The Efficacy of Transcranial Direct Current Stimulation in Enhancing Surgical Skill Acquisition: A Preliminary Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
3. Results
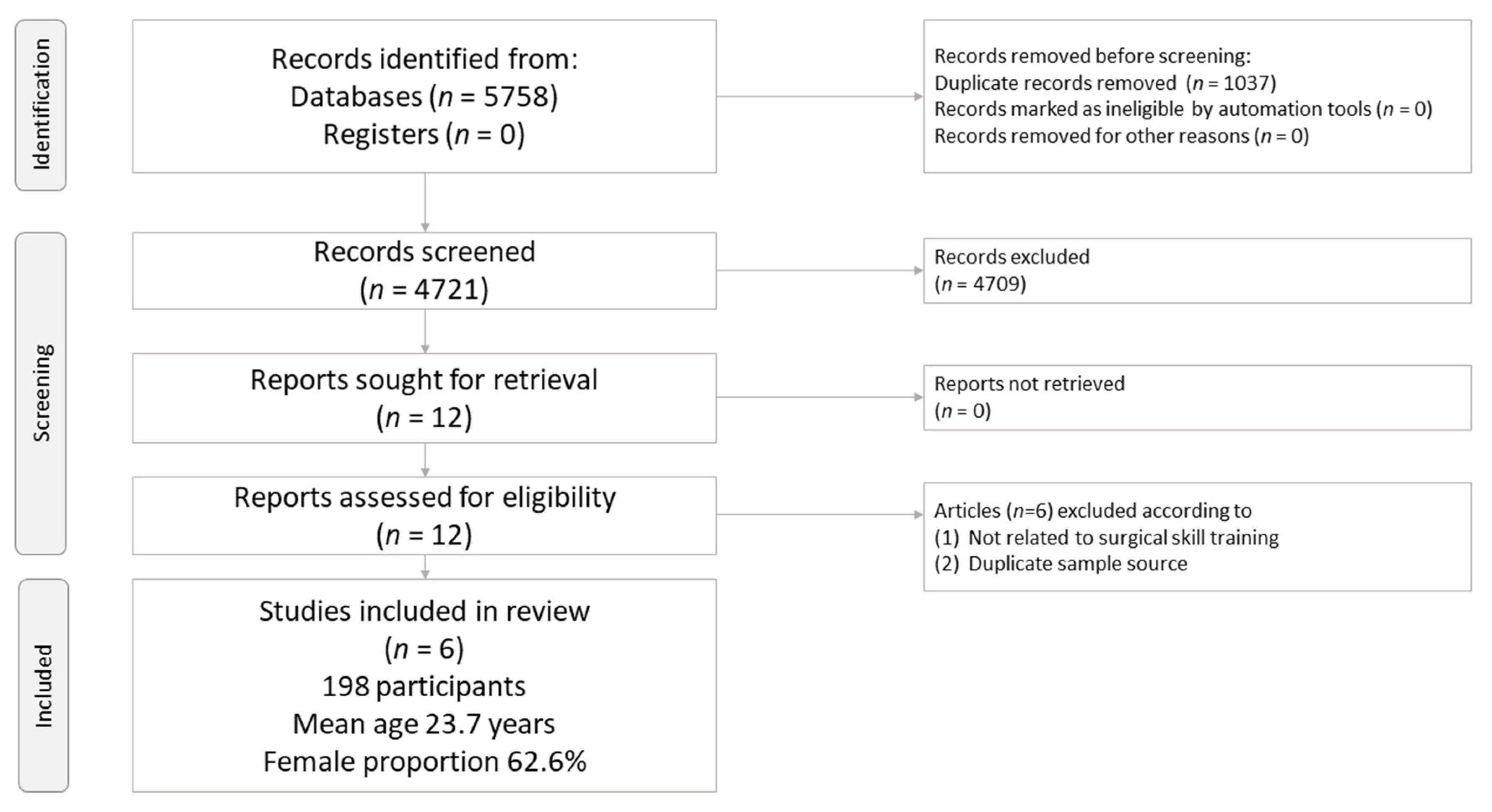
3.1. Study Selection
3.2. Methodological Quality of Included Studies
3.3. Primary Outcome: Changes in Surgical Performance
3.3.1. Sensitivity Test
3.3.2. Meta-Regression
3.3.3. Subgroup Meta-Analysis: Different Targeted Cortices
3.3.4. Subgroup Meta-Analysis: Meeting Abstracts or Formally Published Articles
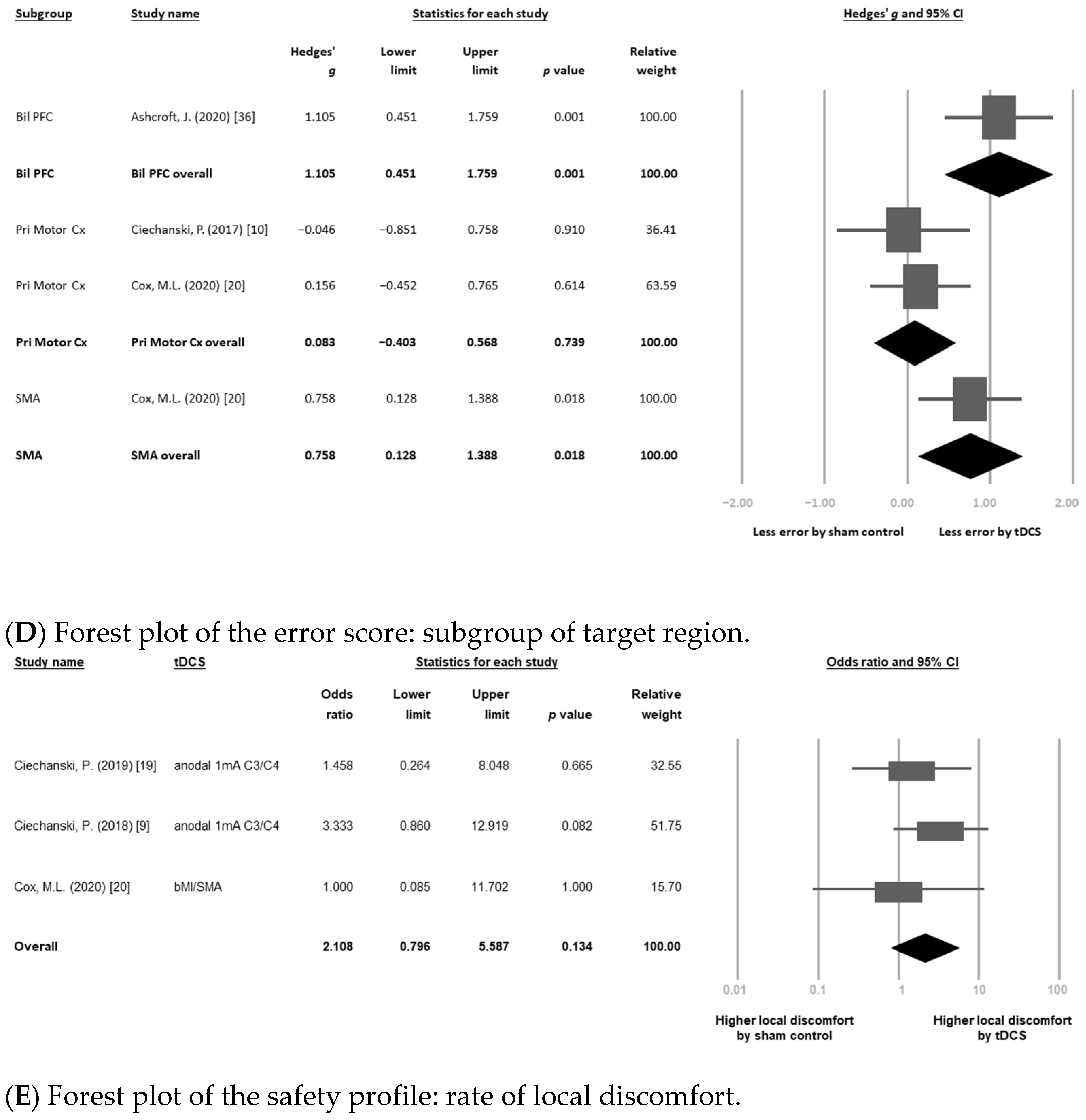
3.4. Secondary Outcome: Changes in Error Score
Subgroup Meta-Analysis: Different Targeted Cortices
3.5. Safety Profile: Rates of Local Discomfort (i.e., Itching, Tingling Pain, or Erythematous)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rose, J.; Weiser, T.G.; Hider, P.; Wilson, L.; Gruen, R.L.; Bickler, S.W. Estimated need for surgery worldwide based on prevalence of diseases: A modelling strategy for the WHO Global Health Estimate. Lancet Glob. Health 2015, 3 (Suppl. 2), S13–S20. [Google Scholar] [CrossRef] [Green Version]
- Mattar, S.G.; Alseidi, A.A.; Jones, D.B.; Jeyarajah, D.R.; Swanstrom, L.L.; Aye, R.W.; Wexner, S.D.; Martinez, J.M.; Ross, S.B.; Awad, M.M.; et al. General surgery residency inadequately prepares trainees for fellowship: Results of a survey of fellowship program directors. Ann. Surg. 2013, 258, 440–449. [Google Scholar] [CrossRef]
- Qureshi, H.A.; Rawlani, R.; Mioton, L.M.; Dumanian, G.A.; Kim, J.Y.; Rawlani, V. Burnout phenomenon in U.S. plastic surgeons: Risk factors and impact on quality of life. Plast. Reconstr. Surg. 2015, 135, 619–626. [Google Scholar] [CrossRef]
- Verret, C.I.; Nguyen, J.; Verret, C.; Albert, T.J.; Fufa, D.T. How Do Areas of Work Life Drive Burnout in Orthopaedic Attending Surgeons, Fellows, and Residents? Clin. Orthop. Relat. Res. 2020. [Google Scholar] [CrossRef]
- Lewis, F.R.; Klingensmith, M.E. Issues in general surgery residency training--2012. Ann. Surg. 2012, 256, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.; Devitt, K.S.; Keshet, I.; Spicer, J.; Imrie, K.; Feldman, L.; Cools-Lartigue, J.; Kayssi, A.; Lipsman, N.; Elmi, M.; et al. A systematic review of the effects of resident duty hour restrictions in surgery: Impact on resident wellness, training, and patient outcomes. Ann. Surg. 2014, 259, 1041–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, D.A.; Hatala, R.; Brydges, R.; Zendejas, B.; Szostek, J.H.; Wang, A.T.; Erwin, P.J.; Hamstra, S.J. Technology-enhanced simulation for health professions education: A systematic review and meta-analysis. JAMA 2011, 306, 978–988. [Google Scholar] [CrossRef] [PubMed]
- McGaghie, W.C.; Issenberg, S.B.; Cohen, E.R.; Barsuk, J.H.; Wayne, D.B. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad. Med. 2011, 86, 706–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciechanski, P.; Cheng, A.; Damji, O.; Lopushinsky, S.; Hecker, K.; Jadavji, Z.; Kirton, A. Effects of transcranial direct-current stimulation on laparoscopic surgical skill acquisition. BJS Open 2018, 2, 70–78. [Google Scholar] [CrossRef]
- Ciechanski, P.; Cheng, A.; Lopushinsky, S.; Hecker, K.; Gan, L.S.; Lang, S.; Zareinia, K.; Kirton, A. Effects of Transcranial Direct-Current Stimulation on Neurosurgical Skill Acquisition: A Randomized Controlled Trial. World Neurosurg. 2017, 108, 876–884. [Google Scholar] [CrossRef]
- Varley, M.; Choi, R.; Kuan, K.; Bhardwaj, N.; Trochsler, M.; Maddern, G.; Hewett, P.; Mees, S.T. Prospective randomized assessment of acquisition and retention of SILS skills after simulation training. Sur.g Endosc. 2015, 29, 113–118. [Google Scholar] [CrossRef]
- Nakashima, S.; Koeda, M.; Ikeda, Y.; Hama, T.; Funayama, T.; Akiyama, T.; Arakawa, R.; Tateno, A.; Suzuki, H.; Okubo, Y. Effects of anodal-tDCS on implicit motor learning and language-related brain function: An fMRI study. Psychiatry Clin. Neurosci. 2021. [Google Scholar] [CrossRef]
- Chan, M.M.Y.; Yau, S.S.Y.; Han, Y.M.Y. The neurobiology of prefrontal transcranial direct current stimulation (tDCS) in promoting brain plasticity: A systematic review and meta-analyses of human and rodent studies. Neurosci. Biobehav. Rev. 2021, 125, 392–416. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Transcranial direct current stimulation--update 2011. Restor. Neurol. Neurosci. 2011, 29, 463–492. [Google Scholar] [CrossRef]
- Forogh, B.; Yazdi-Bahri, S.M.; Ahadi, T.; Fereshtehnejad, S.M.; Raissi, G.R. Comparison of two protocols of transcranial magnetic stimulation for treatment of chronic tinnitus: A randomized controlled clinical trial of burst repetitive versus high-frequency repetitive Transcranial Magnetic Stimulation. Neurol. Sci. 2014, 35, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.C.; Carter, O.; Forte, J.D. Transcranial direct current stimulation: Five important issues we aren’t discussing (but probably should be). Front. Syst. Neurosci. 2014, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Fritsch, B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr. Opin. Neurol. 2011, 24, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Jayaram, G.; Pastor, D.; Kincses, Z.T.; Matthews, P.M.; Johansen-Berg, H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011, 49, 800–804. [Google Scholar] [CrossRef] [Green Version]
- Karok, S.; Fletcher, D.; Witney, A.G. Task-specificity of unilateral anodal and dual-M1 tDCS effects on motor learning. Neuropsychologia 2017, 94, 84–95. [Google Scholar] [CrossRef]
- Ciechanski, P.; Kirton, A.; Wilson, B.; Williams, C.C.; Anderson, S.J.; Cheng, A.; Lopushinsky, S.; Hecker, K.G. Electroencephalography correlates of transcranial direct-current stimulation enhanced surgical skill learning: A replication and extension study. Brain Res. 2019, 1725, 146445. [Google Scholar] [CrossRef]
- Cox, M.L.; Deng, Z.D.; Palmer, H.; Watts, A.; Beynel, L.; Young, J.R.; Lisanby, S.H.; Migaly, J.; Appelbaum, L.G. Utilizing transcranial direct current stimulation to enhance laparoscopic technical skills training: A randomized controlled trial. Brain Stimul. 2020, 13, 863–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Moura, M.; Hazime, F.A.; Marotti Aparicio, L.V.; Grecco, L.A.C.; Brunoni, A.R.; Hasue, R.H. Effects of transcranial direct current stimulation (tDCS) on balance improvement: A systematic review and meta-analysis. Somatosens. Mot. Res. 2019, 36, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Saleem, G.T.; Crasta, J.E.; Slomine, B.S.; Cantarero, G.L.; Suskauer, S.J. Transcranial Direct Current Stimulation in Pediatric Motor Disorders: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 724–738. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Cavuoto, L.; Schwaitzberg, S.; Norfleet, J.E.; Intes, X.; De, S. The Effects of Transcranial Electrical Stimulation on Human Motor Functions: A Comprehensive Review of Functional Neuroimaging Studies. Front. Neurosci. 2020, 14, 744. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Patel, R.; Ashcroft, J.; Darzi, A.; Singh, H.; Leff, D.R. Neuroenhancement in surgeons: Benefits, risks and ethical dilemmas. Br. J. Surg. 2020, 107, 946–950. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2; The Cochrane Collaboration: Oxford, UK, 2009. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Green, S. 10.4.3.1 Recommendations on testing for funnel plot asymmetry. Cochrane Handb. Syst. Rev. Interv. 2011, 10, 24–27. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Tobias, A. Assessing the influence of a single study in meta-analysis. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- Altman, D.G.; Bland, J.M. Interaction revisited: The difference between two estimates. BMJ 2003, 326, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.; Suwaa, Y.; Kinross, J.; von Roon, A.; Woods, A.; Darzi, A.; Singh, H.; Leff, D. P98 Improving accuracy and knot-strength in a robotic surgical task in surgical residents—A single session tDCS study. Clin. Neurophysiol. 2020, 131, e67–e68. [Google Scholar] [CrossRef]
- Ashcroft, J.; Patel, R.; Woods, A.J.; Darzi, A.; Singh, H.; Leff, D.R. Prefrontal transcranial direct-current stimulation improves early technical skills in surgery. Brain Stimul. 2020, 13, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luft, A.R.; Buitrago, M.M.; Ringer, T.; Dichgans, J.; Schulz, J.B. Motor skill learning depends on protein synthesis in motor cortex after training. J. Neurosci. 2004, 24, 6515–6520. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Marangolo, P.; Hampstead, B.M.; Bestmann, S.; Galletta, E.; Knotkova, H.; Bikson, M. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul. 2018, 11, 310–321. [Google Scholar] [CrossRef]
- Beheshti, I.; Ko, J.H. Modulating brain networks associated with cognitive deficits in Parkinson’s disease. Mol. Med. 2021, 27, 24. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.D.; Ward, L.M. The benefits of noise in neural systems: Bridging theory and experiment. Nat. Rev. Neurosci. 2011, 12, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Pascual-Leone, A.; Fregni, F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys. Ther. 2010, 90, 398–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Author (Year) | Task to Learn | tDCS | Comparison | Subjects | Mean Age | Female (%) | Adverse Event | Country |
|---|---|---|---|---|---|---|---|---|
| Ashcroft, J. (2020) [38] | knot-tying task | Anode and cathode tDCS 2 mA over F3 and F4, respectively, for 15 min | Active tDCS | 20 | 21.3 ± 2.5 | 55.0 | Not mentioned | Multiple countries |
| Sham tDCS | 20 | 21.9 ± 2.2 | 55.0 | |||||
| Cox, M.L. (2020) [22] | Fundamentals of laparoscopic surgery | bM1: cathode tDCS 2 mA at right primary motor cortex + anode at left primary motor cortex over 20 min SMA: the anode over Cz and the cathode over Fpz over 20 min | Active tDCS (bM1) | 20 | 21.9 ± 5.2 | 60.0 | Lightheadedness, and dizziness | USA |
| Active tDCS (SMA) | 20 | 23.5 ± 5.4 | 80.0 | |||||
| Sham tDCS | 20 | 22.7 ± 3.7 | 70.0 | |||||
| Patel, R. (2020) [37] | robotic-suturing task | Active tDCS 2 mA at bilateral prefrontal cortex over 15 min | Active tDCS | 15 | NA | 46.7 | Not mentioned | Multiple countries |
| Sham tDCS | ||||||||
| Ciechanski, P. (2019) [21] * | Fundamentals of laparoscopic surgery pattern cutting and peg transfer tasks | anode tDCS 1 mA at dominant primary motor cortex + cathode at contralateral either F3 or F4 for 20 min | Active tDCS | 11 | 25.9 ± 3.6 | 72.7 | Tingling, itching, and warmness | Canada |
| Sham tDCS | 11 | 25.5 ± 4.7 | 72.7 | |||||
| Ciechanski, P. (2018) [9] * | Fundamentals of laparoscopic surgery using simulation-based task training | anode tDCS 1 mA at dominant primary motor cortex + cathode at contralateral supraorbital area over 20 min | Active tDCS | 20 | 26.3 ± 4.1 | 55.0 | Itching, burning, tingling, and pain | Canada |
| Sham tDCS | 19 | 24.7 ± 3.3 | 52.6 | |||||
| Ciechanski, P. (2017) [10] * | ultrasonic aspirator to resect 3 virtual tumors embedded in healthy brain with NeuroTouch Neurosurgical simulator | anode tDCS 1 mA at dominant primary motor cortex + cathode at contralateral supraorbital area for 20 min | Active tDCS | 11 | 25.8 ± 3.0 | 72.7 | Itching, tingling, burning, and pain | Canada |
| Sham tDCS | 11 | 24.6 ± 2.1 | 72.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, C.-M.; Zeng, B.-Y.; Zeng, B.-S.; Sun, C.-K.; Cheng, Y.-S.; Su, K.-P.; Wu, Y.-C.; Chen, T.-Y.; Lin, P.-Y.; Liang, C.-S.; et al. The Efficacy of Transcranial Direct Current Stimulation in Enhancing Surgical Skill Acquisition: A Preliminary Meta-Analysis of Randomized Controlled Trials. Brain Sci. 2021, 11, 707. https://doi.org/10.3390/brainsci11060707
Hung C-M, Zeng B-Y, Zeng B-S, Sun C-K, Cheng Y-S, Su K-P, Wu Y-C, Chen T-Y, Lin P-Y, Liang C-S, et al. The Efficacy of Transcranial Direct Current Stimulation in Enhancing Surgical Skill Acquisition: A Preliminary Meta-Analysis of Randomized Controlled Trials. Brain Sciences. 2021; 11(6):707. https://doi.org/10.3390/brainsci11060707
Chicago/Turabian StyleHung, Chao-Ming, Bing-Yan Zeng, Bing-Syuan Zeng, Cheuk-Kwan Sun, Yu-Shian Cheng, Kuan-Pin Su, Yi-Cheng Wu, Tien-Yu Chen, Pao-Yen Lin, Chih-Sung Liang, and et al. 2021. "The Efficacy of Transcranial Direct Current Stimulation in Enhancing Surgical Skill Acquisition: A Preliminary Meta-Analysis of Randomized Controlled Trials" Brain Sciences 11, no. 6: 707. https://doi.org/10.3390/brainsci11060707
APA StyleHung, C.-M., Zeng, B.-Y., Zeng, B.-S., Sun, C.-K., Cheng, Y.-S., Su, K.-P., Wu, Y.-C., Chen, T.-Y., Lin, P.-Y., Liang, C.-S., Hsu, C.-W., Chu, C.-S., Chen, Y.-W., Wu, M.-K., & Tseng, P.-T. (2021). The Efficacy of Transcranial Direct Current Stimulation in Enhancing Surgical Skill Acquisition: A Preliminary Meta-Analysis of Randomized Controlled Trials. Brain Sciences, 11(6), 707. https://doi.org/10.3390/brainsci11060707







