Imaging of Functional Brain Circuits during Acquisition and Memory Retrieval in an Aversive Feedback Learning Task: Single Photon Emission Computed Tomography of Regional Cerebral Blood Flow in Freely Behaving Rats
Abstract
1. Introduction
2. Materials and Methods
Subjects and Housing
3. Behavioral Experiments
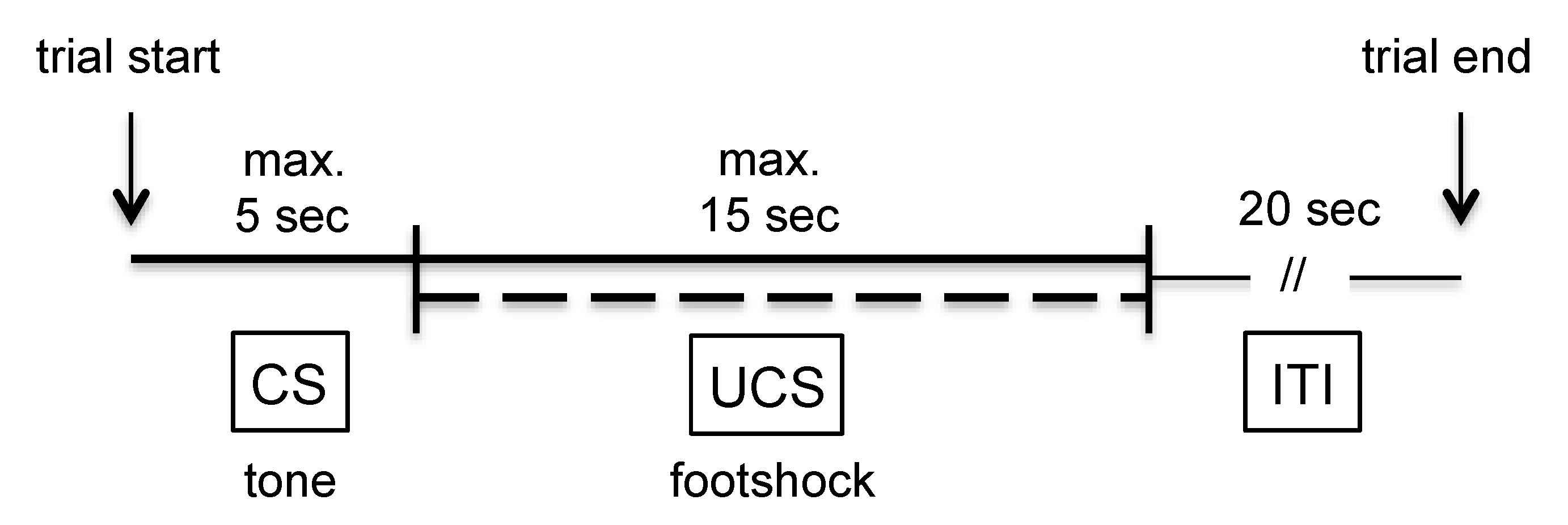
3.1. Two-Way Avoidance (TWA) Training
3.2. Experimental Groups
3.3. Imaging Experiments
3.3.1. Jugular Vein Catheterization
3.3.2. Intravenous 99mTc-HMPAO Application in Freely Behaving Animals
3.3.3. In Vivo SPECT-Imaging of Regional Cerebral Blood Flow
3.3.4. SPECT Data Analysis
4. Results
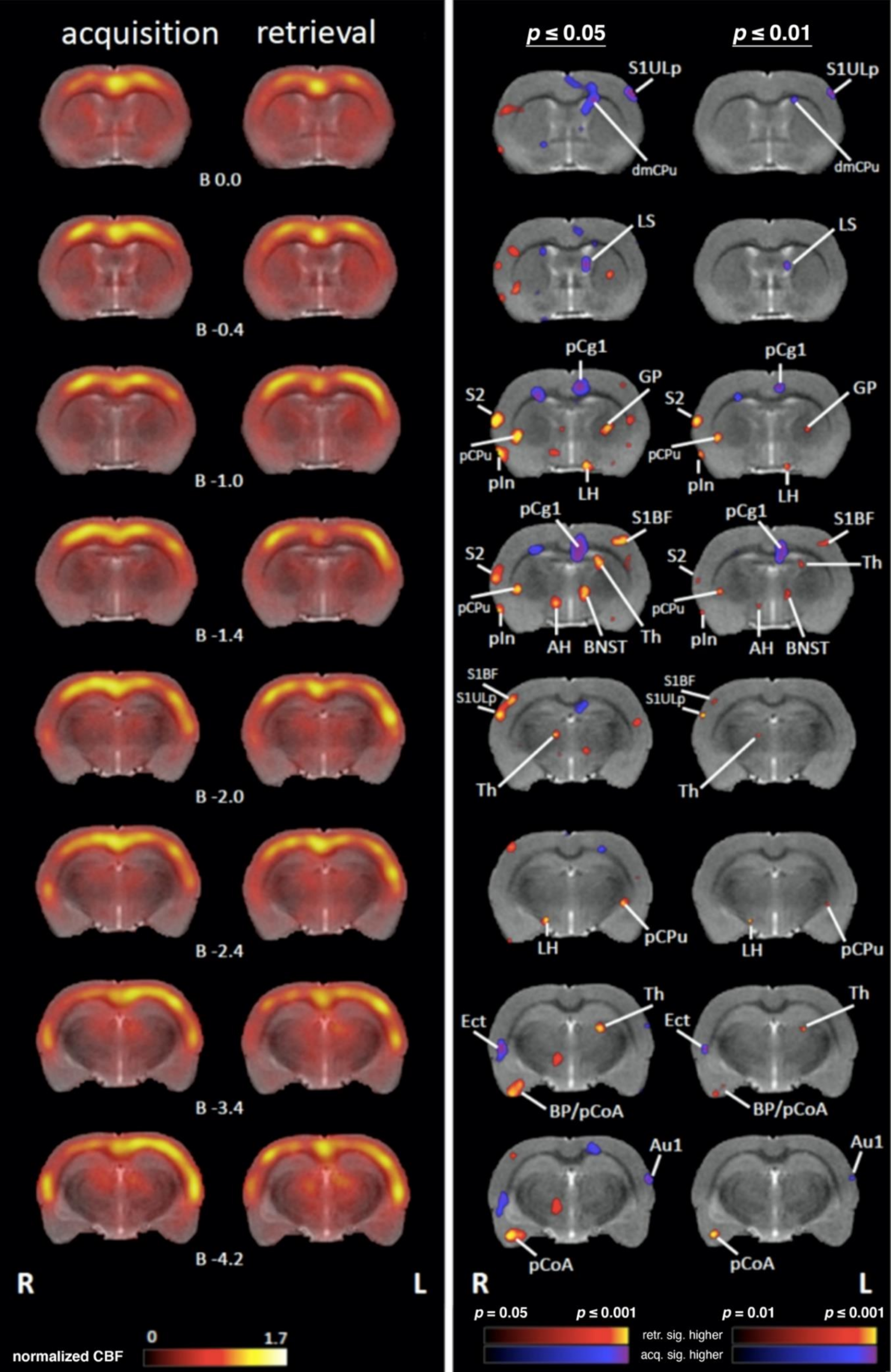
4.1. Comparing Regional Cerebral Blood Flow during Acquisition and Retrieval
4.1.1. Sensory/Motor Cortex
4.1.2. Subcortical Systems
4.1.3. Association and Prefrontal Cortices
4.1.4. Medial Temporal Lobe (MTL)/Limbic Regions
4.1.5. Limbic Output Regions
5. Discussion
5.1. Learning to Avoid an Aversive Situation—Switching from Escape to Avoidance Strategy
5.2. Acquisition
5.2.1. The Role of Sensory and Motor Pathways in CS-UCS Association and the Escape Strategy
5.2.2. The Role of Prefrontal/Association Cortices and Limbic Regions in CS-UCS Association, Working Memory and Avoidance Strategy
5.3. Retrieval
The Role of Prefrontal, Subcortical Sensory and Limbic Systems in Memory Recall and Behavioral Strategy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Glossary
| Acb | nucleus accumbens |
| shAcb | n. accumbens—shell region |
| cAcb | n. accumbens—core region |
| AH | anterior hypothalamus |
| Au1 | primary auditory cortex |
| Au2 | secondary auditory cortex |
| BA | anterior basal amygdala |
| BNST | bed nucleus of the stria terminalis |
| BP | posterior basal amygdala |
| CeA | central amygdala |
| Cg1 | cingular cortex |
| CoA | cortical amygdala |
| CoM | corpus mammillare |
| CPu | caudate putamen |
| Ect | ectorhinal cortex |
| Ent | entorhinal cortex |
| GP | globus pallidus |
| Hip | hippocampus |
| IC | inferior colliculus |
| IL | infralimbic cortex |
| aIn | anterior insular cortex |
| pIn | posterior insular cortex |
| IP | interpeduncular nucleus |
| LA | lateral amygdala |
| LG | lateral geniculatum |
| LH | lateral hypothalamus |
| LS | lateral septum |
| M1 | primary motor cortex |
| M2 | secondary motor cortex |
| MeA | medial amygdala |
| MG | medial geniculatum |
| MH | medial hypothalamus |
| MO | medial orbitofrontal cortex |
| MS | medial septum |
| MTL | medial temporal lobe |
| ON | olfactory nucleus |
| OT | olfactory tubercle |
| PAG | periaqueductal grey |
| Pir | piriform cortex |
| Pn | pontine nucleus |
| PRh | perirhinal cortex |
| PrL | prelimbic cortex |
| PTA | area pretectalis |
| PtA | parietal association cortex |
| RNc | raphe nuclei |
| RSA | agranular retrosplenial cortex |
| RSG | granular retrosplenial cortex |
| S1BF | primary somatosensory cortex—barrel cortex |
| S1FL | primary somatosensory cortex—forelimbs |
| S1HL | primary somatosensory cortex—hindlimbs |
| S1J | primary somatosensory cortex—jaw region |
| S1ULp | primary somatosensory cortex—upper lip |
| S2 | secondary somatosensory cortex |
| SC | superior colliculus |
| SF | septofimbrial nucleus |
| SG | subgeniculatum |
| SN | substantia nigra |
| Sub | subiculum |
| TeA | temporal association cortex |
| Tg | tegmentum |
| Th | thalamus |
| TS | triangular septum |
| TT | taenia tecta |
| V1B | primary visual cortex—binocular area |
| V1M | primary visual cortex—monocular area |
| V2L | secondary visual cortex—lateral |
| V2M | secondary visual cortex—medial |
| VG | ventral geniculatum |
| VO/LO | ventral/lateral orbitofrontal cortex |
| VP | ventral pallidum |
| VTA | ventral tegmental area |
References
- Cartoni, E.; Puglisi-Allegra, S.; Baldassarre, G. The three principles of action: A Pavlovian-instrumental transfer hypothesis. Front. Behav. Neurosci. 2013, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Cartoni, E.; Balleine, B.; Baldassarre, G. Appetitive Pavlovian-instrumental Transfer: A review. Neurosci. Biobehav. Rev. 2016, 71, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Jung, M.W. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neurosci. Biobehav. Rev. 2006, 30, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Stark, H.; Bischof, A.; Scheich, H. Increase of extracellular dopamine in prefrontal cortex of gerbils during acquisition of the avoidance strategy in the shuttle-box. Neurosci. Lett. 1999, 264, 77–80. [Google Scholar] [CrossRef]
- Stark, H.; Bischof, A.; Wagner, T.; Scheich, H. Stages of avoidance strategy formation in gerbils are correlated with dopa-minergic transmission activity. Eur. J. Pharmacol. 2000, 405, 263–275. [Google Scholar] [CrossRef]
- Oelschlegel, A.M.; Goldschmidt, J. Functional Neuroimaging in Rodents Using Cerebral Blood Flow SPECT. Front. Phys. 2020, 8, 152. [Google Scholar] [CrossRef]
- Mannewitz, A.; Bock, J.; Kreitz, S.; Hess, A.; Goldschmidt, J.; Scheich, H.; Braun, K. Comparing brain activity patterns dur-ing spontaneous exploratory and cue-instructed learning using single photon-emission computed tomography (SPECT) im-aging of regional cerebral blood flow in freely behaving rats. Brain Struct. Funct. 2018, 223, 2025–2038. [Google Scholar] [CrossRef]
- Goldschmidt, J.; Wanger, T.; Engelhorn, A.; Friedrich, H.; Happel, M.; Ilango, A.; Engelmann, M.; Stuermer, I.W.; Ohl, F.W.; Scheich, H. High-resolution mapping of neuronal activity using the lipophilic thallium chelate complex TlDDC: Protocol and validation of the method. NeuroImage 2010, 49, 303–315. [Google Scholar] [CrossRef]
- Vincenz, D.; Wernecke, K.E.; Fendt, M.; Goldschmidt, J. Habenula and interpeduncular nucleus differentially modulate predator odor-induced innate fear behavior in rats. Behav. Brain Res. 2017, 332, 164–171. [Google Scholar] [CrossRef]
- Kolodziej, A.; Lippert, M.; Angenstein, F.; Neubert, J.; Pethe, A.; Grosser, O.S.; Amthauer, H.; Schroeder, U.H.; Reymann, K.G.; Scheich, H.; et al. SPECT-imaging of activity-dependent changes in regional cerebral blood flow induced by electrical and optogenetic self-stimulation in mice. NeuroImage 2014, 103, 171–180. [Google Scholar] [CrossRef][Green Version]
- Hess, A.; Axmann, R.; Rech, J.; Finzel, S.; Heindl, C.; Kreitz, S.; Sergeeva, M.; Saake, M.; Garcia, M.; Kollias, G.; et al. Blockade of TNF-α rapidly inhibits pain responses in the central nervous sys-tem. Proc. Natl. Acad. Sci. USA 2011, 108, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Knabl, J.; Witschi, R.; Hösl, K.; Reinold, H.; Zeilhofer, U.B.; Ahmadi, S.; Brockhaus, J.; Sergejeva, M.; Hess, A.; Brune, K.; et al. Reversal of pathological pain through specific spinal GABAA recep-tor subtypes. Nature 2008, 451, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Endepols, H.; Sommer, S.; Backes, H.; Wiedermann, D.; Graf, R.; Hauber, W. Effort-Based Decision Making in the rat: An [18F]Fluorodeoxyglucose Micro Positron Emission Tomography Study. J. Neurosci. 2010, 30, 9708–9714. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Anderson, S.A.R.; Ananth, M.; Smirnov, D.; Thanos, P.K.; Neumaier, J.F.; Wang, G.-J.; Volkow, N.D.; Hurd, Y.L. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J. Clin. Investig. 2013, 123, 5342–5350. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C.R.; Emson, P.C. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J. Neurosci. Methods 1980, 3, 129–149. [Google Scholar] [CrossRef]
- Schäble, S.; Poeggel, G.; Braun, K.; Gruss, M. Long-term consequences of early experience on adult avoidance learning in fe-male rats: Role of the dopaminergic system. Neurobiol. Learn. Mem. 2007, 87, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Gruss, M.; Abraham, A.; Schäble, S.; Becker, S.; Braun, K. Cognitive training during infancy and adolescence facilitates adult associative learning: Critical impact of age, stimulus contingency and training intensity. Neurobiol. Learn. Mem. 2010, 94, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Riedel, A.; Gruss, M.; Bock, J.; Braun, K. Impaired active avoidance learning in infant rats may be related to insufficient met-abolic recruitment of the lateral septum. Neurobiol. Learn. Mem. 2010, 93, 275–282. [Google Scholar] [CrossRef]
- Neirinckx, R.D.; Canning, L.R.; Piper, I.M.; Nowotnik, D.P.; Pickett, R.D.; Holmes, R.A.; Volkert, W.A.; Forster, A.M.; Weisner, P.S.; Marriott, J.A. Technetium-99m d,l-HM-PAO: A new radiopharmaceutical for SPECT imaging of regional cerebral blood perfusion. J. Nucl. Med. 1987, 28, 191–202. [Google Scholar]
- Geurts, D.E.M.; Huys, Q.J.M.; Ouden, H.E.M.D.; Cools, R. Aversive Pavlovian Control of Instrumental Behavior in Humans. J. Cogn. Neurosci. 2013, 25, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Scheich, H.; Brosch, M. Task-related activation of auditory cortex. In Neural Correlates of Auditory Cognition; Springer: New York, NY, USA, 2013; pp. 45–81. [Google Scholar]
- Cain, C.K.; LeDoux, J.E. Emotional processing and motivation: In search of brain mechanisms. In Handbook of Approach and Avoidance Motivation; Elliot, A.J., Ed.; Psychology Press: London, UK, 2008; pp. 17–34. [Google Scholar]
- Spröwitz, A.; Bock, J.; Braun, K. Sex-specific positive and negative consequences of avoidance training during childhood on adult active avoidance learning in mice. Front. Behav. Neurosci. 2013, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992, 15, 353–375. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. The Emotional Brain, Fear, and the Amygdala. Cell. Mol. Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Goosens, K.A.; Maren, S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygda-loid nuclei in rats. Learn. Mem. 2001, 8, 148–155. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Gale, G.D. The amygdala, fear, and memory. Ann. N. Y. Acad. Sci. 2003, 985, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Gilbertson, M.W.; Orr, S.P.; Herzallah, M.M.; Servatius, R.J.; Myers, C.E. A model of amygdala–hippocampal–prefrontal interaction in fear conditioning and extinction in animals. Brain Cogn. 2013, 81, 29–43. [Google Scholar] [CrossRef]
- Staib, J.M.; Della Valle, R.; Knox, D.K. Disruption of medial septum and diagonal bands of Broca cholinergic projections to the ventral hippocampus disrupt auditory fear memory. Neurobiol. Learn. Mem. 2018, 152, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, N.M. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear. Res. 2007, 229, 54–68. [Google Scholar] [CrossRef]
- Scheich, H.; Brechmann, A.; Brosch, M.; Budinger, E.; Ohl, F.W.; Selezneva, E.; Stark, H.; Tischmeyer, W.; Wetzel, W. Behav-ioral semantics of learning and crossmodal processing in auditory cortex: The semantic processor concept. Hear. Res. 2011, 27, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Brosch, M.; Selezneva, E.; Scheich, H. Representation of Reward Feedback in Primate Auditory Cortex. Front. Syst. Neurosci. 2011, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.R. Auditory perceptual learning and changes in the conceptualization of auditory cortex. Hear. Res. 2018, 366, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Ohl, F.W.; Scheich, H. Learning-induced plasticity in animal and human auditory cortex. Curr. Opin. Neurobiol. 2005, 15, 470–477. [Google Scholar] [CrossRef]
- Matsumoto, N.; Hanakawa, T.; Maki, S.; Graybiel, A.M.; Kimura, M. Nigrostriatal Dopamine System in Learning to Perform Sequential Motor Tasks in a Predictive Manner. J. Neurophysiol. 1999, 82, 978–998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiotani, K.; Tanisumi, Y.; Murata, K.; Hirokawa, J.; Sakurai, Y.; Manabe, H. Tuning of olfactory cortex ventral tenia tecta neurons to distinct task elements of goal-directed behavior. eLife 2020, 9, 57268. [Google Scholar] [CrossRef]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 2000, 1, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. The Prefrontal Cortex, 4th ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Pearson, J.M.; Heilbronner, S.R.; Barack, D.L.; Hayden, B.Y.; Platt, M.L. Posterior cingulate cortex: Adapting behavior to a changing world. Trends Cogn. Sci. 2011, 15, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Klein, T.A.; Endrass, T.; Kathmann, N.; Neumann, J.; von Cramon, D.Y.; Ullsperger, M. Neural correlates of error awareness. NeuroImage 2007, 34, 1774–1781. [Google Scholar] [CrossRef]
- Kennerley, S.W.; Walton, M.E.; Behrens, T.E.; Buckley, M.J.; Rushworth, M.F. Optimal decision making and the anterior cin-gulate cortex. Nat. Neurosci. 2006, 7, 940–947. [Google Scholar] [CrossRef] [PubMed]
- van Veen, V.; Cohen, J.D.; Botvinick, M.M.; Stenger, V.A.; Carter, C.S. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 2001, 6, 1302–1308. [Google Scholar] [CrossRef]
- Gianaros, P.J.; Derbtshire, S.W.; May, J.C.; Siegle, G.J.; Gamalo, M.A.; Jennings, J.R. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology 2005, 42, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Yeung, N.; Botvinick, M.M.; Cohen, J.D. The Neural Basis of Error Detection: Conflict Monitoring and the Error-Related Negativity. Psychol. Rev. 2004, 111, 931–959. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.A.; Ullsperger, M.M.P.; Danielmeier, C. Error awareness and the insula: Links to neurological and psychiatric diseases. Front. Hum. Neurosci. 2013, 7, 14. [Google Scholar] [CrossRef]
- Gogolla, N. The insular cortex. Curr. Biol. 2017, 27, R580–R586. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Miezin, F.M.; Cohen, A.L.; Wenger, K.K.; Dosenbach, R.A.T.; Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E.; et al. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11073–11078. [Google Scholar] [CrossRef]
- Lovero, K.L.; Simmons, A.N.; Aron, J.L.; Paulus, M.P. Anterior insular cortex anticipates impending stimulus significance. NeuroImage 2009, 45, 976–983. [Google Scholar] [CrossRef]
- Méndez-Ruette, M.; Linsambarth, S.; Moraga-Amaro, R.; Quintana-Donoso, D.; Méndez, L.; Tamburini, G.; Cornejo, F.; Torres, R.F.; Stehberg, J. The Role of the Rodent Insula in Anxiety. Front. Physiol. 2019, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damasio, A.R. The somatic marker hypothesis: A neural theory of economic decision. Games Econ. Behav. 2005, 52, 336–372. [Google Scholar] [CrossRef]
- Tabbert, K.; Stark, R.; Kirsch, P.; Vaitl, D. Hemodynamic responses of the amygdala, the orbitofrontal cortex and the visual cortex during a fear conditioning paradigm. Int. J. Psychophysiol. 2005, 57, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damasio, H.; Damasio, A.R. Emotion, Decision Making and the Orbitofrontal Cortex. Cereb. Cortex 2000, 10, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The Orbitofrontal Cortex and Reward. Cereb. Cortex 2000, 10, 284–294. [Google Scholar] [CrossRef]
- Roberts, A. Primate orbitofrontal cortex and adaptive behaviour. Trends Cogn. Sci. 2006, 10, 83–90. [Google Scholar] [CrossRef]
- Schoenbaum, G.; Roesch, M.R.; Stalnaker, T.A.; Takahashi, Y.K. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat. Rev. Neurosci. 2009, 10, 885–892. [Google Scholar] [CrossRef]
- Kim, H.; Shimojo, S.; O’Doherty, J.P. Is Avoiding an Aversive Outcome Rewarding? Neural Substrates of Avoidance Learn-ing in the Human Brain. PLoS Biol. 2006, 4, e233. [Google Scholar] [CrossRef] [PubMed]
- Calandreau, L.; Jaffard, R.; Desmedt, A. Dissociated roles for the lateral and medial septum in elemental and contextual fear conditioning. Learn. Mem. 2007, 14, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Mogenson, G.J.; Jones, D.L.; Yim, C.Y. From motivation to action: Functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980, 14, 69–97. [Google Scholar] [CrossRef]
- Hart, G.; Leung, B.K.; Balleine, B.W. Dorsal and ventral streams: The distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol. Learn. Mem. 2014, 108, 104–118. [Google Scholar] [CrossRef]
- Malenka, R.C.; Nestler, E.J.; Hyman, S.E. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, 2nd ed.; Sydor, A., Brown, R.Y., Eds.; McGraw-Hill Medical: New York, NY, USA, 2009; pp. 147–148. [Google Scholar]
- Fenu, S.; Bassareo, V.; Di Chiara, G. A Role for Dopamine D1 Receptors of the Nucleus Accumbens Shell in Conditioned Taste Aversion Learning. J. Neurosci. 2001, 21, 6897–6904. [Google Scholar] [CrossRef]
- Ambroggi, F.; Ishikawa, A.; Fields, H.L.; Nicola, S.M. Basolateral Amygdala Neurons Facilitate Reward-Seeking Behavior by Exciting Nucleus Accumbens Neurons. Neuron 2008, 59, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Bissonette, G.B.; Burton, A.C.; Gentry, R.N.; Goldstein, B.L.; Hearn, T.N.; Barnett, B.R.; Kashtelyan, V.; Roesch, M.R. Separate Populations of Neurons in Ventral Striatum Encode Value and Motivation. PLoS ONE 2013, 8, e64673. [Google Scholar] [CrossRef]
- Maia, T.V. Two-factor theory, the actor--critic model, and conditioned avoidance. Learn. Behav. 2010, 38, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Lex, A.; Hauber, W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn. Mem. 2008, 15, 483–491. [Google Scholar] [CrossRef]
- Kitanishi, T.; Umaba, R.; Mizuseki, K. Robust information routing by dorsal subiculum neurons. Sci. Adv. 2021, 7eabf191. [Google Scholar] [CrossRef]
- Nelson, A.J.D.; Kinnavane, L.; Amin, E.; O’Mara, S.M.; Aggleton, J.P. Deconstructing the direct reciprocal hippocam-pal-anterior thalamic pathways for spatial learning. J. Neurosci. 2020, 40, 6978–6990. [Google Scholar] [CrossRef] [PubMed]
- Hafting, T.; Fyhn, M.; Molden, S.; Moser, M.; Moser, E. Microstructure of a spatial map in the entorhinal cortex. Nature 2005, 436, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Gehrlach, D.A.; Dolensek, N.; Klein, A.S.; Chowdhury, R.R.; Matthys, A.; Junghänel, M.; Gaitanos, T.N.; Podgornik, A.; Black, T.D.; Vaka, N.R.; et al. Aversive state processing in the posterior insular cortex. Nat. Neurosci. 2019, 22, 1424–1437. [Google Scholar] [CrossRef]
- Casanova, J.P.; Madrid, C.; Contreras, M.; Rodríguez, M.; Vasquez, M.; Torrealba, F. A role for the interoceptive insular cor-tex in the consolidation of learned fear. Behav. Brain Res. 2016, 296, 70–77. [Google Scholar] [CrossRef]
- Berret, E.; Kintscher, M.; Palchaudhuri, S.; Tang, W.; Osypenko, D.; Kochubey, O.; Schneggenburger, R. Insular cortex pro-cesses aversive somatosensory information and is crucial for threat learning. Science 2019, 364, 6443. [Google Scholar] [CrossRef]
- Christianson, J.P.; Jennings, J.H.; Ragole, T.; Flyer, J.G.; Benison, A.M.; Barth, D.S.; Watkins, L.R.; Maier, S.F. Safety sig-nals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol. Psychiatry 2011, 70, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Foilb, A.R.; Flyer-Adams, J.G.; Maier, S.F.; Christianson, J.P. Posterior insular cortex is necessary for conditioned inhibition of fear. Neurobiol. Learn. Mem. 2016, 134, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Rogers-Carter, M.M.; Varela, J.A.; Gribbons, K.B.; Pierce, A.F.; McGoey, M.T.; Ritchey, M.; Christianson, J.P. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat. Neurosci. 2018, 21, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.M.; Benison, A.M.; Klein, A.; Barth, D.S. Auditory, Somatosensory, and Multisensory Insular Cortex in the Rat. Cereb. Cortex 2008, 18, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, W.; Wang, S.; Zhou, Q.; Wang, H.; Zhang, B.; Huang, J.; Hong, B.; Wang, X. The Roles of Subdivisions of Human Insula in Emotion Perception and Auditory Processing. Cereb. Cortex 2019, 29, 517–528. [Google Scholar] [CrossRef]
- Vaudano, E.; Legg, C.R.; Glickstein, M. Afferent and efferent connections of temporal association cortex in the rat: A horse-radish peroxidase study. Eur. J. Neurosci. 1991, 3, 317–330. [Google Scholar] [CrossRef]
- Budinger, E.; Scheich, H. Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex. Hear. Res. 2009, 258, 16–27. [Google Scholar] [CrossRef]
- Tasaka, G.I.; Feigin, L.; Maor, I.; Groysman, M.; DeNardo, L.A.; Schiavo, J.K.; Froemke, R.C.; Luo, L.; Mizrahi, A. The tem-poral association cortex plays a key role in auditory-driven maternal plasticity. Neuron 2020, 107, 566–579. [Google Scholar] [CrossRef]
- Zingg, B.; Hintiryan, H.; Gou, L.; Song, M.Y.; Bay, M.; Bienkowski, M.S.; Foster, N.N.; Yamashita, S.; Bowman, I.; Toga, A.W.; et al. Neural networks of the mouse neocortex. Cell 2014, 156, 1096–1111. [Google Scholar] [CrossRef]
- Weinberger, N.M.; Bakin, J.S. Learning-induced physiological memory in adult primary auditory cortex: Receptive fields plasticity, model, and mechanisms. Audiol. Neurotol. 1998, 3, 145–167. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.; Sakaguchi, A.; Reis, D. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J. Neurosci. 1984, 4, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Poremba, A.; Gabriel, M. Amygdala Neurons Mediate Acquisition But Not Maintenance of Instrumental Avoidance Behavior in Rabbits. J. Neurosci. 1999, 19, 9635–9641. [Google Scholar] [CrossRef] [PubMed]
- Maren, S.; Ferrario, C.R.; Corcoran, K.A.; Desmond, T.J.; Frey, K.A. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur. J. Neurosci. 2003, 18, 3080–3088. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.M.; Rabinak, C.A.; McLachlan, I.G.; Maren, S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn. Mem. 2007, 14, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, S.; Jolkkonen, E.; Pitkänen, A. Projections from the posterior cortical nucleus of the amygdala to the hippocampal formation and parahippocampal region in rat. Hippocampus 2002, 12, 735–755. [Google Scholar] [CrossRef]
- Goode, T.D.; Maren, S. Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn. Mem. 2017, 24, 480–491. [Google Scholar] [CrossRef]
- Kim, E.J.; Horovitz, O.; Pellman, B.A.; Tan, L.M.; Li, Q.; Richter-Levin, G.; Kim, J.J. Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. Proc. Natl. Acad. Sci. USA 2013, 110, 14795–14800. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; McNaughton, N. Are periaqueductal gray and dorsal raphe the foundation of appetitive and aversive control? A comprehensive review. Prog. Neurobiol. 2019, 177, 33–72. [Google Scholar] [CrossRef]
- Brandão, M.L.; Lovick, T.A. Role of the dorsal periaqueductal gray in posttraumatic stress disorder: Mediation by dopamine and neurokinin. Transl. Psychiatry 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McNally, G.P.; Johansen, J.P.; Blair, H.T. Placing prediction into the fear circuit. Trends Neurosci. 2011, 34, 283–292. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, K.; Mannewitz, A.; Bock, J.; Kreitz, S.; Hess, A.; Scheich, H.; Goldschmidt, J. Imaging of Functional Brain Circuits during Acquisition and Memory Retrieval in an Aversive Feedback Learning Task: Single Photon Emission Computed Tomography of Regional Cerebral Blood Flow in Freely Behaving Rats. Brain Sci. 2021, 11, 659. https://doi.org/10.3390/brainsci11050659
Braun K, Mannewitz A, Bock J, Kreitz S, Hess A, Scheich H, Goldschmidt J. Imaging of Functional Brain Circuits during Acquisition and Memory Retrieval in an Aversive Feedback Learning Task: Single Photon Emission Computed Tomography of Regional Cerebral Blood Flow in Freely Behaving Rats. Brain Sciences. 2021; 11(5):659. https://doi.org/10.3390/brainsci11050659
Chicago/Turabian StyleBraun, Katharina, Anja Mannewitz, Joerg Bock, Silke Kreitz, Andreas Hess, Henning Scheich, and Jürgen Goldschmidt. 2021. "Imaging of Functional Brain Circuits during Acquisition and Memory Retrieval in an Aversive Feedback Learning Task: Single Photon Emission Computed Tomography of Regional Cerebral Blood Flow in Freely Behaving Rats" Brain Sciences 11, no. 5: 659. https://doi.org/10.3390/brainsci11050659
APA StyleBraun, K., Mannewitz, A., Bock, J., Kreitz, S., Hess, A., Scheich, H., & Goldschmidt, J. (2021). Imaging of Functional Brain Circuits during Acquisition and Memory Retrieval in an Aversive Feedback Learning Task: Single Photon Emission Computed Tomography of Regional Cerebral Blood Flow in Freely Behaving Rats. Brain Sciences, 11(5), 659. https://doi.org/10.3390/brainsci11050659




