048 0.19 |
| Low Beta (13–15 Hz) | 0.40 (0.33) (0.48) | 0.31 (0.25) (0.43) | 0.002 0.29 | 0.15 (0.13) (0.16) | 0.16 (0.14) (0.17) | 0.067 0.17 | 0.15 (0.15) (0.16) | 0.15 (0.15) (0.16) | 0.74 0.03 |
| High Beta (15–30 Hz) | 0.34 (0.27) (0.45) | 0.27 (0.22) (0.37) | 0.006 0.26 | 0.13 (0.11) (0.15) | 0.14 (0.12) (0.15) | 0.077 0.17 | 0.13 (0.13) (0.13) | 0.13 (0.13) (0.14) | 0.10 0.15 |
| Gamma (30–45 Hz) | 0.18 (0.13) (0.24) | 0.13 (0.09) (0.59) | 0.003 0.29 | 0.06 (0.05) (0.8) | 0.07 (0.05) (0.8) | 0.77 0.03 | 0.10 (0.10) (0.11) | 0.10 (0.10) (0.11) | 0.69 0.04 |
| Absolute Power Frequency Bands | Pearson Correlation | p-Value |
|---|---|---|
| Delta (1–4 Hz) | 0.35 | p < 0.01 |
| Theta (4–7 Hz) | 0.31 | p < 0.05 |
| Alpha (7–13 Hz) | 0.32 | p < 0.05 |
| Low Beta (13–15 Hz) | 0.32 | p < 0.05 |
| High Beta (15–30 Hz) | 0.06 | n.s |
| Gamma (30–45 Hz) | −0.06 | n.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchanan, D.M.; Ros, T.; Nahas, R. Elevated and Slowed EEG Oscillations in Patients with Post-Concussive Syndrome and Chronic Pain Following a Motor Vehicle Collision. Brain Sci. 2021, 11, 537. https://doi.org/10.3390/brainsci11050537
Buchanan DM, Ros T, Nahas R. Elevated and Slowed EEG Oscillations in Patients with Post-Concussive Syndrome and Chronic Pain Following a Motor Vehicle Collision. Brain Sciences. 2021; 11(5):537. https://doi.org/10.3390/brainsci11050537
Chicago/Turabian StyleBuchanan, Derrick Matthew, Tomas Ros, and Richard Nahas. 2021. "Elevated and Slowed EEG Oscillations in Patients with Post-Concussive Syndrome and Chronic Pain Following a Motor Vehicle Collision" Brain Sciences 11, no. 5: 537. https://doi.org/10.3390/brainsci11050537
APA StyleBuchanan, D. M., Ros, T., & Nahas, R. (2021). Elevated and Slowed EEG Oscillations in Patients with Post-Concussive Syndrome and Chronic Pain Following a Motor Vehicle Collision. Brain Sciences, 11(5), 537. https://doi.org/10.3390/brainsci11050537






