Reaction Time and Visual Memory in Connection with Alcohol Use in Schizophrenia and Schizoaffective Disorder
Abstract
1. Introduction
- The association between hazardous drinking and reaction time and visual memory in persons with schizophrenia and schizoaffective disorder.
- The association between alcohol use disorder and reaction time and visual memory in persons with schizophrenia and schizoaffective disorder.
2. Materials and Methods
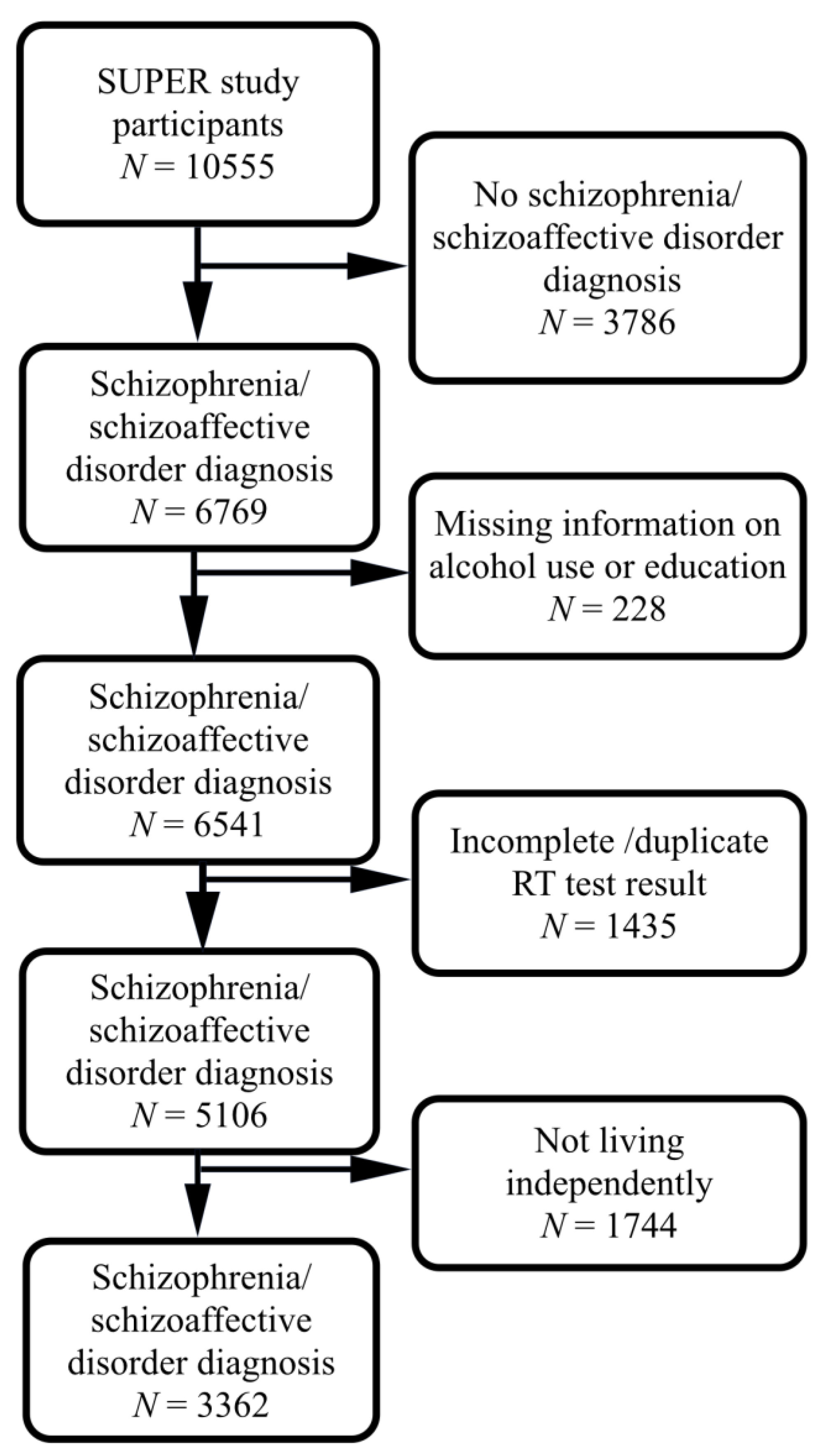
2.1. Participants
2.2. Schizophrenia and Schizoaffective Disorder Diagnoses
2.3. Hazardous Drinking Screening
2.4. Alcohol Use Disorder Diagnoses
2.5. Cognitive Measures
2.6. Confounding Factors
2.6.1. Age
2.6.2. Education
2.6.3. Household Pattern
2.6.4. Age of First Psychotic Episode
2.7. Statistical Methods
3. Results
3.1. Background Factors and Alcohol Use Patterns
3.2. Association between Reaction Time and Visual Memory and Hazardous Drinking in Schizophrenia and Schizoaffective Disorder
3.3. Association between Reaction Time and Visual Memory and Alcohol Use Disorder in Schizophrenia and Schizoaffective Disorder
4. Discussion
4.1. Main Findings
4.2. Comparison with Other Studies
4.3. Strengths
4.4. Limitations
4.5. What Is Already Known on This Subject?
- Alcohol use disorder reduces cognition in schizophrenia and schizoaffective disorder patients.
- Mild alcohol use is not associated with impaired cognition in the normal population.
4.6. What Does This Study Add?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosia, M.; Buonocore, M.; Bechi, M.; Santarelli, L.; Spangaro, M.; Cocchi, F.; Guglielmino, C.; Bianchi, L.; Bringheli, S.; Bosinelli, F.; et al. Improving Cognition to Increase Treatment Efficacy in Schizophrenia: Effects of Metabolic Syndrome on Cognitive Remediation’s Outcome. Front. Psychiatry 2018, 9, 647. [Google Scholar] [CrossRef]
- Vacca, A.; Longo, R.; Mencar, C. Identification and evaluation of cognitive deficits in schizophrenia using “Machine learning”. Psychiatr. Danub. 2019, 31, 261–264. [Google Scholar] [PubMed]
- Anda, L.; Brønnick, K.K.; Johannessen, J.O.; Joa, I.; Kroken, R.A.; Johnsen, E.; Rettenbacher, M.; Fathian, F.; Løberg, E.-M. Cognitive Profile in Ultra High Risk for Psychosis and Schizophrenia: A Comparison Using Coordinated Norms. Front. Psychiatry 2019, 10, 695. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.M.; Karcher, N.R.; Barch, D.M. Cognitive Deficits in Psychotic Disorders: A Lifespan Perspective. Neuropsychol. Rev. 2018, 28, 509–533. [Google Scholar] [CrossRef]
- Potvin, S.; Stavro, K.; Pelletier, J. Paradoxical Cognitive Capacities in Dual Diagnosis Schizophrenia: The Quest for Explanatory Factors. J. Dual Diagn. 2012, 8, 35–47. [Google Scholar] [CrossRef]
- Manning, V.; Betteridge, S.; Wanigaratne, S.; Best, D.; Strang, J.; Gossop, M. Cognitive impairment in dual diagnosis inpatients with schizophrenia and alcohol use disorder. Schizophr. Res. 2009, 114, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Bondi, M.W.; Kasckow, J.W.; Golshan, S.; Jeste, D.V. Neurocognitive functioning in dually diagnosed middle aged and elderly patients with alcoholism and schizophrenia. Int. J. Geriatr. Psychiatry 2006, 21, 711–718. [Google Scholar] [CrossRef]
- Tyburski, E.; Pełka-Wysiecka, J.; Mak, M.; Samochowiec, A.; Bieńkowski, P.; Samochowiec, J. Neuropsychological Profile of Specific Executive Dysfunctions in Patients with Deficit and Non-deficit Schizophrenia. Front. Psychol. 2017, 8, 1459. [Google Scholar] [CrossRef]
- Yu, M.; Tang, X.; Wang, X.; Zhang, X.; Zhang, X.; Sha, W.; Yao, S.; Shu, N.; Zhang, X.; Zhang, Z. Neurocognitive Impairments in Deficit and Non-Deficit Schizophrenia and Their Relationships with Symptom Dimensions and Other Clinical Variables. PLoS ONE 2015, 10, e0138357. [Google Scholar] [CrossRef]
- Hartz, S.M.; Pato, C.N.; Medeiros, H.; Cavazos-Rehg, P.; Sobell, J.L.; Knowles, J.A.; Bierut, L.J.; Pato, M.T. Comorbidity of Severe Psychotic Disorders With Measures of Substance Use. JAMA Psychiatry 2014, 71, 248–254. [Google Scholar] [CrossRef]
- Leposavić, L.; Dimitrijević, D.; Đorđević, S.; Leposavić, I.; Balkoski, G.N. Comorbidity of harmful use of alcohol in population of schizophrenic patients. Psychiatr. Danub. 2015, 27, 84–89. [Google Scholar] [PubMed]
- Hunt, G.E.; Large, M.M.; Cleary, M.; Lai, H.M.X.; Saunders, J.B. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: Systematic review and meta-analysis. Drug Alcohol Depend. 2018, 191, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Esser, M.B.; Hedden, S.L.; Kanny, D.; Brewer, R.D.; Gfroerer, J.C.; Naimi, T.S. Prevalence of Alcohol Dependence Among US Adult Drinkers, 2009–2011. Prev. Chronic Dis. 2014, 11, E206. [Google Scholar] [CrossRef]
- Fat, L.N.; Bell, S.; Britton, A. A life-time of hazardous drinking and harm to health among older adults: Findings from the Whitehall II prospective cohort study. Addiction 2020, 115, 1855–1866. [Google Scholar] [CrossRef]
- Fujii, H.; Nishimoto, N.; Yamaguchi, S.; Kurai, O.; Miyano, M.; Ueda, W.; Oba, H.; Naoki, N.; Kawada, N.; Okawa, K. The Alcohol Use Disorders Identification Test for Consumption (AUDIT-C) is more useful than pre-existing laboratory tests for predicting hazardous drinking: A cross-sectional study. BMC Public Health 2016, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Anderson, P.; Manthey, J.; Shield, K.D.; Struzzo, P.; Wojnar, M.; Gual, A. Alcohol Use Disorders in Primary Health Care: What Do We Know and Where Do We Go? Alcohol Alcohol. 2016, 51, 422–427. [Google Scholar] [CrossRef]
- Babor, T.F.; Longabaugh, R.; Zweben, A.; Fuller, R.K.; Stout, R.L.; Anton, R.F.; Randall, C.L. Issues in the definition and measurement of drinking outcomes in alcoholism treatment research. J. Stud. Alcohol Suppl. 1994, s12, 101–111. [Google Scholar] [CrossRef]
- Wilsnack, R.W.; Wilsnack, S.C.; Kristjanson, A.F.; Vogeltanz-Holm, N.D.; Gmel, G. Gender and alcohol consumption: Patterns from the multinational GENACIS project. Addiction 2009, 104, 1487–1500. [Google Scholar] [CrossRef]
- Slade, T.; Chapman, C.; Swift, W.; Keyes, K.; Tonks, Z.; Teesson, M. Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: Systematic review and metaregression. BMJ Open 2016, 6, e011827. [Google Scholar] [CrossRef]
- Mäkelä, P.; Tigerstedt, C.; Mustonen, H. The Finnish drinking culture: Change and continuity in the past 40 years. Drug Alcohol Rev. 2012, 31, 831–840. [Google Scholar] [CrossRef]
- Brennan, S.E.; McDonald, S.; Page, M.J.; Reid, J.; Ward, S.; Forbes, A.B.; McKenzie, J.E. Long-term effects of alcohol consumption on cognitive function: A systematic review and dose-response analysis of evidence published between 2007 and 2018. Syst. Rev. 2020, 9, 1–39. [Google Scholar] [CrossRef]
- Rehm, J.; Hasan, O.S.M.; Black, S.E.; Shield, K.D.; Schwarzinger, M. Alcohol use and dementia: A systematic scoping review. Alzheimer’s Res. Ther. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Koch, M.; Fitzpatrick, A.L.; Rapp, S.R.; Nahin, R.L.; Williamson, J.D.; Lopez, O.L.; DeKosky, S.T.; Kuller, L.H.; Mackey, R.H.; Mukamal, K.J.; et al. Alcohol Consumption and Risk of Dementia and Cognitive Decline Among Older Adults With or Without Mild Cognitive Impairment. JAMA Netw. Open 2019, 2, e1910319. [Google Scholar] [CrossRef] [PubMed]
- Piumatti, G.; Moore, S.C.; Berridge, D.M.; Sarkar, C.; Gallacher, J. The relationship between alcohol use and long-term cognitive decline in middle and late life: A longitudinal analysis using UK Biobank. J. Public Health 2018, 40, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamaa, J.; Suvisaari, J.; Henriksson, M.; Heilä, H.; Karjalainen, E.; Koskela, J.; Cannon, M.; Lönnqvist, J. The validity of schizophrenia diagnosis in the Finnish Hospital Discharge Register: Findings from a 10-year birth cohort sample. Nord. J. Psychiatry 2008, 62, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; DeBenedetti, A.F.; Volk, R.J.; Williams, E.; Kivlahan, D.R.; Bradley, K.A. Effectiveness of the AUDIT-C as a Screening Test for Alcohol Misuse in Three Race/Ethnic Groups. J. Gen. Intern. Med. 2008, 23, 781–787. [Google Scholar] [CrossRef]
- Public Health England. Alcohol Use Screening Tests. 2017. Available online: https://www.gov.uk/government/publications/alcohol-use-screening-tests (accessed on 28 October 2020).
- Lintonen, T.; Niemelä, S.; Mäkelä, P. Alkoholinkäytön hälytysrajan ylittäviä käyttäjiä on Suomessa vähintään viisi prosenttia väestöstä. Lääketieteellinen Aikakauskirja Duodecim 2019, 135, 1459–1466. Available online: https://www.duodecimlehti.fi/lehti/2019/16/duo15071 (accessed on 28 October 2020).
- Barnett, J.H.; Sahakian, B.J.; Werners, U.; Hill, K.E.; Brazil, R.; Gallagher, O.; Bullmore, E.T.; Jones, P.B. Visuospatial learning and executive function are independently impaired in first-episode psychosis. Psychol. Med. 2005, 35, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Taivalantti, M.; Barnett, J.H.; Halt, A.-H.; Koskela, J.; Auvinen, J.; Timonen, M.; Järvelin, M.-R.; Veijola, J. Depressive symptoms as predictors of visual memory deficits in middle-age. J. Affect. Disord. 2020, 264, 29–34. [Google Scholar] [CrossRef]
- Van Hooren, S.A.H.; Valentijn, A.M.; Bosma, H.; Ponds, R.W.H.M.; Van Boxtel, M.P.J.; Jolles, J. Cognitive Functioning in Healthy Older Adults Aged 64–81: A Cohort Study into the Effects of Age, Sex, and Education. Aging Neuropsychol. Cogn. 2007, 14, 40–54. [Google Scholar] [CrossRef]
- Biddle, K.D.; Jacobs, H.I.L.; Uquillas, F.D.; Zide, B.S.; Kirn, D.R.; Properzi, M.R.; Rentz, D.M.; Johnson, K.A.; Sperling, R.A.; Donovan, N.J. Associations of Widowhood and β-Amyloid With Cognitive Decline in Cognitively Unimpaired Older Adults. JAMA Netw. Open 2020, 3, e200121. [Google Scholar] [CrossRef]
- Andersson, C.; Marklund, K.; Walles, H.; Hagman, G.; Miley-Akerstedt, A. Lifestyle Factors and Subjective Cognitive Impairment in Patients Seeking Help at a Memory Disorder Clinic: The Role of Negative Life Events. Dement. Geriatr. Cogn. Disord. 2019, 48, 196–206. [Google Scholar] [CrossRef]
- Strandberg, A.Y.; Trygg, T.; Pitkälä, K.H.; E Strandberg, T. Alcohol consumption in midlife and old age and risk of frailty. Age Ageing 2018, 47, 248–254. [Google Scholar] [CrossRef]
- Krahn, D.; Freese, J.; Hauser, R.; Barry, K.; Goodman, B. Alcohol Use and Cognition at Mid-Life: The Importance of Adjusting for Baseline Cognitive Ability and Educational Attainment. Alcohol. Clin. Exp. Res. 2003, 27, 1162–1166. [Google Scholar] [CrossRef]
- Donovan, N.J.; Okereke, O.I.; Vannini, P.; Amariglio, R.E.; Rentz, D.M.; Marshall, G.A.; Johnson, K.A.; Sperling, R.A. Association of Higher Cortical Amyloid Burden With Loneliness in Cognitively Normal Older Adults. JAMA Psychiatry 2016, 73, 1230–1237. [Google Scholar] [CrossRef]
- Bora, E. Neurodevelopmental origin of cognitive impairment in schizophrenia. Psychol. Med. 2015, 45, 1–9. [Google Scholar] [CrossRef]
- Frangou, S. Cognitive function in early onset schizophrenia: A selective review. Front. Hum. Neurosci. 2009, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Son, Y.-J. Gender Differences in the Impact of Cognitive Function on Health Literacy among Older Adults with Heart Failure. Int. J. Environ. Res. Public Health 2018, 15, 2711. [Google Scholar] [CrossRef] [PubMed]
- Voyer, D.; Voyer, S.D.; Saint-Aubin, J. Sex differences in visual-spatial working memory: A meta-analysis. Psychon. Bull. Rev. 2017, 24, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, A.; Dekhtyar, S.; Asperholm, M.; Weber, D. Gender differences in memory and cognition. In Encyclopedia of Geropsychology; Springer: Singapore, 2016; pp. 1–7. ISBN 978-981-287-080-3. [Google Scholar] [CrossRef]
- Hughes, T.L.; Wilsnack, S.C.; Kantor, L.W. The Influence of Gender and Sexual Orientation on Alcohol Use and Alco-hol-Related Problems: Toward a Global Perspective. Alcohol Res. Curr. Rev. 2016, 38, 121–132. [Google Scholar]
- Manning, V.; Wanigaratne, S.; Best, D.; Strathdee, G.; Schrover, I.; Gossop, M. Screening for cognitive functioning in psychiatric outpatients with schizophrenia, alcohol dependence, and dual diagnosis. Schizophr. Res. 2007, 91, 151–158. [Google Scholar] [CrossRef]
- Thoma, R.J.; Hanlon, F.M.; Miller, G.A.; Huang, M.; Weisend, M.P.; Sanchez, F.P.; Waldorf, V.A.; Jones, A.; Smith, A.; Formoso, M.J.; et al. Neuropsychological and sensory gating deficits related to remote alcohol abuse history in schizophrenia. J. Int. Neuropsychol. Soc. 2006, 12, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; Serper, M.R.; Riggio, S.; Harvey, P.D. Neurocognition, symptomatology, and functional skills in older alco-hol-abusing schizophrenia patients. Schizophr Bull. 2005, 31, 175–182. [Google Scholar] [CrossRef]
- Allen, D.N.; Goldstein, G.; Forman, S.D.; Keshavan, M.S.; Van Kammen, D.P.; Sanders, R.D. Neurologic examination abnormalities in schizophrenia with and without a history of alcoholism. Neuropsychiatry, Neuropsychol. Behav. Neurol. 2000, 13, 184–187. [Google Scholar]
- Buleyko, A.A.; Soldatkin, V.A.; Murina, I.V.; Ruban, D.A.; Simak, O.Y.; Krysenko, P.B.; Kryuchkova, M.N. Does alcohol influence the cognitive functions of schizophrenic patients? Zhurnal Nevrol. Psikhiatrii Im S S Korsakova 2019, 119, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.N.; Goldstein, G.; Aldarondo, F. Neurocognitive dysfunction in patients diagnosed with schizophrenia and alcoholism. Neuropsychology 1999, 13, 62–68. [Google Scholar] [CrossRef]
- Nixon, S.J.; Hallford, H.; Tivis, R.D. Neurocognitive function in alcoholic, schizophrenic, and dually diagnosed patients. Psychiatry Res. 1996, 64, 35–45. [Google Scholar] [CrossRef]
- Gizewski, E.R.; Müller, B.W.; Scherbaum, N.; Lieb, B.; Forsting, M.; Wiltfang, J.; Leygraf, N.; Schiffer, B. The impact of alcohol dependence on social brain function. Addict. Biol. 2012, 18, 109–120. [Google Scholar] [CrossRef]
- Potvin, S.; Mancini-Marïe, A.; Fahim, C.; Mensour, B.; Stip, E. Processing of social emotion in patients with schizophrenia and substance use disorder: An fMRI study. Soc. Neurosci. 2007, 2, 106–116. [Google Scholar] [CrossRef]
- Mancini-Marïe, A.; Potvin, S.; Fahim, C.; Beauregard, M.; Mensour, B.; Stip, E. Neural correlates of the affect regulation model in schizophrenia patients with substance use history: A functional magnetic resonance imaging study. J. Clin. Psychiatry 2006, 67, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Carey, K.B.; Carey, M.P.; Simons, J.S. Correlates of substance use disorder among psychiatric outpatients: Focus on cognition, social role functioning, and psychiatric status. J. Nerv. Ment. Dis. 2003, 191, 300–308. [Google Scholar] [CrossRef][Green Version]
- Potvin, S.; Joyal, C.C.; Pelletier, J.; Stip, E. Contradictory cognitive capacities among substance-abusing patients with schiz-ophrenia: A meta-analysis. Schizophr. Res. 2008, 100, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Thoma, P.; Daum, I. Comorbid substance use disorder in schizophrenia: A selective overview of neurobiological and cognitive underpinnings. Psychiatry Clin. Neurosci. 2013, 67, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Bartholow, B.D.; Fleming, K.A.; Wood, P.K.; Cowan, N.; Saults, J.S.; Altamirano, L.; Miyake, A.; Martins, J.; Sher, K.J. Alcohol effects on response inhibition: Variability across tasks and individuals. Exp. Clin. Psychopharmacol. 2018, 26, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Mayhugh, R.E.; Moussa, M.N.; Simpson, S.L.; Lyday, R.G.; Burdette, J.H.; Porrino, L.J.; Laurienti, P.J. Moderate-Heavy Alcohol Consumption Lifestyle in Older Adults Is Associated with Altered Central Executive Network Community Structure during Cognitive Task. PLoS ONE 2016, 11, e0160214. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, P.E.M.; Dos Santos, M.B.M.; Piasecki, L.; Jorge, A.C. Doença de Marchiafava-Bignami: Uma rara entidade com prognóstico sombrio. Rev. Bras. Ter. Intensiva 2013, 25, 68–72. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, D.Y.; Lee, B.C.; Jung, M.H.; Kim, H.; Choi, Y.S.; Choi, I.-G. Alcohol and Cognition in the Elderly: A Review. Psychiatry Investig. 2012, 9, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.N.; Simpson, S.L.; Mayhugh, R.E.; Grata, M.E.; Burdette, J.H.; Porrino, L.J.; Laurienti, P.J. Long-term moderate alcohol consumption does not exacerbate age-related cognitive decline in healthy, community-dwelling older adults. Front. Aging Neurosci. 2015, 6, 341. [Google Scholar] [CrossRef]
- Sabia, S.; Elbaz, A.; Britton, A.; Bell, S.; Dugravot, A.; Shipley, M.; Kivimaki, M.; Singh-Manoux, A. Alcohol consumption and cognitive decline in early old age. Neurology 2014, 82, 332–339. [Google Scholar] [CrossRef]
- Reas, E.; Laughlin, G.; Kritz-Silverstein, D.; Barrett-Connor, E.; McEvoy, L. Moderate, Regular Alcohol Consumption is Associated with Higher Cognitive Function in Older Community-Dwelling Adults. J. Prev. Alzheimer’s Dis. 2016, 3, 105–113. [Google Scholar]
- Panza, F.; Frisardi, V.; Seripa, D.; Logroscino, G.; Santamato, A.; Imbimbo, B.P.; Scafato, E.; Pilotto, A.; Solfrizzi, V. Alcohol consumption in mild cognitive impairment and dementia: Harmful or neuroprotective? Int. J. Geriatr. Psychiatry 2012, 27, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.E.; Burr, R.; McCurry, S.M.; Graves, A.B.; Larson, E.B. Alcohol, aging, and cognitive performance in a cohort of Japanese Americans aged 65 and older: The Kame project. Int. Psychogeriatr. 2001, 13, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.L.; Hutchison, K.E. Alcohol, Aging, and the Stress Response. Alcohol Res. Health 1999, 23, 272–283. [Google Scholar] [PubMed]
- Zhang, R.; Shen, L.; Miles, T.; Shen, Y.; Cordero, J.; Qi, Y.; Liang, L.; Li, C. Association of Low to Moderate Alcohol Drinking With Cognitive Functions From Middle to Older Age Among US Adults. JAMA Netw. Open 2020, 3, e207922. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, M.; Bilt, J.V.; Saxton, J.A.; Shen, C.; Dodge, H.H. Alcohol consumption and cognitive function in late life: A longitudinal community study. Neurology 2005, 65, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Britton, A.; Singh-Manoux, A.; Marmot, M. Alcohol Consumption and Cognitive Function in the Whitehall II Study. Am. J. Epidemiol. 2004, 160, 240–247. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Zhang, J.; Li, W.; Nie, J.; Qiu, Q.; Liu, Y.; Fang, Y.; Yang, Z.; Li, X.; et al. Alcohol Consumption and Subclinical Findings on Cognitive Function, Biochemical Indexes, and Cortical Anatomy in Cognitively Normal Aging Han Chinese Population. Front. Aging Neurosci. 2018, 10, 182. [Google Scholar] [CrossRef]
- Topiwala, A.; Allan, C.L.; Valkanova, V.; Zsoldos, E.; Filippini, N.; Sexton, C.; Mahmood, A.; Fooks, P.; Singh-Manoux, A.; E Mackay, C.; et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ 2017, 357, j2353. [Google Scholar] [CrossRef]
- Lobo, E.; Dufouil, C.; Marcos, G.; Quetglas, B.; Saz, P.; Guallar, E.; Lobo, A.; Workgroup, F.T.Z. Is There an Association Between Low-to-Moderate Alcohol Consumption and Risk of Cognitive Decline? Am. J. Epidemiol. 2010, 172, 708–716. [Google Scholar] [CrossRef]
- Richard, E.L.; Kritz-Silverstein, D.; Laughlin, G.A.; Fung, T.T.; Barrett-Connor, E.; McEvoy, L.K. Alcohol Intake and Cognitively Healthy Longevity in Community-Dwelling Adults: The Rancho Bernardo Study. J. Alzheimer’s Dis. 2017, 59, 803–814. [Google Scholar] [CrossRef]
- Parker, E.S.; A Parker, D.; Harford, T.C. Specifying the relationship between alcohol use and cognitive loss: The effects of frequency of consumption and psychological distress. J. Stud. Alcohol 1991, 52, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.J.K.; Vidal, J.-S.; Garcia, M.; Aspelund, T.; Van Buchem, M.A.; Jónsdóttir, M.K.; Sigurdsson, S.; Harris, T.B.; Gudnason, V.; Launer, L.J.; et al. The Alcohol Paradox: Light-to-Moderate Alcohol Consumption, Cognitive Function, and Brain Volume. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 69, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, H.-M.; Payette, M.-C.; Berbiche, D.; Grenier, S.; Hudon, C. Cognitive decline and alcohol consumption adjusting for functional status over a 3-year period in French speaking community living older adults. J. Public Health 2019, 41, e177–e184. [Google Scholar] [CrossRef] [PubMed]
- Day, A.M.; Kahler, C.W.; Ahern, D.C.; Clark, U.S. Executive Functioning in Alcohol Use Studies: A Brief Review of Findings and Challenges in Assessment. Curr. Drug Abus. Rev. 2015, 8, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Neafsey, E.J.; A Collins, M. Moderate alcohol consumption and cognitive risk. Neuropsychiatr. Dis. Treat. 2011, 7, 465–484. [Google Scholar] [CrossRef]
- Kim, J.W.; Byun, M.S.; Yi, D.; Lee, J.H.; Ko, K.; Jeon, S.Y.; Sohn, B.K.; Lee, J.-Y.; Kim, Y.K.; A Shin, S.; et al. Association of moderate alcohol intake with in vivo amyloid-beta deposition in human brain: A cross-sectional study. PLoS Med. 2020, 17, e1003022. [Google Scholar] [CrossRef]
- Wallach, J.D.; Serghiou, S.; Chu, L.; Egilman, A.C.; Vasiliou, V.; Ross, J.S.; Ioannidis, J.P.A. Evaluation of confounding in epidemiologic studies assessing alcohol consumption on the risk of ischemic heart disease. BMC Med. Res. Methodol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Emberson, J.R.; A Bennett, D. Effect of alcohol on risk of coronary heart disease and stroke: Causality, bias, or a bit of both? Vasc. Health Risk Manag. 2006, 2, 239–249. [Google Scholar] [CrossRef]
- Wootton, R.E.; Greenstone, H.; Abdellaoui, A.; Denys, D.; Verweij, K.; Munafò, M.R.; Treur, J.L. Bidirectional effects between loneliness, smoking and alcohol use: Evidence from a Mendelian randomization study. Addiction 2020, 116, 400–406. [Google Scholar] [CrossRef]
- Schutte, R.; Papageorgiou, M.; Najlah, M.; Huisman, H.W.; Ricci, C.; Zhang, J.; Milner, N.; Schutte, A.E. Drink types unmask the health risks associated with alcohol intake–Prospective evidence from the general population. Clin. Nutr. 2020, 39, 3168–3174. [Google Scholar] [CrossRef]
- Trevisan, M.; Schisterman, E.; Mennotti, A.; Farchi, G.; Conti, S. Drinking pattern and mortality: The Italian Risk Factor and Life Expectancy pooling project. Ann. Epidemiol. 2001, 11, 312–319. [Google Scholar] [CrossRef]
- Hakulinen, C.; Elovainio, M.; Batty, G.D.; Virtanen, M.; Kivimäki, M.; Jokela, M. Personality and alcohol consumption: Pooled analysis of 72,949 adults from eight cohort studies. Drug Alcohol Depend. 2015, 151, 110–114. [Google Scholar] [CrossRef]
- Stephenson, M.; Barr, P.; Ksinan, A.; Aliev, F.; Latvala, A.; Viken, R.; Rose, R.; Kaprio, J.; Dick, D.; Salvatore, J.E. Which adolescent factors predict alcohol misuse in young adulthood? A co-twin comparisons study. Addiction 2020, 115, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Der, G.; Batty, G.D.; Deary, I.J. The association between IQ in adolescence and a range of health outcomes at 40 in the 1979 US National Longitudinal Study of Youth. Intelligence 2009, 37, 573–580. [Google Scholar] [CrossRef]
- Cao, M.; Cui, B. Association of Educational Attainment with Adiposity, Type 2 Diabetes, and Coronary Artery Diseases: A Mendelian Randomization Study. Front. Public Health 2020, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Sun, D.; Li, X.; Ma, H.; Heianza, Y.; Qi, L. Educational attainment and drinking behaviors: Mendelian randomization study in UK Biobank. Mol. Psychiatry 2019, 25, 1–12. [Google Scholar] [CrossRef]
- Mugavin, J.; MacLean, S.; Room, R.; Callinan, S. Adult low-risk drinkers and abstainers are not the same. BMC Public Health 2020, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Haber, J.R.; Harris-Olenak, B.; Burroughs, T.; Jacob, T. Residual Effects: Young Adult Diagnostic Drinking Predicts Late-Life Health Outcomes. J. Stud. Alcohol Drugs 2016, 77, 859–867. [Google Scholar] [CrossRef][Green Version]
- Saarni, S.I.; Joutsenniemi, K.; Koskinen, S.; Suvisaari, J.; Pirkola, S.; Sintonen, H.; Poikolainen, K.; Lönnqvist, J. Alcohol consumption, abstaining, health utility, and quality of life–a general population survey in finland. Alcohol Alcohol. 2008, 43, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, K.M.; Stockwell, T.; Chikritzhs, T.; Bostrom, A.; Kerr, W. Moderate Alcohol Use and Reduced Mortality Risk: Systematic Error in Prospective Studies and New Hypotheses. Ann. Epidemiol. 2007, 17, S16–S23. [Google Scholar] [CrossRef]
- Gémes, K.; Janszky, I.; Strand, L.B.; László, K.D.; Ahnve, S.; Vatten, L.J.; Dalen, H.; Mukamal, K.J. Light–moderate alcohol consumption and left ventricular function among healthy, middle-aged adults: The HUNT study. BMJ Open 2018, 8, e020777. [Google Scholar] [CrossRef] [PubMed]
- Kilian, C.; Manthey, J.; Probst, C.; Brunborg, G.S.; Bye, E.K.; Ekholm, O.; Kraus, L.; Moskalewicz, J.; Sieroslawski, J.; Rehm, J. Why Is Per Capita Consumption Underestimated in Alcohol Surveys? Results from 39 Surveys in 23 European Countries. Alcohol Alcohol. 2020, 55, 554–563. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Jensen, M.K.; Grønbaek, M.; Stampfer, M.J.; Manson, J.E.; Pischon, T.; Rimm, E.B. Drinking Frequency, Mediating Biomarkers, and Risk of Myocardial Infarction in Women and Men. Circulation 2005, 112, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, J.; Heidrich, J.; Berger, K.; Döring, A.; Heuschmann, P.U.; Keil, U. Changes in alcohol intake and risk of coronary heart disease and all-cause mortality in the MONICA/KORA-Augsburg cohort 1987-97. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 48–55. [Google Scholar] [CrossRef]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef]
- Seid, A.K. Social interactions, trust and risky alcohol consumption. Health Econ. Rev. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Himelstein, R.; Bredemeier, K.; Silverstein, S.M.; Grant, P. What accounts for poor func-tioning in people with schizophrenia: A re-evaluation of the contributions of neurocognitive v. attitudinal and motivational factors. Psychol. Med. 2018, 48, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Moritz, S.; Irshaid, S.; Lüdtke, T.; Schäfer, I.; Hauschildt, M.; Lipp, M. Neurocognitive Functioning in Alcohol Use Disorder: Cognitive Test Results Do not Tell the Whole Story. Eur. Addict. Res. 2018, 24, 217–225. [Google Scholar] [CrossRef]
- Campellone, T.R.; Sanchez, A.H.; Kring, A.M. Defeatist Performance Beliefs, Negative Symptoms, and Functional Outcome in Schizophrenia: A Meta-analytic Review. Schizophr. Bull. 2016, 42, 1343–1352. [Google Scholar] [CrossRef]
- Charlton, A.J.; May, C.; Luikinga, S.J.; Burrows, E.L.; Kim, J.H.; Lawrence, A.J.; Perry, C.J. Chronic voluntary alcohol consumption causes persistent cognitive deficits and cortical cell loss in a rodent model. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Galaj, E.; Kipp, B.; Floresco, S.; Savage, L. Persistent Alterations of Accumbal Cholinergic Interneurons and Cognitive Dysfunction after Adolescent Intermittent Ethanol Exposure. Neuroscience 2019, 404, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, X.; Ma, X.; Garcia, R.; Belfield, K.; Haorah, J. Alcohol promotes waste clearance in the CNS via brain vascular reactivity. Free. Radic. Biol. Med. 2019, 143, 115–126. [Google Scholar] [CrossRef]
- Ayabe, T.; Fukuda, T.; Ano, Y. Improving Effects of Hop-Derived Bitter Acids in Beer on Cognitive Functions: A New Strategy for Vagus Nerve Stimulation. Biomolecules 2020, 10, 131. [Google Scholar] [CrossRef]
- Ano, Y.; Takaichi, Y.; Uchida, K.; Kondo, K.; Nakayama, H.; Takashima, A. Iso-α-Acids, the Bitter Components of Beer, Suppress Microglial Inflammation in rTg4510 Tauopathy. Molecules 2018, 23, 3133. [Google Scholar] [CrossRef]
- Lu, S.; Liao, L.; Zhang, B.; Yan, W.; Chen, L.; Yan, H.; Guo, L.; Lu, S.; Xiong, K.; Yan, J. Antioxidant cascades confer neuroprotection in ethanol, morphine, and methamphetamine preconditioning. Neurochem. Int. 2019, 131, 104540. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhao, Y.; Hook, M.; Zhao, W.; Starlard-Davenport, A.; Cook, M.N.; Jones, B.C.; Hamre, K.M.; Lu, L. Ethanol’s Effect on Coq7 Expression in the Hippocampus of Mice. Front. Genet. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Casañas-Sánchez, V.; Pérez, J.A.; Quinto-Alemany, D.; Díaz, M. Sub-toxic Ethanol Exposure Modulates Gene Expression and Enzyme Activity of Antioxidant Systems to Provide Neuroprotection in Hippocampal HT22 Cells. Front. Physiol. 2016, 7, 312. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, L. Alcohol protects the CNS by activating HSF1 and inducing the heat shock proteins. Neurosci. Lett. 2019, 713, 134507. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.-P.; Leng, T.-D.; Yang, T.; Chen, F.-H.; Xiong, Z.-G. Acute Ethanol Exposure Promotes Autophagy-Lysosome Pathway-Dependent ASIC1a Protein Degradation and Protects Against Acidosis-Induced Neurotoxicity. Mol. Neurobiol. 2019, 56, 3326–3340. [Google Scholar] [CrossRef]
- Su, F.; Guo, A.-C.; Li, W.-W.; Zhao, Y.-L.; Qu, Z.-Y.; Wang, Y.-J.; Wang, Q.; Zhu, Y.-L. Low-Dose Ethanol Preconditioning Protects Against Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Injury By Activating Large Conductance, Ca2+-Activated K+ Channels In Vitro. Neurosci. Bull. 2016, 33, 28–40. [Google Scholar] [CrossRef]
- Larsson, S.C.; Burgess, S.; Mason, A.M.; Michaëlsson, K. Alcohol Consumption and Cardiovascular Disease. Circ. Genom. Precis. Med. 2020, 13, e002814. [Google Scholar] [CrossRef] [PubMed]
- Millwood, I.Y.; Walters, R.G.; Mei, X.W.; Guo, Y.; Yang, L.; Bian, Z.; A Bennett, D.; Chen, Y.; Dong, C.; Hu, R.; et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: A prospective study of 500,000 men and women in China. Lancet 2019, 393, 1831–1842. [Google Scholar] [CrossRef]
- Christensen, A.I.; Nordestgaard, B.G.; Tolstrup, J.S. Alcohol Intake and Risk of Ischemic and Haemorrhagic Stroke: Results from a Mendelian Randomisation Study. J. Stroke 2018, 20, 218–227. [Google Scholar] [CrossRef]
- Kumari, M.; Holmes, M.V.; Dale, C.E.; Hubacek, J.A.; Palmer, T.M.; Pikhart, H.; Peasey, A.; Britton, A.; Horvat, P.; Kubinova, R.; et al. Alcohol consumption and cognitive performance: A Mendelian randomiza-tion study. Addiction 2014, 109, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.-K.; Adams, M.J.; Davies, G.; Howard, D.M.; Hall, L.S.; Padmanabhan, S.; Murray, A.D.; Smith, B.H.; Campbell, A.; Hayward, C.; et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N = 112 117). Mol. Psychiatry 2017, 22, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.C.; Magnotta, V. Hippocampal volume deficits and shape deformities in young biological relatives of schizo-phrenia probands. Neuroimage 2010, 49, 3385–3393. [Google Scholar] [CrossRef]
- Haijma, S.V.; Van Haren, N.; Cahn, W.; Koolschijn, P.C.M.P.; Pol, H.E.H.; Kahn, R.S. Brain Volumes in Schizophrenia: A Meta-Analysis in Over 18 000 Subjects. Schizophr. Bull. 2012, 39, 1129–1138. [Google Scholar] [CrossRef]
- Lawrie, S.M.; Abukmeil, S.S. Brain abnormality in schizophrenia. Br. J. Psychiatry 1998, 172, 110–120. [Google Scholar] [CrossRef]
- Lawrie, S.M. Are structural brain changes in schizophrenia related to antipsychotic medication? A narrative review of the evidence from a clinical perspective. Ther. Adv. Psychopharmacol. 2018, 8, 319–326. [Google Scholar] [CrossRef]
| Male | Female | |
|---|---|---|
| N = 1711 | N = 1651 | |
| Age (mean (SD)) | 44.5 (12.8) | 46.3 (13.2) |
| Education | ||
| No matriculation examination (%) | 1191 (69.6) | 981 (59.4) |
| Matriculation examination (%) | 520 (30.4) | 670 (40.6) |
| Age of first psychotic episode (mean (SD)) | 26.84 (8.25) | 28.01 (9.51) |
| Household patterns | ||
| With spouse | 200 (11.7) | 441 (26.7) |
| Other | 1511 (88.3) | 1210 (73.3) |
| Current psychotropic medications | ||
| No (%) | 42 (2.45) | 33 (2.00) |
| Yes (%) | 1668 (97.49) | 1614 (97.76) |
| Missing (%) | 1 (0.06) | 4 (0.24) |
| Hazardous drinking * | ||
| No (%) | 1276 (74.6) | 1390 (84.2) |
| Yes (%) | 435 (25.4) | 261 (15.8) |
| Alcohol use disorder | ||
| No (%) | 1212 (70.8) | 1395 (84.5) |
| Yes (%) | 499 (29.2) | 256 (15.5) |
| Five-Choice Reaction Time * | Five-Choice Reaction Time * | |||||||
|---|---|---|---|---|---|---|---|---|
| Median | SD | |||||||
| Crude | Adjusted a | Crude | Adjusted a | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Male | ||||||||
| Hazardous drinking | 0.97 (0.95–0.99) | 0.002 | 0.99 (0.97–1.01) | 0.379 | 0.86 (0.81–0.91) | <001 | 0.93 (0.88–0.98) | 0.008 |
| Female | ||||||||
| Hazardous drinking | 0.94 (0.92–0.97) | <0.001 | 0.97 (0.95–0.99) | 0.016 | 0.89 (0.83–0.96) | 0.002 | 0.99 (0.92–1.06) | 0.760 |
| PAL first trial memory score ** | PAL total errors adjusted score ** | |||||||
| Better performance than 15% of NFBC 1966 members | Higher error scores than 50% of NFBC 1966 members | |||||||
| Crude | Adjusted a | Crude | Adjusted a | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Male | ||||||||
| Hazardous drinking | 1.76 (1.13–2.66) | 0.001 | 1.24 (0.74–2.02) | 0.399 | 0.56 (0.43–0.73) | <0.001 | 0.92 (0.67–1.28) | 0.253 |
| Female | ||||||||
| Hazardous drinking | 1.58 (0.95–2.52) | 0.064 | 1.02 (0.60–1.71) | 0.893 | 0.58 (0.43–0.80) | <0.001 | 0.97 (0.75–1.34) | 0.743 |
| Five-Choice Reaction Time * | Five-Choice Reaction Time * | |||||||
|---|---|---|---|---|---|---|---|---|
| Median | SD | |||||||
| Crude | Adjusted a | Crude | Adjusted a | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Male | ||||||||
| Alcohol use disorder | 1.01 (0.99–1.02) | 0.545 | 1.00 (0.99–1.02) | 0.871 | 1.02 (0.96–1.08) | 0.482 | 1.00 (0.95–1.06) | 0.899 |
| Female | ||||||||
| Alcohol use disorder | 0.99 (0.96–1.01) | 0.246 | 0.99 (0.96–1.01) | 0.225 | 1.03 (0.96–1.11) | 0.413 | 1.04 (0.97–1.11) | 0.324 |
| PAL first trial memory score ** | PAL total errors adjusted score ** | |||||||
| Better performance than 15% of NFBC 1966 members | Higher error scores than 50% of NFBC 1966 members | |||||||
| Crude | Adjusted | Crude | Adjusted | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Male | ||||||||
| Alcohol use disorder | 0.71 (0.45–1.09) | 0.131 | 0.83 (0.46–1.45) | 0.543 | 1.36 (1.05–1.77) | 0.021 | 1.18 (0.84–1.67) | 0.358 |
| Female | ||||||||
| Alcohol use disorder | 0.53 (0.28–0.94) | 0.043 | 0.62 (0.29–1.17) | 0.102 | 1.57 (1.13–2.22) | 0.009 | 1.34 (1.02–1.93) | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazumder, A.H.; Barnett, J.; Lindberg, N.; Torniainen-Holm, M.; Lähteenvuo, M.; Lahdensuo, K.; Kerkelä, M.; Hietala, J.; Isometsä, E.T.; Kampman, O.; et al. Reaction Time and Visual Memory in Connection with Alcohol Use in Schizophrenia and Schizoaffective Disorder. Brain Sci. 2021, 11, 688. https://doi.org/10.3390/brainsci11060688
Mazumder AH, Barnett J, Lindberg N, Torniainen-Holm M, Lähteenvuo M, Lahdensuo K, Kerkelä M, Hietala J, Isometsä ET, Kampman O, et al. Reaction Time and Visual Memory in Connection with Alcohol Use in Schizophrenia and Schizoaffective Disorder. Brain Sciences. 2021; 11(6):688. https://doi.org/10.3390/brainsci11060688
Chicago/Turabian StyleMazumder, Atiqul Haq, Jennifer Barnett, Nina Lindberg, Minna Torniainen-Holm, Markku Lähteenvuo, Kaisla Lahdensuo, Martta Kerkelä, Jarmo Hietala, Erkki Tapio Isometsä, Olli Kampman, and et al. 2021. "Reaction Time and Visual Memory in Connection with Alcohol Use in Schizophrenia and Schizoaffective Disorder" Brain Sciences 11, no. 6: 688. https://doi.org/10.3390/brainsci11060688
APA StyleMazumder, A. H., Barnett, J., Lindberg, N., Torniainen-Holm, M., Lähteenvuo, M., Lahdensuo, K., Kerkelä, M., Hietala, J., Isometsä, E. T., Kampman, O., Kieseppä, T., Jukuri, T., Häkkinen, K., Cederlöf, E., Haaki, W., Kajanne, R., Wegelius, A., Männynsalo, T., Niemi-Pynttäri, J., ... Veijola, J. (2021). Reaction Time and Visual Memory in Connection with Alcohol Use in Schizophrenia and Schizoaffective Disorder. Brain Sciences, 11(6), 688. https://doi.org/10.3390/brainsci11060688









