Differential Alterations in Resting State Functional Connectivity Associated with Depressive Symptoms and Early Life Adversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Data Acquisition and Analysis
2.2.1. fMRI Data Acquisition
2.2.2. fMRI Data Analysis
2.2.3. Statistical Analysis
3. Results
3.1. Associations between Functional Connectivity and BDI-II
3.2. Associations between Functional Connectivity and CTQ Abuse and CTQ Neglect
3.3. Moderation Effects of CTQ Abuse and CTQ Neglect on the Association between BDI-II and Functional Connectivity
4. Discussion
4.1. Functional Connectivity Associated with Severity of Depressive Symptoms
4.2. Functional Connectivity Associated with ELA
4.3. Moderation Effects of ELA on the Association of Severity of Depressive Symptoms and FC
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Rojas, L.; Porras-Segovia, A.; Dunne, H.; Andrade-González, N.; Cervilla, J.A. Prevalence and correlates of major depressive disorder: A systematic review. Braz. J. Psychiatry 2020, 42, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Kaltenboeck, A.; Harmer, C. The neuroscience of depressive disorders: A brief review of the past and some considerations about the future. Brain Neurosci. Adv. 2018, 2, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machmutow, K.; Meister, R.; Jansen, A.; Kriston, L.; Watzke, B.; Härter, M.C.; Liebherz, S. Comparative effectiveness of continuation and maintenance treatments for persistent depressive disorder in adults. Cochrane Database Syst. Rev. 2019, 5, 1–3. [Google Scholar] [CrossRef]
- Kraus, C.; Kadriu, B.; Lanzenberger, R.; Zarate, C.A., Jr.; Kasper, S. Prognosis and improved outcomes in major depression: A review. Transl. Psychiatry 2019, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Scheepens, D.S.; van Waarde, J.A.; Lok, A.; de Vries, G.; Denys, D.A.J.P.; van Wingen, G.A. The link between structural and functional brain abnormalities in depression: A systematic review of multimodal neuroimaging studies. Front. Psychiatry 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Disabato, B.; Bauer, I.E.; Soares, J.C.; Sheline, Y. Neural structure and organization of mood pathology. In The Oxford Handbook of Mood Disorders; DeRubeis, R.J., Strunk, D.R., Eds.; Oxford University Press: Oxford, UK, 2016. [Google Scholar] [CrossRef]
- Zhuo, C.; Li, G.; Lin, X.; Jiang, D.; Xu, Y.; Tian, H.; Wang, W.; Song, X. The rise and fall of MRI studies in major depressive disorder. Transl. Psychiatry 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Raichle, M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Hermans, E.J.; Henckens, M.J.A.G.; Joëls, M.; Fernández, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014, 37, 304–314. [Google Scholar] [CrossRef]
- Chen, T.; Cai, W.; Ryali, S.; Supekar, K.; Menon, V. Distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS Biol. 2016, 14, e1002469. [Google Scholar] [CrossRef] [Green Version]
- Goulden, N.; Khusnulina, A.; Davis, N.J.; Bracewell, R.M.; Bokde, A.L.; McNulty, J.P.; Mullins, P.G. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. NeuroImage 2014, 99, 180–190. [Google Scholar] [CrossRef]
- Brakowski, J.; Spinelli, S.; Dörig, N.; Bosch, O.G.; Manoliu, A.; Grosse Holtforth, M.; Seifritz, S. Resting state brain network function in major depression—Depression symptomatology, antidepressant treatment effects, future research. J. Psychiatr. Res. 2017, 92, 147–159. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, H.; Xu, X.; Zuo, Z. Brain structural and functional changes in patients with major depressive disorder: A literature review. PeerJ 2019, 7, 1–17. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Farmer, M.; Fogelman, P.; Gotlib, I.H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry 2015, 78, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef]
- Li, B.; Friston, K.; Mody, M.; Wang, H.; Lu, H.; Hu, D. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. Ther. 2018, 24, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Duan, M.; Chen, X.; Chang, X.; He, H.; Li, Y.; Luo, C.; Yao, D. Common and distinct dysfunctional patterns contribute to triple network model in schizophrenia and depression: A preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Linn, K.A.; Shinohara, R.T.; Oathes, D.J.; Cook, P.A.; Duprat, R.; Moore, T.M.; Oquendo, M.A.; Phillips, M.L.; McInnis, M.; et al. Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc. Natl. Acad. Sci. USA 2019, 116, 8582–8590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulders, P.C.; van Eijndhoven, P.F.; Schene, A.H.; Beckmann, C.F.; Tendolkar, I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015, 56, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Helm, K.; Viol, K.; Weiger, T.M.; Tass, P.A.; Grefkes, C.; del Monte, D.; Schiepek, G. Neuronal connectivity in major depressive disorder: A systematic review. Neuropsychiatr. Dis. Treat. 2018, 14, 2715–2737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeroff, C.B. Paradise lost: The neurobiological and clinical consequences of child abuse and neglect. Neuron 2016, 89, 892–909. [Google Scholar] [CrossRef] [Green Version]
- Lippard, E.T.C.; Nemeroff, C.B. The devastating clinical consequences of child abuse and neglect: Increased disease vulnerability and poor treatment response in mood disorders. Am. J. Psychiatry 2020, 177, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Baram, T.Z. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacol. Rev. 2016, 41, 197–206. [Google Scholar] [CrossRef] [Green Version]
- VanTieghem, M.R.; Tottenham, N. Neurobiological programming of early life stress: Functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr. Top. Behav. Neurosci. 2018, 38, 117–136. [Google Scholar] [CrossRef]
- Hart, H.; Lim, L.; Mehta, M.A.; Chatzieffraimidou, A.; Curtis, C.; Xu, X.; Breen, G.; Simmons, A.; Mirza, K.; Rubia, K. Reduced functional connectivity of fronto-parietal sustained attention networks in severe childhood abuse. PLoS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Marusak, H.A.; Etkin, A.; Thomason, M.E. Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. NeuroImage Clin. 2015, 8, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Gao, W.; Wei, Z.; Wang, D.; Hu, S.; Huang, M.; Xu, Y.; Li, L. Intrinsic brain abnormalities in young healthy adults with childhood trauma: A resting-state functional magnetic resonance imaging study of regional homogeneity and functional connectivity. Aust. N. Z. J. Psychiatry 2017, 51, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Samson, J.A.; Anderson, C.M.; Ohashi, K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016, 17, 652–666. [Google Scholar] [CrossRef]
- Wang, L.; Dai, Z.; Peng, H.; Tan, L.; Ding, Y.; He, Z.; Zhang, Y.; Xia, M.; Li, Z.; Li, W.; et al. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum. Brain Mapp. 2014, 35, 1154–1166. [Google Scholar] [CrossRef]
- Suzuki, H.; Luby, J.L.; Botteron, K.N.; Dietrich, R.; McAvoy, M.P.; Barch, D.M. Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 800–813. [Google Scholar] [CrossRef] [Green Version]
- Grant, M.M.; White, D.; Hadley, J.; Hutcheson, N.; Shelton, R.; Sreenivasan, K.; Deshpande, G. Early life trauma and directional brain connectivity within major depression. Hum. Brain Mapp. 2014, 35, 4815–4826. [Google Scholar] [CrossRef]
- Saleh, A.; Potter, G.G.; McQuoid, D.R.; Boyd, B.; Turner, R.; MacFall, J.R.; Taylor, W.D. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 2017, 47, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Teicher, M.H.; Samson, J.A. Annual research review: Enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry 2016, 57, 241–266. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.W.; Li, D.; Whipple, S.S. Cumulative risk and child development. Psychol. Bull. 2013, 139, 1342–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, K.A.; Sheridan, M.A. Beyond cumulative risk: A dimensional approach to childhood adversity. Curr. Dir. Psychol. Sci. 2016, 25, 239–245. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, K.A.; Sheridan, M.A.; Lambert, H.K. Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 2014, 47, 578–591. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, M.; McLaughlin, K. Dimensions of early experience and neural development: Deprivation and threat. Trends Cogn. Neurosci. 2014, 18, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Cassiers, L.L.M.; Sabbe, B.G.C.; Schmaal, L.; Veltman, D.J.; Penninx, B.W.J.H.; Van Den Eede, F. Structural and functional brain abnormalities associated with exposure to different childhood trauma subtypes: A systematic review of neuroimaging findings. Front. Psychiatry 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, L.L.; Tyrka, A.R.; Ross, N.S.; Khoury, L.; Anderson, G.M.; Price, L.H. Effect of childhood emotional abuse and age oncortisol responsivity in adulthood. Biol. Psychiatry 2009, 66, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Margraf, J.; Cwik, J.C.; Pflug, V.; Schneider, S. Strukturierte klinische Interviews zur Erfassung psychischer Störungen über die Lebensspanne. Zeitschrift Für Klinische Psychologie Und Psychotherapie 2017, 46, 176–186. [Google Scholar] [CrossRef]
- Wingenfeld, K.; Spitzer, C.; Mensebach, C.; Grabe, H.J.; Hill, A.; Gast, U.; Schlosser, N.; Höpp, H.; Beblo, T.; Driessen, M. Die deutsche Version des Childhood Trauma Questionnaire (CTQ): Erste Befunde zu den psychometrischen Kennwerten. Psychother. Psych. Med. 2010, 60, 442–450. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II, Beck Depression Inventory: Manual; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Wang, Y.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Braz. J. Psychiatry 2013, 35, 416–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruessmann, K.P.; Weiger, M.; Scheidegger, M.B.; Boesiger, P. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med. 1999, 42, 952–962. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Manoliu, A.; Meng, C.; Brandl, F.; Doll, A.; Tahmasian, M.; Scherr, M.; Schwerthöffer, D.; Zimmer, C.; Förstl, H.; Bäuml, J.; et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2014, 7, 1–17. [Google Scholar] [CrossRef]
- Pannekoek, J.N.; van der Werff, S.J.A.; Meens, P.H.F.; van den Bulk, B.G.; Jolles, D.D.; Veer, I.M.; van Lang, N.D.J.; Rombouts, S.A.R.B.; van der Wee, N.J.A.; Vermeiren, R.R.J.M. Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naive clinically depressed adolescents. J. Child Psychol. Psychiatry 2014, 55, 1317–1327. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Glover, G.H.; Bagarinao, E.; Chang, C.; Mackey, S.; Sacchet, M.D.; Gotlib, I.H. Effects of salience-network-node neurofeedback training on affective biases in major depressive disorder. Psychiatry Res. Neuroimaging 2016, 249, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Hilland, E.; Landrø, N.I.; Harmer, C.J.; Maglanoc, L.A.; Jonassen, R. Within-Network connectivity in salience network after attention bias. Modification training in residual depression: Report from a preregistered clinical trial. Front. Hum. Neurosci. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Liu, C.; Ma, X.; Song, L.; Tang, L.; Jing, B.; Zhang, Y.; Feng, L.; Zhou, Z.; Fan, J.; Wang, C. Alteration of spontaneous neuronal activity within the salience network in partially remitted depression. Brain Res. 2015, 1599, 93–102. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, N.; Imamura, K.; Lu, S.; Li, M.; Zhou, H.; Li, H.; Yang, X.; Wan, Z.; Wang, G.; et al. Task and resting-state fMRI reveal altered salience responses to positive stimuli in patients with major depressive disorder. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, R.G.; Gilbert, S.J.; Frith, C.D.; Burgess, P.W. Rostral prefrontal cortex and the focus of attention in prospective memory. Cereb. Cortex 2012, 22, 1876–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, R.G.; Gilbert, S.J.; Volle, E.; Burgess, P.W. When I think about me and simulate you: Medial rostral prefrontal cortex and self-referential processes. NeuroImage 2010, 50, 1340–1349. [Google Scholar] [CrossRef]
- Burgess, P.W.; Dumontheil, I.; Gilbert, S.J. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn. Sci. 2007, 11, 290–298. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Price, J.L.; Yan, Z.; Mintun, M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. USA 2010, 107, 11020–11025. [Google Scholar] [CrossRef] [Green Version]
- Dutta, A.; McKie, S.; Deakin, J.F.W. Resting state networks in major depressive disorder. Psychiatry Res. Neuroimaging 2014, 224, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Chen, X.; Li, L.; Castellanos, F.X.; Bai, T.; Bo, Q.; Cao, J.; Chen, G.; Chen, N.; Chen, W.; et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci. USA 2019, 116, 9078–9083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bellis, M.D. The psychobiology of neglect. Child Maltreat. 2005, 10, 150–172. [Google Scholar] [CrossRef]
- Sheridan, M.A.; McLaughlin, K.A. Chapter 13—Neurodevelopmental mechanisms linking ACEs with psychopathology. In Adverse Childhood Experiences; Asmundson, G.J.G., Afifi, T.O., Eds.; Academic Press: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Yuen, G.S.; Gunning-Dixon, F.M.; Hoptman, M.J.; AbdelMalak, B.; McGovern, A.R.; Seirup, J.K.; Alexopoulos, G.S. The salience network in the apathy of late-life depression. Int. J. Geriatr. Psychiatry 2014, 29, 1116–1124. [Google Scholar] [CrossRef] [Green Version]
- Geugies, H.; Opmeer, E.M.; Marsman, J.B.C.; Figueroa, C.A.; van Tol, M.J.; Schmaal, L.; van der Wee, N.J.A.; Aleman, A.; Penninx, B.W.J.H.; Veltman, D.J.; et al. Decreased functional connectivity of the insula within the salience network as an indicator for prospective insufficient response to antidepressants. NeuroImage Clin. 2019, 24, 1–8. [Google Scholar] [CrossRef]
- Nelson, J.; Klumparendt, A.; Doebler, P.; Ehring, T. Childhood maltreatment and characteristics of adult depression: Meta-analysis. Br. J. Psychiatry 2017, 201, 96–104. [Google Scholar] [CrossRef]
- Miller, A.B.; Sheridan, M.; Hanson, J.L.; McLaughlin, K.A.; Bates, J.E.; Lansford, J.E.; Pettit, G.S.; Dodge, K.A. Dimensions of deprivation and threat, psychopathology, and potential mediators: A multi-year longitudinal analysis. J. Abnorm. Psychol. 2019, 127, 160–170. [Google Scholar] [CrossRef]
- Duerden, E.G.; Arsalidou, M.; Lee, M.; Taylor, M.J. Lateralization of affective processing in the insula. NeuroImage 2013, 78, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Patil, K.R.; Hoffstaedter, F.; Nostro, A.; Yeo, B.T.T.; Eickhoff, S.B. Sex classification by resting state brain connectivity. Cereb. Cortex 2020, 30, 824–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardt, J.; Rutter, M. Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. J. Child Psychol. Psychiatry 2004, 45, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pechtel, P.; Pizzagalli, D.A. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology 2011, 214, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.C.; Davis, C.G.; Kendler, K.S. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol. Med. 1997, 27, 1101–1119. [Google Scholar] [CrossRef] [PubMed]
| Variables | Healthy Control Group (n = 42) | Depressive Patients (n = 41) |
|---|---|---|
| Age | M = 30.74 (SD = 11.22) | M = 28.2 (SD = 10.06) |
| Gender | ||
| Female Male | n = 32 n = 10 | n = 31 n = 10 |
| BDI-II | M = 2.43 (SD = 2.92) | M = 27.1 (SD = 9.04) |
| CTQ: Neglect | M = 14.55 (SD = 5.31) | M = 16.73 (SD = 6.27) |
| CTQ: Abuse | M = 12.38 (SD = 3.65) | M = 15.27 (SD = 6.34) |
| CTQ subscales | ||
| CTQ: SA | M = 5.12 (SD = 0.55) | M = 5.44 (SD = 1.23) |
| CTQ: EN | M = 8.12 (SD = 3.83) | M = 10.22 (SD = 4.53) |
| CTQ: PN | M = 6.43 (SD = 2.06) | M = 6.51 (SD = 2.27) |
| CTQ: EA | M = 7 (SD = 2.66) | M = 9 (SD = 3.80) |
| CTQ: PA | M = 5.38 (SD = 1.58) | M = 6.27 (SD = 3.05) |
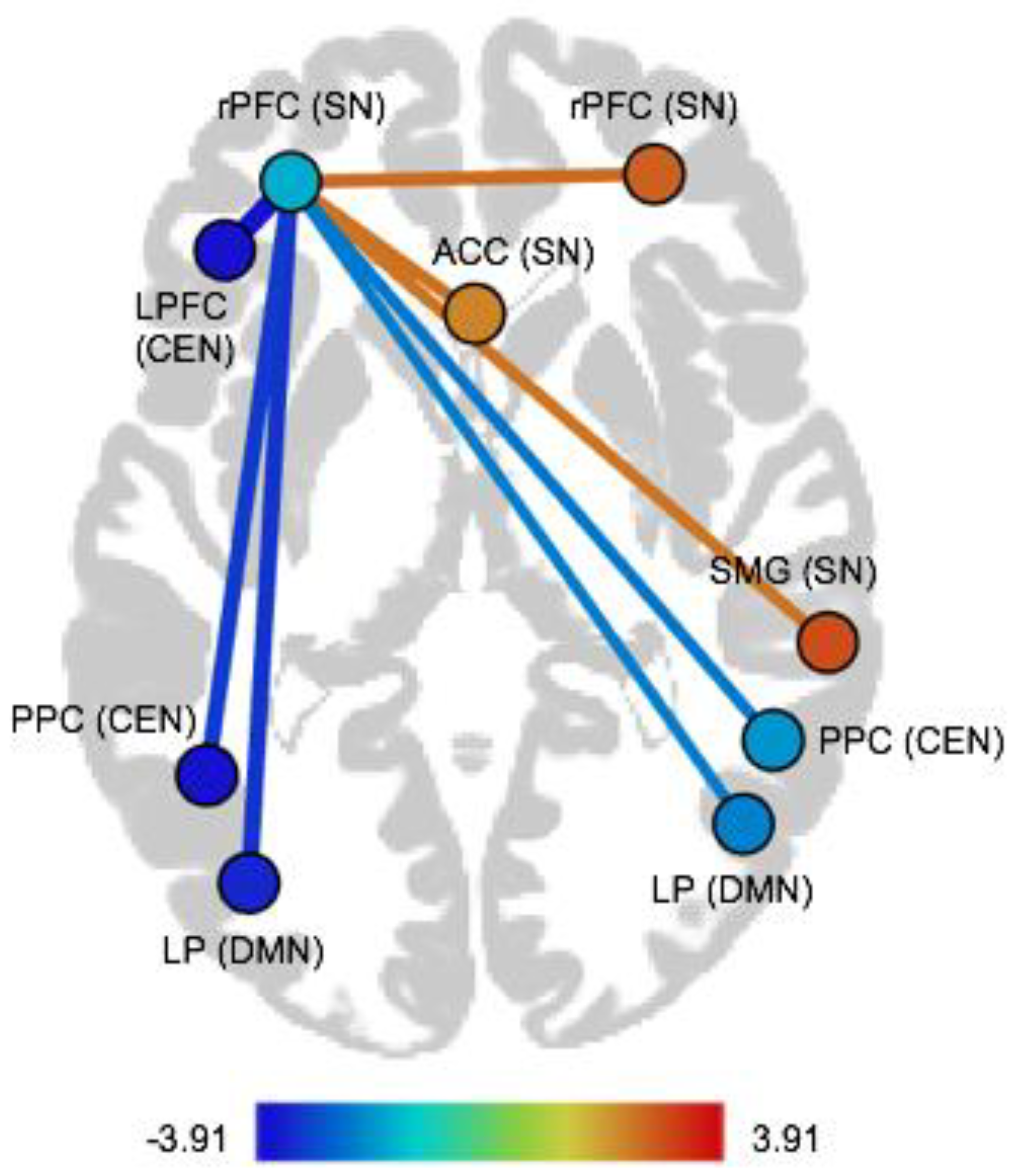
| Pair of ROIs | Dir | T(75) | p-FDR |
|---|---|---|---|
| Within-network FC | |||
| Salience network | |||
| rPFC (left)–rPFC (right) | pos | 2.58 | 0.04 |
| rPFC (left)–ACC | pos | 2.49 | 0.04 |
| rPFC (left)–SMG (right) | pos | 2.45 | 0.04 |
| Between-network FC | |||
| Salience network–Default mode network | |||
| rPFC (left)–LP (left) | neg | −3.23 | 0.01 |
| rPFC (left)–LP (right) | neg | −2.29 | 0.04 |
| Salience network–Central executive network | |||
| rPFC (left)–LPFC (left) | neg | −3.91 | 0.003 |
| rPFC (left)–PPC (left) | neg | −3.12 | 0.01 |
| rPFC (left)–PPC (right) | neg | −2.37 | 0.04 |
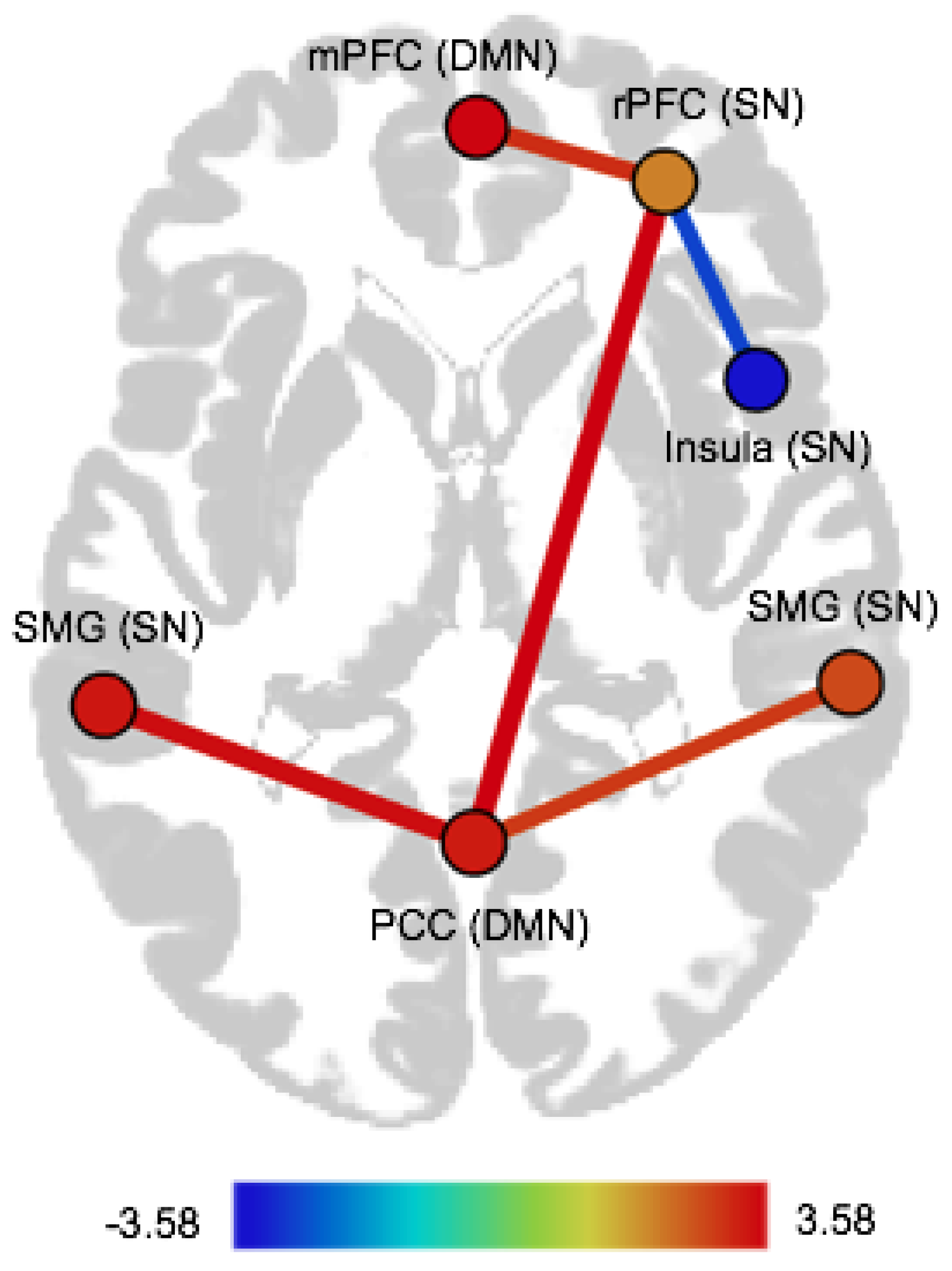
| Pair of ROIs | Dir | T(75) | p-FDR |
|---|---|---|---|
| CTQ abuse | |||
| Within-network FC | |||
| Salience network | |||
| rPFC (right)–Insula (right) | pos | 3.40 | 0.02 |
| CTQ neglect | |||
| Within-network FC | |||
| Salience network | |||
| rPFC (right)–Insula (right) | neg | −2.75 | 0.03 |
| Between-network FC | |||
| Salience network–Default mode network | |||
| rPFC (right)–PCC | pos | 3.58 | 0.01 |
| rPFC (right)–mPFC | pos | 3.05 | 0.02 |
| SMG (left)–PCC | pos | 3.44 | 0.01 |
| SMG (right)–PCC | pos | 2.91 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadel, E.; Boeker, H.; Gaertner, M.; Richter, A.; Kleim, B.; Seifritz, E.; Grimm, S.; Wade-Bohleber, L.M. Differential Alterations in Resting State Functional Connectivity Associated with Depressive Symptoms and Early Life Adversity. Brain Sci. 2021, 11, 591. https://doi.org/10.3390/brainsci11050591
Fadel E, Boeker H, Gaertner M, Richter A, Kleim B, Seifritz E, Grimm S, Wade-Bohleber LM. Differential Alterations in Resting State Functional Connectivity Associated with Depressive Symptoms and Early Life Adversity. Brain Sciences. 2021; 11(5):591. https://doi.org/10.3390/brainsci11050591
Chicago/Turabian StyleFadel, Eleonora, Heinz Boeker, Matti Gaertner, Andre Richter, Birgit Kleim, Erich Seifritz, Simone Grimm, and Laura M. Wade-Bohleber. 2021. "Differential Alterations in Resting State Functional Connectivity Associated with Depressive Symptoms and Early Life Adversity" Brain Sciences 11, no. 5: 591. https://doi.org/10.3390/brainsci11050591
APA StyleFadel, E., Boeker, H., Gaertner, M., Richter, A., Kleim, B., Seifritz, E., Grimm, S., & Wade-Bohleber, L. M. (2021). Differential Alterations in Resting State Functional Connectivity Associated with Depressive Symptoms and Early Life Adversity. Brain Sciences, 11(5), 591. https://doi.org/10.3390/brainsci11050591






