Preliminary Results Regarding Sleep in a Zebrafish Model of Autism Spectrum Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics and Husbandry
2.2. Chemical Exposure
2.3. Experimental Design
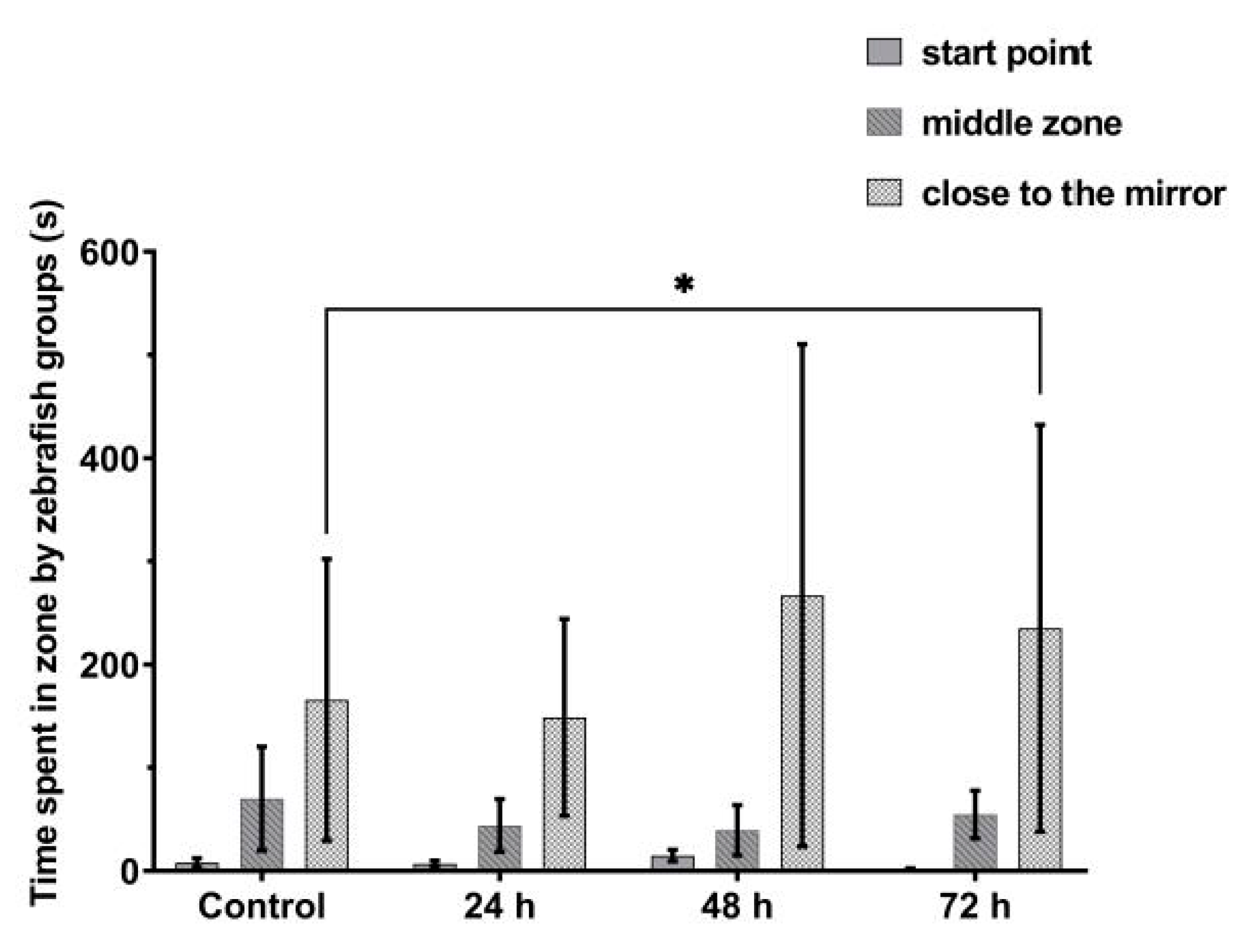
2.4. Behavioral Assessment
2.4.1. Locomotor Activity
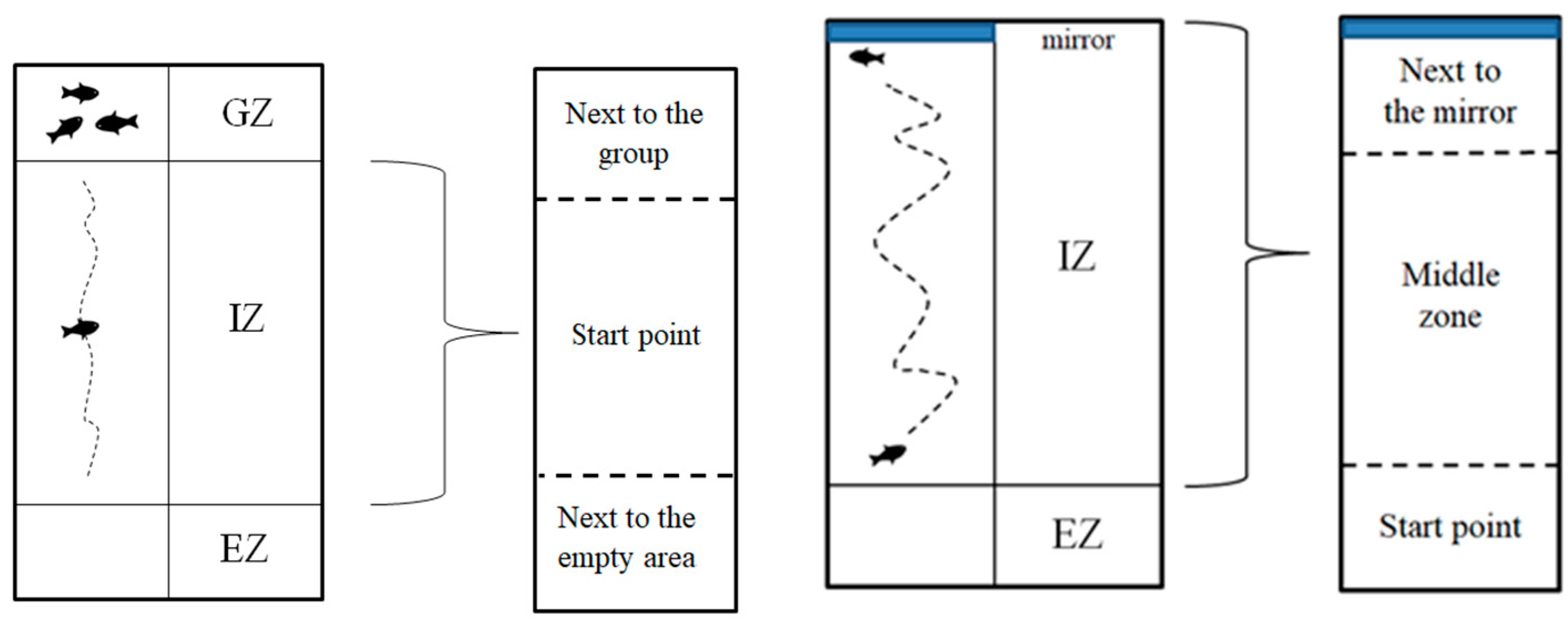
2.4.2. Social Interaction Test
2.4.3. Aggression
2.4.4. Sleep Parameter Recording
2.5. Statistical Analysis
3. Results
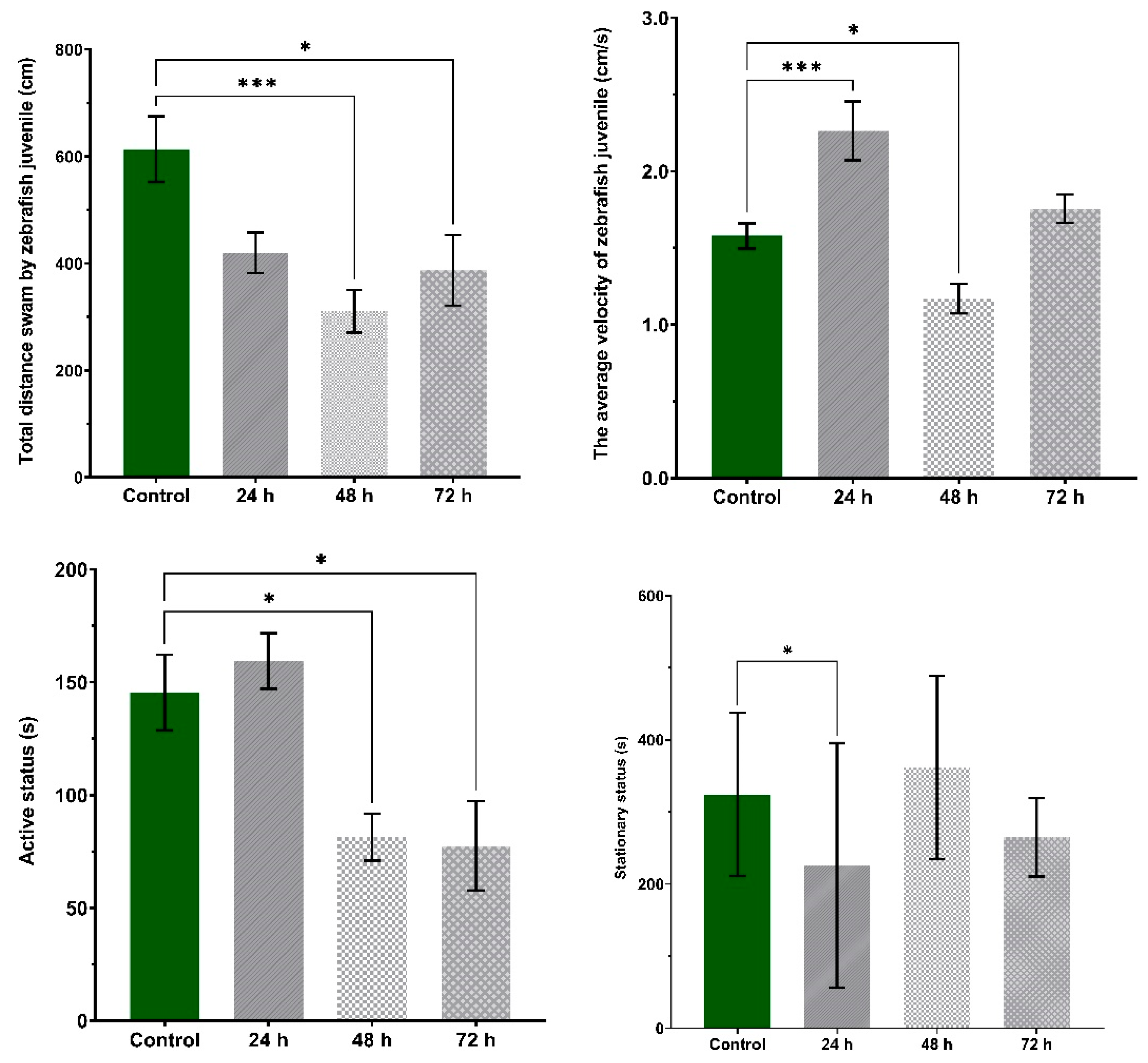
3.1. VPA Administration Leads to Hyperactivity
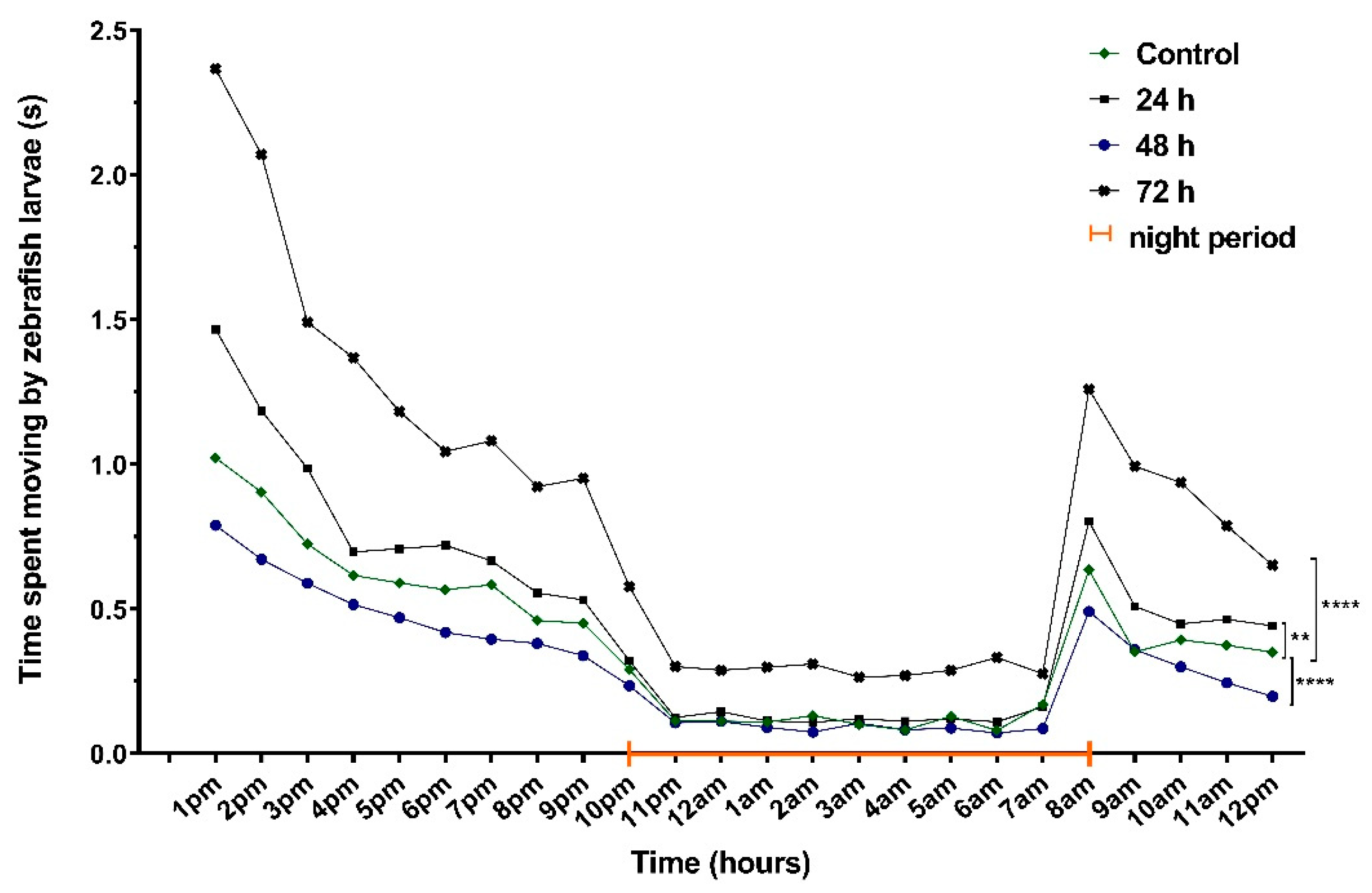
3.2. No Significant Effects on Sleep after VPA Administration in Zebrafish Larvae
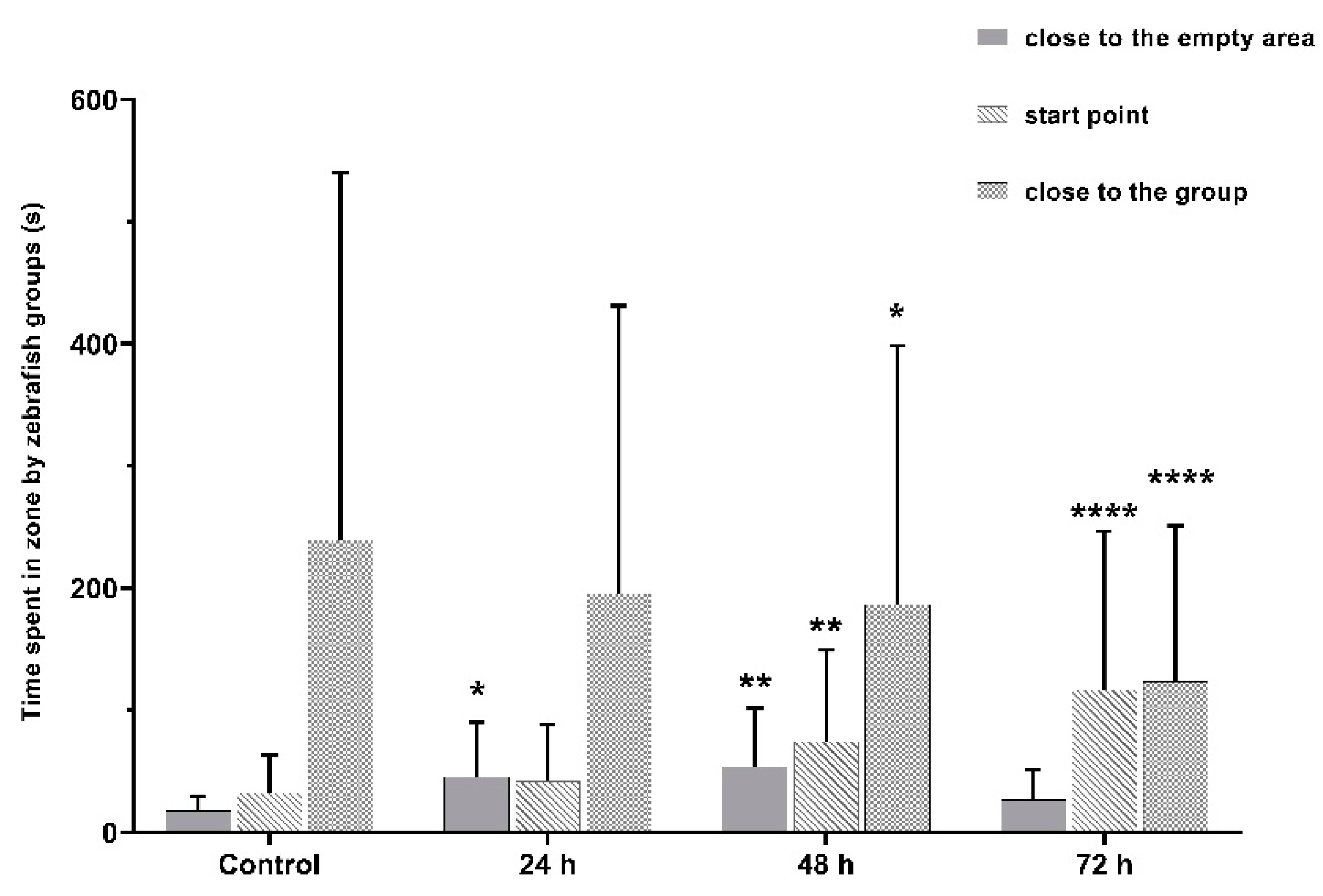
3.3. VPA Administration Leads to Impairments in Juvenile Zebrafish Social Interaction
3.4. VPA Administration Has No Effects on Juvenile Zebrafish Aggression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piazza, C.C.; Fisher, W.W.; Brown, K.A.; Shore, B.A.; Patel, M.R.; Katz, R.M.; Sevin, B.M.; Gulotta, C.S.; Blakely-Smith, A. Functional analysis of inappropriate mealtime behaviors. J. Appl. Behav. Anal. 2003, 36, 187–204. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Maenner, M. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network. 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef]
- Frith, U.; Happé, F. Autism spectrum disorder. Curr. Biol. 2005, 15, R786–R790. [Google Scholar] [CrossRef] [Green Version]
- Mabunga, D.F.N.; Gonzales, E.L.T.; Kim, J.-W.; Kim, K.C.; Shin, C.Y. Exploring the Validity of Valproic Acid Animal Model of Autism. Exp. Neurobiol. 2015, 24, 285–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kas, M.J.; Glennon, J.C.; Buitelaar, J.; Ey, E.; Biemans, B.; Crawley, J.; Ring, R.H.; Lajonchere, C.; Esclassan, F.; Talpos, J.; et al. Assessing behavioural and cognitive domains of autism spectrum disorders in rodents: Current status and future perspectives. Psychopharmacology 2014, 231, 1125–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar]
- Tick, B.; Bolton, P.; Happé, F.; Rutter, M.; Rijsdijk, F. Heritability of autism spectrum disorders: A meta-analysis of twin studies. J. Child Psychol. Psychiatry 2016, 57, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. JAMA 2017, 318, 1182–1184. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Shu, L.-Q.; Zhao, X.-S.; Li, X.-K. Autism spectrum disorders: Autistic phenotypes and complicated mechanisms. World J. Pediatr. 2019, 15, 17–25. [Google Scholar] [CrossRef]
- Blatt, G.J. The Neuropathology of Autism. Scientifica 2012, 2012, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Rogers, T.D.; McKimm, E.; Dickson, P.E.; Goldowitz, D.; Blaha, C.D.; Mittleman, G. Is autism a disease of the cerebellum? An integration of clinical and pre-clinical research. Front. Syst. Neurosci. 2013, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauman, M.L.; Kemper, T.L. Neuroanatomic observations of the brain in autism: A review and future directions. Int. J. Dev. Neurosci. 2005, 23, 183–187. [Google Scholar] [CrossRef]
- Skefos, J.; Cummings, C.; Enzer, K.; Holiday, J.; Weed, K.; Levy, E.; Yuce, T.; Kemper, T.; Bauman, M. Regional Alterations in Purkinje Cell Density in Patients with Autism. PLoS ONE 2014, 9, e81255. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K. Purkinje cell vulnerability and autism: A possible etiological connection. Brain Dev. 2003, 25, 377–382. [Google Scholar] [CrossRef]
- Pizzarelli, R.; Cherubini, E. Alterations of GABAergic Signaling in Autism Spectrum Disorders. Neural Plast. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puts, N.A.; Wodka, E.L.; Harris, A.D.; Crocetti, D.; Tommerdahl, M.; Mostofsky, S.H.; Edden, R.A. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res. 2017, 10, 608–619. [Google Scholar] [CrossRef] [Green Version]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in autism spectrum disorder—A translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fagundes, S.B.R. Valproic acid: Review. Rev. Neurosci. 2008, 16, 130–136. [Google Scholar]
- Star, K.; Edwards, I.R.; Choonara, I. Valproic Acid and Fatalities in Children: A Review of Individual Case Safety Reports in VigiBase. PLoS ONE 2014, 9, e108970. [Google Scholar] [CrossRef] [Green Version]
- Ximenes, J.C.M.; Verde, E.C.L.; Naffah-Mazzacoratti, M.D.G.; Viana, G.S.D.B. Valproic Acid, a Drug with Multiple Molecular Targets Related to Its Potential Neuroprotective Action. Neurosci. Med. 2012, 3, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, D.B.; Berg, M.; Breault, R. Valproic Acid—Induced Pancreatitis in Childhood Epilepsy: Case Series and Review. J. Child Neurol. 2004, 19, 498–502. [Google Scholar] [CrossRef]
- Kudin, A.P.; Mawasi, H.; Eisenkraft, A.; Elger, C.E.; Bialer, M.; Kunz, W.S. Mitochondrial Liver Toxicity of Valproic Acid and Its Acid Derivatives Is Related to Inhibition of α-Lipoamide Dehydrogenase. Int. J. Mol. Sci. 2017, 18, 1912. [Google Scholar] [CrossRef] [Green Version]
- Gayam, V.; Mandal, A.K.; Khalid, M.; Shrestha, B.; Garlapati, P.; Khalid, M. Valproic acid induced acute liver injury resulting in hepatic encephalopathy—A case report and literature review. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2004, 30, 80–89. [Google Scholar] [CrossRef]
- Zimmermann, F.F.; Gaspary, K.V.; Leite, C.E.; Cognato, G.D.P.; Bonan, C.D. Embryological exposure to valproic acid induces social interaction deficits in zebrafish (Danio rerio): A developmental behavior analysis. Neurotoxicol. Teratol. 2015, 52, 36–41. [Google Scholar] [CrossRef]
- Chen, J.; Lei, L.; Tian, L.; Hou, F.; Roper, C.; Ge, X.; Zhao, Y.; Chen, Y.; Dong, Q.; Tanguay, R.L.; et al. Developmental and behavioral alterations in zebrafish embryonically exposed to valproic acid (VPA): An aquatic model for autism. Neurotoxicol. Teratol. 2018, 66, 8–16. [Google Scholar] [CrossRef]
- WLee, S.; Chun, H.-S.; Lee, J.; Park, H.-J.; Kim, K.-T.; Kim, C.-H.; Yoon, S.; Kim, W.-K. Plausibility of the zebrafish embryos/larvae as an alternative animal model for autism: A comparison study of transcriptome changes. PLoS ONE 2018, 13, e0203543. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Turczak, J.; Przewłocki, R. Environmental Enrichment Reverses Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Issues for a Therapeutic Approach in Autism. Neuropsychopharmacology 2006, 31, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grone, B.P.; Qu, T.; Baraban, S.C. Behavioral Comorbidities and Drug Treatments in a Zebrafish scn1lab Model of Dravet Syndrome. ENeuro 2017, 4, 0066-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffer, I.E.; Nabbout, R. SCN1A-related phenotypes: Epilepsy and beyond. Epilepsia 2019, 60, S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Baronio, D.; Puttonen, H.A.J.; Sundvik, M.; Semenova, S.; Lehtonen, E.; Panula, P. Embryonic exposure to valproic acid affects the histaminergic system and the social behaviour of adult zebrafish (Danio rerio). Br. J. Pharmacol. 2018, 175, 797–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caramillo, E.M.; Echevarria, D.J. Alzheimer’s disease in the zebrafish: Where can we take it? Behav. Pharmacol. 2017, 28, 179–186. [Google Scholar] [CrossRef]
- Tropepe, V.; Sive, H.L. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003, 2, 268–281. [Google Scholar] [CrossRef]
- Stewart, A.M.; Nguyen, M.; Wong, K.; Poudel, M.K.; Kalueff, A.V. Developing zebrafish models of autism spectrum disorder (ASD). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 50, 27–36. [Google Scholar] [CrossRef]
- Meshalkina, D.A.; Kizlyk, M.N.; Kysil, E.V.; Collier, A.D.; Echevarria, D.J.; Abreu, M.S.; Barcellos, L.J.; Song, C.; Warnick, J.E.; Kyzar, E.J.; et al. Zebrafish models of autism spectrum disorder. Exp. Neurol. 2018, 299, 207–216. [Google Scholar] [CrossRef]
- Liu, C.-X.; Li, C.-Y.; Hu, C.-C.; Wang, Y.; Lin, J.; Jiang, Y.-H.; Li, Q.; Xu, X. CRISPR/Cas9-induced shank3b mutant zebrafish display autism-like behaviors. Mol. Autism 2018, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Vaz, R.; Hofmeister, W.; Lindstrand, A. Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques. Int. J. Mol. Sci. 2019, 20, 1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, E.J.; Turner, K.J.; Fernandez, J.M.; Cifuentes, D.; Ghosh, M.; Ijaz, S.; Jain, R.A.; Kubo, F.; Bill, B.R.; Baier, H.; et al. Estrogens Suppress a Behavioral Phenotype in Zebrafish Mutants of the Autism Risk Gene, CNTNAP2. Neuron 2016, 89, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devnani, P.A.; Hegde, A.U. Autism and sleep disorders. J. Pediatr. Neurosci. 2015, 10, 304–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souders, M.C.; Zavodny, S.; Eriksen, W.; Sinko, R.; Connell, J.; Kerns, C.; Schaaf, R.; Pinto-Martin, J. Sleep in Children with Autism Spectrum Disorder. Curr. Psychiatry Rep. 2017, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.O.; Dovgan, K.; Neumeyer, A.M.; Malow, B.A. Course and Predictors of Sleep and Co-occurring Problems in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 2101–2115. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.J.; Ben Simon, E.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef]
- Reynolds, S.; Lane, S.J.; Thacker, L. Sensory Processing, Physiological Stress, and Sleep Behaviors in Children with and without Autism Spectrum Disorders. OTJR Occup. Particip. Health 2012, 32, 246–257. [Google Scholar] [CrossRef]
- Tzischinsky, O.; Meiri, G.; Manelis, L.; Bar-Sinai, A.; Flusser, H.; Michaelovski, A.; Zivan, O.; Ilan, M.; Faroy, M.; Menashe, I.; et al. Sleep disturbances are associated with specific sensory sensitivities in children with autism. Mol. Autism 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Vriend, B.J.L.; Corkum, P.V.; Moon, B.E.C.; Smith, I.M. Behavioral Interventions for Sleep Problems in Children with Autism Spectrum Disorders: Current Findings and Future Directions. J. Pediatr. Psychol. 2011, 36, 1017–1029. [Google Scholar] [CrossRef] [Green Version]
- Turner, K.S.; Johnson, C.R. Behavioral Interventions to Address Sleep Disturbances in Children with Autism Spectrum Disorders. Top. Early Child. Spéc. Educ. 2012, 33, 144–152. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Sciberras, E.; Hiscock, H.; Mulraney, M.; McGillivray, J.; Rinehart, N. The Efficacy of a Brief Behavioral Sleep Intervention in School-Aged Children with ADHD and Comorbid Autism Spectrum Disorder. J. Atten. Disord. 2015, 23, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Relia, S.; Ekambaram, V. Pharmacological Approach to Sleep Disturbances in Autism Spectrum Disorders with Psychiatric Comorbidities: A Literature Review. Med. Sci. 2018, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, B.M.; Vaz, S.; Lee, E.A.L.; Thompson, C.; Rogerson, J.M.; Falkmer, T. Effectiveness of Sleep-Based Interventions for Children with Autism Spectrum Disorder: A Meta-Synthesis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, I.V. Sleep and its regulation in zebrafish. Rev. Neurosci. 2011, 22, 27–36. [Google Scholar] [CrossRef]
- Leung, L.C.; Wang, G.X.; Madelaine, R.; Skariah, G.; Kawakami, K.; Deisseroth, K.; Urban, A.E.; Mourrain, P. Neural signatures of sleep in zebrafish. Nat. Cell Biol. 2019, 571, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Chen, Y.-C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef]
- Chiu, C.N.; Prober, D.A. Regulation of zebrafish sleep and arousal states: Current and prospective approaches. Front. Neural Circuits 2013, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorribes, A.; Þorsteinsson, H.; Arnardóttir, H.; Jóhannesdóttir, I.Þ.; Sigurgeirsson, B.; De Polavieja, G.G.; Karlsson, K.Æ. The ontogeny of sleep–wake cycles in zebrafish: A comparison to humans. Front. Neural Circuits 2013, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Rihel, J.; Prober, D.A.; Schier, A.F. Monitoring sleep and arousal in zebrafish. Methods Cell Biol. 2010, 100, 281–294. [Google Scholar] [CrossRef]
- Clift, D.; Richendrfer, H.; Thorn, R.J.; Colwill, R.M.; Creton, R. High-Throughput Analysis of Behavior in Zebrafish Larvae: Effects of Feeding. Zebrafish 2014, 11, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. Zebra Fish: An Uncharted Behavior Genetic Model. Behav. Genet. 2003, 33, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R.; Lahav, M.; Guo, S.; Rosenthal, A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000, 67, 773–782. [Google Scholar] [CrossRef]
- Zabegalov, K.N.; Kolesnikova, T.O.; Khatsko, S.L.; Volgin, A.D.; Yakovlev, O.A.; Amstislavskaya, T.G.; Friend, A.J.; Bao, W.; Alekseeva, P.A.; Lakstygal, A.M.; et al. Understanding zebrafish aggressive behavior. Behav. Process. 2019, 158, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, T.; Marin, W.; Faraco, J.; Pézeron, G.; Appelbaum, L.; Zhang, J.; Rosa, F.; Mourrain, P.; Mignot, E. Characterization of Sleep in Zebrafish and Insomnia in Hypocretin Receptor Mutants. PLoS Biol. 2007, 5, e277. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro-Da-Silva, J.; Tran, S.; Luchiari, A.C. Sleep deprivation impairs cognitive performance in zebrafish: A matter of fact? Behav. Process. 2018, 157, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ye, Z.; Houston, C.M.; Zecharia, A.Y.; Ma, Y.; Zhang, Z.; Uygun, D.S.; Parker, S.; Vyssotski, A.L.; Yustos, R.; et al. Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron 2015, 87, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Singh, C.; Oikonomou, G.; Prober, D.A. Genetic Analysis of Histamine Signaling in Larval Zebrafish Sleep. eNeuro 2017, 4, 0286. [Google Scholar] [CrossRef]
- Eban-Rothschild, A.; Appelbaum, L.; De Lecea, L. Neuronal Mechanisms for Sleep/Wake Regulation and Modulatory Drive. Neuropsychopharmacology 2018, 43, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Scammell, T.E.; Jackson, A.C.; Franks, N.P.; Wisden, W.; Dauvilliers, Y. Histamine: Neural circuits and new medications. Sleep 2019, 42. [Google Scholar] [CrossRef] [PubMed]
- Sundvik, M.; Panula, P. Organization of the histaminergic system in adult zebrafish (Danio rerio) brain: Neuron number, location, and cotransmitters. J. Comp. Neurol. 2012, 520, 3827–3845. [Google Scholar] [CrossRef]
- Yu, X.; Franks, N.P.; Wisden, W. Sleep and Sedative States Induced by Targeting the Histamine and Noradrenergic Systems. Front. Neural Circuits 2018, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.D.; Soanes, K.H. A larval zebrafish model of bipolar disorder as a screening platform for neuro-therapeutics. Behav. Brain Res. 2012, 233, 450–457. [Google Scholar] [CrossRef]
- Bailey, J.M.; Oliveri, A.N.; Karbhari, N.; Brooks, R.A.; De La Rocha, A.J.; Janardhan, S.; Levin, E.D. Persistent behavioral effects following early life exposure to retinoic acid or valproic acid in zebrafish. NeuroToxicology 2016, 52, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Lin, J.; Liu, X.; Li, W.; Ding, Y.; Zhang, Y.; Zhou, S.; Guo, N.; Li, Q. Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs. Ann. Transl. Med. 2018, 6, 173. [Google Scholar] [CrossRef]
- Steindal, I.A.F.; Whitmore, D. Circadian Clocks in Fish—What Have We Learned so far? Biology 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, D.; Hoerger, M.; Dyer, T.; Graham, N.; Penney, H.; Mace, F.C. Sodium valproate withdrawal correlates with reduced aggression. Br. J. Learn. Disabil. 2014, 42, 162–167. [Google Scholar] [CrossRef]
- Battaglia, C.; Averna, R.; Labonia, M.; Riccioni, A.; Vicari, S. Intravenous Valproic Acid Add-On Therapy in Acute Agitation Adolescents with Suspected Substance Abuse: A Report of Six Cases. Clin. Neuropharmacol. 2018, 41, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Huband, N.; Ferriter, M.; Nathan, R.; Jones, H. Antiepileptics for aggression and associated impulsivity. Cochrane Database Syst. Rev. 2010, 2010, CD003499. [Google Scholar] [CrossRef] [PubMed]
- Smagin, D.A.; Bondar, N.P.; Kudriavtseva, N.N. Effect of sodium valproate on aggressive behavior of male mice with various aggression experience. Eksp. Klin. Farmakol. 2010, 73, 10–15. [Google Scholar]




| Fragmentation | Sleep Ratio | Velocity | Total Distance Moved | Wake Bout Duration | Sleep Bout Duration | |
|---|---|---|---|---|---|---|
| Group 1 | 142.19 (±2.93) | 0.445 (±0.02) | 0.18 (±0.01) | 296.7 (±30.1) | 14.094 (±1.05) | 11.513 (±0.65) |
| Group 2 | 147.74 (±0.02) | 0.455 (±0.02) | 0.21 (±0.01) | 256.7 (±34.9) | 13.293 (±1.07) | 11.208 (±0.67) |
| Group 3 | 147.67 (±3.03) | 0.456 (±0.02) | 0.18 (±0.01) | 237.4 (±24.9) | 13.261 (±1.09) | 11.237 (±0.68) |
| Group 4 | 133.78 (±2.75) | 0.426 (±0.02) | 0.31 (±0.02) | 467.4 (±47.4) | 16.701 (±0.99) | 11.379 (±0.61) |
| p-value | 0.002 | N.S. | 0.001 | 0.001 | N.S. | N.S. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robea, M.A.; Ciobica, A.; Curpan, A.-S.; Plavan, G.; Strungaru, S.; Lefter, R.; Nicoara, M. Preliminary Results Regarding Sleep in a Zebrafish Model of Autism Spectrum Disorder. Brain Sci. 2021, 11, 556. https://doi.org/10.3390/brainsci11050556
Robea MA, Ciobica A, Curpan A-S, Plavan G, Strungaru S, Lefter R, Nicoara M. Preliminary Results Regarding Sleep in a Zebrafish Model of Autism Spectrum Disorder. Brain Sciences. 2021; 11(5):556. https://doi.org/10.3390/brainsci11050556
Chicago/Turabian StyleRobea, Madalina Andreea, Alin Ciobica, Alexandrina-Stefania Curpan, Gabriel Plavan, Stefan Strungaru, Radu Lefter, and Mircea Nicoara. 2021. "Preliminary Results Regarding Sleep in a Zebrafish Model of Autism Spectrum Disorder" Brain Sciences 11, no. 5: 556. https://doi.org/10.3390/brainsci11050556
APA StyleRobea, M. A., Ciobica, A., Curpan, A.-S., Plavan, G., Strungaru, S., Lefter, R., & Nicoara, M. (2021). Preliminary Results Regarding Sleep in a Zebrafish Model of Autism Spectrum Disorder. Brain Sciences, 11(5), 556. https://doi.org/10.3390/brainsci11050556








