Social Behavior and Ultrasonic Vocalizations in a Genetic Rat Model Haploinsufficient for the Cross-Disorder Risk Gene Cacna1c
Abstract
:1. Introduction
2. Social Behavior in Rats
3. Ultrasonic Vocalizations in Rats
3.1. Rough-and-Tumble Play and Ultrasonic Vocalizations in Rats
3.2. Playback of Ultrasonic Vocalizations in Rats
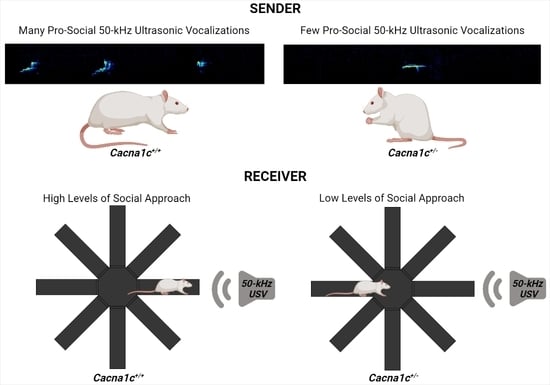
4. Social Behavior and Ultrasonic Vocalizations in Cacna1c Haploinsufficient Rats
5. Other Behavioral Phenotypes Displayed by Cacna1c Haploinsufficient Rats
6. Neurobiological Alterations in Cacna1c Haploinsufficient Rats
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, E.K.; Grozeva, D.; Jones, I.; Jones, L.; Kirov, G.; Caesar, S.; Gordon-Smith, K.; Fraser, C.; Forty, L.; Russell, E.; et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol. Psychiatry 2010, 15, 1016–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.A.; O’Donovan, M.C.; Meng, Y.A.; Jones, I.R.; Ruderfer, D.M.; Jones, L.; Fan, J.; Kirov, G.; Perlis, R.H.; Green, E.K.; et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008, 40, 1056–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Splawski, I.; Timothy, K.W.; Sharpe, L.M.; Decher, N.; Kumar, P.; Bloise, R.; Napolitano, C.; Schwartz, P.J.; Joseph, R.M.; Condouris, K.; et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 2004, 119, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyegaard, M.; Demontis, D.; Foldager, L.; Hedemand, A.; Flint, T.J.; Sørensen, K.M.; Andersen, P.S.; Nordentoft, M.; Werge, T.; Pedersen, C.B.; et al. CACNA1C (rs1006737) is associated with schizophrenia. Mol. Psychiatry 2010, 15, 119–121. [Google Scholar] [CrossRef] [Green Version]
- Zamponi, G.W. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat. Rev. Drug Discov. 2016, 15, 19–34. [Google Scholar] [CrossRef]
- Kabir, Z.D.; Lee, A.S.; Rajadhyaksha, A.M. L-type Ca2+ channels in mood, cognition and addiction: Integrating human and rodent studies with a focus on behavioural endophenotypes. J. Physiol. 2016, 594, 5823–5837. [Google Scholar] [CrossRef] [Green Version]
- Bigos, K.L.; Mattay, V.S.; Callicott, J.H.; Straub, R.E.; Vakkalanka, R.; Kolachana, B.; Hyde, T.M.; Lipska, B.K.; Kleinman, J.E.; Weinberger, D.R. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch. Gen. Psychiatry 2010, 67, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Eckart, N.; Song, Q.; Yang, R.; Wang, R.; Zhu, H.; McCallion, A.S.; Avramopoulos, D. Functional Characterization of Schizophrenia-Associated Variation in CACNA1C. PLoS ONE 2016, 11, e0157086. [Google Scholar] [CrossRef] [Green Version]
- Gershon, E.S.; Grennan, K.; Busnello, J.; Badner, J.A.; Ovsiew, F.; Memon, S.; Alliey-Rodriguez, N.; Cooper, J.; Romanos, B.; Liu, C. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol. Psychiatry 2014, 19, 890–894. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, A.E.; Hoeppner, D.J.; Saito, T.; Blanpain, L.; Ukaigwe, J.; Burke, E.E.; Collado-Torres, L.; Tao, R.; Tajinda, K.; Maynard, K.R.; et al. Profiling gene expression in the human dentate gyrus granule cell layer reveals insights into schizophrenia and its genetic risk. Nat. Neurosci. 2020, 23, 510–519. [Google Scholar] [CrossRef]
- Roussos, P.; Mitchell, A.C.; Voloudakis, G.; Fullard, J.F.; Pothula, V.M.; Tsang, J.; Stahl, E.A.; Georgakopoulos, A.; Ruderfer, D.M.; Charney, A.; et al. A role for noncoding variation in schizophrenia. Cell Rep. 2014, 9, 1417–1429. [Google Scholar] [CrossRef]
- Yoshimizu, T.; Pan, J.Q.; Mungenast, A.E.; Madison, J.M.; Su, S.; Ketterman, J.; Ongur, D.; McPhie, D.; Cohen, B.; Perlis, R.; et al. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol. Psychiatry 2015, 20, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.D.; Kisko, T.M.; Vecchia, D.D.; Andreatini, R.; Schwarting, R.K.W.; Wöhr, M. Sex-specific effects of Cacna1c haploinsufficiency on object recognition, spatial memory, and reversal learning capabilities in rats. Neurobiol. Learn. Mem. 2018, 155, 543–555. [Google Scholar] [CrossRef]
- Braun, M.D.; Kisko, T.M.; Witt, S.H.; Rietschel, M.; Schwarting, R.K.W.; Wöhr, M. Long-term environmental impact on object recognition, spatial memory and reversal learning capabilities in Cacna1c-haploinsufficient rats. Hum. Mol. Genet. 2019, 28, 4113–4131. [Google Scholar] [CrossRef]
- Kisko, T.M.; Braun, M.D.; Michels, S.; Witt, S.H.; Rietschel, M.; Culmsee, C.; Schwarting, R.K.W.; Wöhr, M. Cacna1c haploinsufficiency leads to pro-social 50-kHz ultrasonic communication deficits in rats. Dis. Models Mech. 2018, 11, dmm034116. [Google Scholar] [CrossRef] [Green Version]
- Kisko, T.M.; Braun, M.D.; Michels, S.; Witt, S.H.; Rietschel, M.; Culmsee, C.; Schwarting, R.K.W.; Wöhr, M. Sex-dependent effects of Cacna1c haploinsufficiency on juvenile social play behavior and pro-social 50-kHz ultrasonic communication in rats. Genes Brain Behav. 2020, 19, e12552. [Google Scholar] [CrossRef]
- Kisko, T.M.; Schwarting, R.K.W.; Wöhr, M. Sex differences in the acoustic features of social play-induced 50-kHz ultrasonic vocalizations: A detailed spectrographic analysis in wild-type Sprague-Dawley and Cacna1c haploinsufficient rats. Dev. Psychobiol. 2021. [Google Scholar] [CrossRef]
- Redecker, T.M.; Kisko, T.M.; Schwarting, R.K.W.; Wöhr, M. Effects of Cacna1c haploinsufficiency on social interaction behavior and 50-kHz ultrasonic vocalizations in adult female rats. Behav. Brain Res. 2019, 367, 35–52. [Google Scholar] [CrossRef]
- Wöhr, M.; Willadsen, M.; Kisko, T.M.; Schwarting, R.K.W.; Fendt, M. Sex-dependent effects of Cacna1c haploinsufficiency on behavioral inhibition evoked by conspecific alarm signals in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, e109849. [Google Scholar] [CrossRef]
- Michels, S.; Dolga, A.M.; Braun, M.D.; Kisko, T.M.; Sungur, A.Ö.; Witt, S.H.; Rietschel, M.; Dempfle, A.; Wöhr, M.; Schwarting, R.K.W.; et al. Interaction of the psychiatric risk gene Cacna1c with post-weaning social isolation or environmental enrichment does not affect brain mitochondrial bioenergetics in rats. Front. Cell Neurosci. 2019, 13, e483. [Google Scholar] [CrossRef] [Green Version]
- Michels, S.; Ganjam, G.K.; Martins, H.; Schratt, G.M.; Wöhr, M.; Schwarting, R.K.W.; Culmsee, C. Downregulation of the psychiatric susceptibility gene Cacna1c promotes mitochondrial resilience to oxidative stress in neuronal cells. Cell Death Discov. 2018, 4, e54. [Google Scholar] [CrossRef] [Green Version]
- Michels, S.; Wöhr, M.; Schwarting, R.K.W.; Culmsee, C. Psychiatric risk gene Cacna1c determines mitochondrial resilience against oxidative stress in neurons. Cell Death Discov. 2018, 9, e645. [Google Scholar] [CrossRef]
- Redecker, T.M.; Kisko, T.M.; Wöhr, M.; Schwarting, R.K.W. Cacna1c haploinsufficiency lacks effects on adult hippocampal neurogenesis and volumetric properties of prefrontal cortex and hippocampus in female rats. Physiol. Behav. 2020, 223, e112974. [Google Scholar] [CrossRef]
- Krautheim, J.T.; Straube, B.; Dannlowski, U.; Pyka, M.; Schneider-Hassloff, H.; Drexler, R.; Krug, A.; Sommer, J.; Rietschel, M.; Witt, S.H.; et al. Outgroup emotion processing in the vACC is modulated by childhood trauma and CACNA1C risk variant. Soc. Cogn. Affect. Neurosci. 2018, 13, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Nieratschker, V.; Brückmann, C.; Plewnia, C. CACNA1C risk variant affects facial emotion recognition in healthy individuals. Sci. Rep. 2015, 5, e17349. [Google Scholar] [CrossRef] [Green Version]
- Soeiro-de-Souza, M.G.; Otaduy, M.C.; Dias, C.Z.; Bio, D.S.; Machado-Vieira, R.; Moreno, R.A. The impact of the CACNA1C risk allele on limbic structures and facial emotions recognition in bipolar disorder subjects and healthy controls. J. Affect. Disord. 2012, 141, 94–101. [Google Scholar] [CrossRef]
- Krug, A.; Nieratschker, V.; Markov, V.; Krach, S.; Jansen, A.; Zerres, K.; Eggermann, T.; Stöcker, T.; Shah, N.J.; Treutlein, J.; et al. Effect of CACNA1C rs1006737 on neural correlates of verbal fluency in healthy individuals. Neuroimage 2010, 49, 1831–1836. [Google Scholar] [CrossRef]
- Schweinfurth, M.K. The social life of Norway rats (Rattus norvegicus). eLife 2020, 9, e54040. [Google Scholar] [CrossRef]
- Lukas, M.; de Jong, T.R. Conspecific interactions in adult laboratory rodents: Friends or foes? Curr. Top. Behav. Neurosci. 2017, 30, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Vanderschuren, L.J.; Achterberg, E.J.; Trezza, V. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 2016, 70, 86–105. [Google Scholar] [CrossRef] [Green Version]
- Pellis, S.M.; Field, E.F.; Smith, L.K.; Pellis, V.C. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci. Biobehav. Rev. 1997, 21, 105–120. [Google Scholar] [CrossRef]
- Trezza, V.; Baarendse, P.J.; Vanderschuren, L.J. The pleasures of play: Pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010, 31, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perna, J.C.; Engelmann, M. Recognizing others: Rodent’s social memories. Curr. Top. Behav. Neurosci. 2017, 30, 25–45. [Google Scholar] [CrossRef]
- Galef, B.G.; Wigmore, S.W. Transfer of information concerning distant foods: A laboratory investigation of the ‘information-centre’ hypothesis. Anim. Behav. 1983, 31, 748–758. [Google Scholar] [CrossRef]
- Fendt, M.; Gonzalez-Guerrero, C.P.; Kahl, E. Observational fear learning in rats: Role of trait anxiety and ultrasonic vocalization. Brain Sci. 2021, 11, 423. [Google Scholar] [CrossRef]
- Avital, A.; Aga-Mizrachi, S.; Zubedat, S. Evidence for social cooperation in rodents by automated maze. Sci. Rep. 2016, 6, e29517. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Lallement, J.; van Wingerden, M.; Marx, C.; Srejic, M.; Kalenscher, T. Rats prefer mutual rewards in a prosocial choice task. Front. Neurosci. 2015, 8, e443. [Google Scholar] [CrossRef] [Green Version]
- Rutte, C.; Taborsky, M. Generalized reciprocity in rats. PLoS Biol. 2007, 5, e196. [Google Scholar] [CrossRef] [Green Version]
- Bartal, I.B.A.; Decety, J.; Mason, P. Empathy and pro-social behavior in rats. Science 2011, 334, 1427–1430. [Google Scholar] [CrossRef] [Green Version]
- Brudzynski, S.M. Ethotransmission: Communication of emotional states through ultrasonic vocalization in rats. Curr. Opin. Neurobiol. 2013, 23, 310–317. [Google Scholar] [CrossRef]
- Wöhr, M.; Schwarting, R.K.W. Affective communication in rodents: Ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013, 354, 81–97. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Biological functions of rat ultrasonic vocalizations, Arousal mechanisms and call initiation. Brain Sci. 2021, 11, 605. [Google Scholar] [CrossRef]
- Hofer, M.A. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology 1996, 21, 203–217. [Google Scholar] [CrossRef]
- Allin, J.T.; Banks, E.M. Functional aspects of ultrasound production by infant albino rats (Rattus norvegicus). Anim. Behav. 1972, 20, 175–185. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Blanchard, D.C.; Agullana, R.; Weiss, S.M. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol. Behav. 1991, 50, 967–972. [Google Scholar] [CrossRef]
- Fendt, M.; Brosch, M.; Wernecke, K.E.A.; Willadsen, M.; Wöhr, M. Predator odour but not TMT induces 22-kHz ultrasonic vocalizations in rats that lead to defensive behaviours in conspecifics upon replay. Sci. Rep. 2018, 8, e11041. [Google Scholar] [CrossRef]
- Knutson, B.; Burgdorf, J.; Panksepp, J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J. Comp. Psychol. 1998, 112, 65–73. [Google Scholar] [CrossRef]
- Sales, G.D. Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. J. Zool. 1972, 168, 149–164. [Google Scholar] [CrossRef]
- Panksepp, J. Psychology. Beyond a joke: From animal laughter to human joy? Science 2005, 308, 62–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgdorf, J.; Panksepp, J.; Moskal, J.R. Frequency-modulated 50 kHz ultrasonic vocalizations: A tool for uncovering the molecular substrates of positive affect. Neurosci. Biobehav. Rev. 2011, 35, 1831–1836. [Google Scholar] [CrossRef]
- Pereira, M.; Andreatini, R.; Schwarting, R.K.W.; Brenes, J.C. Amphetamine-induced appetitive 50-kHz calls in rats: A marker of affect in mania? Psychopharmacology 2014, 231, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M.; Gourdon, J.C.; Clarke, P.B. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: Effects of amphetamine and social context. Psychopharmacology 2010, 211, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M. Ultrasonic communication in rats: Appetitive 50-kHz ultrasonic vocalizations as social contact calls. Behav. Ecol. Sociobiol. 2018, 72, e14. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E.; Aldridge, J.W. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009, 9, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Burke, C.J.; Modlinska, K.; Mauro, M.H.; Aleksandrova, L.R.; Pellis, S.M.; Phillips, A.G.; Euston, D.R. A naturalistic method to test depression: Anticipation of play. Behav. Brain Res. 2021, 398, e112975. [Google Scholar] [CrossRef]
- Burgdorf, J.; Kroes, R.A.; Moskal, J.R.; Pfaus, J.G.; Brudzynski, S.M.; Panksepp, J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J. Comp. Psychol. 2008, 122, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Himmler, B.T.; Kisko, T.M.; Euston, D.R.; Kolb, B.; Pellis, S.M. Are 50-kHz calls used as play signals in the playful interactions of rats? I. Evidence from the timing and context of their use. Behav. Process. 2014, 106, 60–66. [Google Scholar] [CrossRef]
- Manduca, A.; Campolongo, P.; Palmery, M.; Vanderschuren, L.J.; Cuomo, V.; Trezza, V. Social play behavior, ultrasonic vocalizations and their modulation by morphine and amphetamine in Wistar and Sprague-Dawley rats. Psychopharmacology 2014, 231, 1661–1673. [Google Scholar] [CrossRef]
- Manduca, A.; Servadio, M.; Campolongo, P.; Palmery, M.; Trabace, L.; Vanderschuren, L.J.; Cuomo, V.; Trezza, V. Strain-and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behavior in rats. Eur. Neuropsychopharmacol. 2014, 24, 1337–1348. [Google Scholar] [CrossRef] [Green Version]
- Siviy, S.M.; Panksepp, J. Sensory modulation of juvenile play in rats. Dev. Psychobiol. 1987, 20, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Kisko, T.M.; Euston, D.R.; Pellis, S.M. Are 50-kHz calls used as play signals in the playful interactions of rats? III. The effects of devocalization on play with unfamiliar partners as juveniles and as adults. Behav. Process. 2015, 113, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kisko, T.M.; Himmler, B.T.; Himmler, S.M.; Euston, D.R.; Pellis, S.M. Are 50-kHz calls used as play signals in the playful interactions of rats? II. Evidence from the effects of devocalization. Behav. Process. 2015, 111, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kisko, T.M.; Wöhr, M.; Pellis, V.C.; Pellis, S.M. From Play to Aggression: High-Frequency 50-kHz Ultrasonic Vocalizations as Play and Appeasement Signals in Rats. Curr. Top. Behav. Neurosci. 2017, 30, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J.; Burgdorf, J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: Effects of social housing and genetic variables. Behav. Brain Res. 2000, 115, 25–38. [Google Scholar] [CrossRef]
- Panksepp, J.; Burgdorf, J.; Gordon, N. Towards a genetics of joy: Breeding rats for “laughter”. In Emotions, Qualia, and Consciousness; Kazniak, A., Ed.; World Scientific: Singapore, 2001; pp. 123–136. [Google Scholar] [CrossRef]
- Burgdorf, J.; Panksepp, J.; Brudzynski, S.M.; Beinfeld, M.C.; Cromwell, H.C.; Kroes, R.A.; Moskal, J.R. The effects of selective breeding for differential rates of 50-kHz ultrasonic vocalizations on emotional behavior in rats. Dev. Psychobiol. 2009, 51, 34–46. [Google Scholar] [CrossRef]
- Burgdorf, J.; Panksepp, J.; Brudzynski, S.M.; Kroes, R.; Moskal, J.R. Breeding for 50-kHz positive affective vocalization in rats. Behav. Genet. 2005, 35, 67–72. [Google Scholar] [CrossRef]
- Webber, E.S.; Harmon, K.M.; Beckwith, T.J.; Peña, S.; Burgdorf, J.; Panksepp, J.; Cromwell, H.C. Selective breeding for 50 kHz ultrasonic vocalization emission produces alterations in the ontogeny and regulation of rough-and-tumble play. Behav. Brain Res. 2012, 229, 138–144. [Google Scholar] [CrossRef]
- Burgdorf, J.; Moskal, J.R.; Brudzynski, S.M.; Panksepp, J. Rats selectively bred for low levels of play-induced 50 kHz vocalizations as a model for autism spectrum disorders: A role for NMDA receptors. Behav. Brain Res. 2013, 251, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, S.A.; Nie, R.; Whipple, C.; Winiger, V.; Hofer, M.A.; Zimmerberg, B. The effects of selective breeding for infant ultrasonic vocalizations on play behavior in juvenile rats. Physiol. Behav. 2006, 87, 527–536. [Google Scholar] [CrossRef]
- Dichter, G.S.; Brunelli, S.A.; Hofer, M.A. Elevated plus-maze behavior in adult offspring of selectively bred rats. Physiol. Behav. 1996, 60, 299–304. [Google Scholar] [CrossRef]
- Brunelli, S.A. Selective breeding for an infant phenotype: Rat pup ultrasonic vocalization (USV). Behav. Genet. 2005, 35, 53–65. [Google Scholar] [CrossRef]
- Lukas, M.; Wöhr, M. Endogenous vasopressin, innate anxiety, and the emission of pro-social 50-kHz ultrasonic vocalizations during social play behavior in juvenile rats. Psychoneuroendocrinology 2015, 56, 35–44. [Google Scholar] [CrossRef]
- Schneider, P.; Pätz, M.; Spanagel, R.; Schneider, M. Adolescent social rejection alters pain processing in a CB1 receptor dependent manner. Eur. Neuropsychopharmacol. 2016, 26, 1201–1212. [Google Scholar] [CrossRef]
- Burgdorf, J.; Kroes, R.A.; Weiss, C.; Oh, M.M.; Disterhoft, J.F.; Brudzynski, S.M.; Panksepp, J.; Moskal, J.R. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience 2011, 192, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Moskal, J.R.; Burgdorf, J.; Kroes, R.A.; Brudzynski, S.M.; Panksepp, J. A novel NMDA receptor glycine-site partial agonist, GLYX-13, has therapeutic potential for the treatment of autism. Neurosci. Biobehav. Rev. 2011, 35, 1982–1988. [Google Scholar] [CrossRef]
- Burgdorf, J.; Kroes, R.A.; Beinfeld, M.C.; Panksepp, J.; Moskal, J.R. Uncovering the molecular basis of positive affect using rough-and-tumble play in rats: A role for insulin-like growth factor I. Neuroscience 2010, 168, 769–777. [Google Scholar] [CrossRef]
- Burgdorf, J.; Panksepp, J.; Beinfeld, M.C.; Kroes, R.A.; Moskal, J.R. Regional brain cholecystokinin changes as a function of rough-and-tumble play behavior in adolescent rats. Peptides 2006, 27, 172–177. [Google Scholar] [CrossRef]
- Willey, A.R.; Varlinskaya, E.I.; Spear, L.P. Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav. Brain Res. 2009, 202, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Waddell, J.; Yang, T.; Ho, E.; Wellmann, K.A.; Mooney, S.M. Prenatal ethanol exposure and whisker clipping disrupt ultrasonic vocalizations and play behavior in adolescent rats. Brain Sci. 2016, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Wellmann, K.A.; George, F.; Brnouti, F.; Mooney, S.M. Docosahexaenoic acid partially ameliorates deficits in social behavior and ultrasonic vocalizations caused by prenatal ethanol exposure. Behav. Brain Res. 2015, 286, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Wellmann, K.A.; Varlinskaya, E.I.; Mooney, S.M. D-Cycloserine ameliorates social alterations that result from prenatal exposure to valproic acid. Brain Res. Bull. 2014, 108, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Raza, S.; Himmler, B.T.; Himmler, S.M.; Harker, A.; Kolb, B.; Pellis, S.M.; Gibb, R. Effects of prenatal exposure to valproic acid on the development of juvenile-typical social play in rats. Behav. Pharmacol. 2015, 26, 707–719. [Google Scholar] [CrossRef]
- Shahrier, M.A.; Wada, H. Effects of prenatal ethanol exposure on acoustic characteristics of play fighting-induced ultrasonic vocalizations in juvenile rats. Neurotoxicology 2020, 79, 25–39. [Google Scholar] [CrossRef]
- Gzielo, K.; Potasiewicz, A.; Hołuj, M.; Litwa, E.; Popik, P.; Nikiforuk, A. Valproic acid exposure impairs ultrasonic communication in infant, adolescent and adult rats. Eur. Neuropsychopharmacol. 2020, 41, 52–62. [Google Scholar] [CrossRef]
- Gzielo, K.; Potasiewicz, A.; Litwa, E.; Piotrowska, D.; Popik, P.; Nikiforuk, A. The effect of maternal immune activation on social play-induced ultrasonic vocalization in rats. Brain Sci. 2021, 11, 344. [Google Scholar] [CrossRef]
- Kentner, A.C.; Scalia, S.; Shin, J.; Migliore, M.M.; Rondón-Ortiz, A.N. Targeted sensory enrichment interventions protect against behavioral and neuroendocrine consequences of early life stress. Psychoneuroendocrinology 2018, 98, e74–e85. [Google Scholar] [CrossRef]
- Wöhr, M.; Schwarting, R.K.W. Ultrasonic communication in rats: Can playback of 50-kHz calls induce approach behavior? PLoS ONE 2007, 2, e1365. [Google Scholar] [CrossRef] [Green Version]
- Wöhr, M.; Schwarting, R.K.W. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav. Neurosci. 2008, 122, 310–330. [Google Scholar] [CrossRef]
- Wöhr, M.; Schwarting, R.K.W. Testing social acoustic memory in rats: Effects of stimulus configuration and long-term memory on the induction of social approach behavior by appetitive 50-kHz ultrasonic vocalizations. Neurobiol. Learn. Mem. 2012, 98, 154–164. [Google Scholar] [CrossRef]
- Willadsen, M.; Seffer, D.; Schwarting, R.K.W.; Wöhr, M. Rodent ultrasonic communication: Male prosocial 50-kHz ultrasonic vocalizations elicit social approach behavior in female rats (Rattus norvegicus). J. Comp. Psychol. 2014, 128, 56–64. [Google Scholar] [CrossRef]
- Engelhardt, K.A.; Schwarting, R.K.W.; Wöhr, M. Mapping trait-like socio-affective phenotypes in rats through 50-kHz ultrasonic vocalizations. Psychopharmacology 2018, 235, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Seffer, D.; Rippberger, H.; Schwarting, R.K.W.; Wöhr, M. Pro-Social 50-kHz ultrasonic communication in rats: Post-weaning but not post-adolescent social isolation leads to social impairments-phenotypic rescue by re-socialization. Front. Behav. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenes, J.C.; Lackinger, M.; Höglinger, G.U.; Schratt, G.; Schwarting, R.K.W.; Wöhr, M. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J. Comp. Neurol. 2016, 524, 1586–1607. [Google Scholar] [CrossRef] [PubMed]
- Berz, A.; de Souza, C.P.; Wöhr, M.; Schwarting, R.K.W. Limited generalizability, pharmacological modulation, and state-dependency of habituation towards pro-social 50-kHz calls in rats. IScience 2021, 24, 102426. [Google Scholar] [CrossRef]
- Sadananda, M.; Wöhr, M.; Schwarting, R.K.W. Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci. Lett. 2008, 435, 17–23. [Google Scholar] [CrossRef]
- Willuhn, I.; Tose, A.; Wanat, M.J.; Hart, A.S.; Hollon, N.G.; Phillips, P.E.; Schwarting, R.K.W.; Wöhr, M. Phasic dopamine release in the nucleus accumbens in response to pro-social 50 kHz ultrasonic vocalizations in rats. J. Neurosci. 2014, 34, 10616–10623. [Google Scholar] [CrossRef]
- Engelhardt, K.A.; Fuchs, E.; Schwarting, R.K.W.; Wöhr, M. Effects of amphetamine on pro-social ultrasonic communication in juvenile rats: Implications for mania models. Eur. Neuropsychopharmacol. 2017, 27, 261–273. [Google Scholar] [CrossRef]
- Wöhr, M.; Schwarting, R.K.W. Ultrasonic communication in rats: Effects of morphine and naloxone on vocal and behavioral responses to playback of 50-kHz vocalizations. Pharmacol. Biochem. Behav. 2009, 94, 285–295. [Google Scholar] [CrossRef]
- Berg, E.L.; Copping, N.A.; Rivera, J.K.; Pride, M.C.; Careaga, M.; Bauman, M.D.; Berman, R.F.; Lein, P.J.; Harony-Nicolas, H.; Buxbaum, J.D.; et al. Developmental social communication deficits in the Shank3 rat model of Phelan-McDermid syndrome and autism spectrum disorder. Autism Res. 2018, 11, 587–601. [Google Scholar] [CrossRef]
- Bourgeron, T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat. Rev. Neurosci. 2015, 16, 551–563. [Google Scholar] [CrossRef]
- Berg, E.L.; Pride, M.C.; Petkova, S.P.; Lee, R.D.; Copping, N.A.; Shen, Y.; Adhikari, A.; Fenton, T.A.; Pedersen, L.R.; Noakes, L.S.; et al. Translational outcomes in a full gene deletion of ubiquitin protein ligase E3A rat model of Angelman syndrome. Transl. Psychiatry 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Berg, E.L.; Ching, T.M.; Bruun, D.A.; Rivera, J.K.; Careaga, M.; Ellegood, J.; Lerch, J.P.; Wöhr, M.; Lein, P.J.; Silverman, J.L. Translational outcomes relevant to neurodevelopmental disorders following early life exposure of rats to chlorpyrifos. J. Neurodev. Disord. 2020, 12, 1–15. [Google Scholar] [CrossRef]
- Kircher, T.; Wöhr, M.; Nenadic, I.; Schwarting, R.K.W.; Schratt, G.; Alferink, J.; Culmsee, C.; Garn, H.; Hahn, T.; Müller-Myhsok, B.; et al. Neurobiology of the major psychoses: A translational perspective on brain structure and function-the FOR2107 consortium. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 949–962. [Google Scholar] [CrossRef]
- Trezza, V.; Damsteegt, R.; Vanderschuren, L.J. Conditioned place preference induced by social play behavior: Parametrics, extinction, reinstatement and disruption by methylphenidate. Eur. Neuropsychopharmacol. 2009, 19, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Pellis, S.M.; Pellis, V.C.; McKenna, M.M. Feminine dimension in the play fighting of rats (Rattus norvegicus) and its defeminization neonatally by androgens. J. Comp. Psychol. 1994, 108, 68–73. [Google Scholar] [CrossRef]
- Albert, D.J.; Walsh, M.L.; Gorzalka, B.B.; Siemens, Y.; Louie, H. Testosterone removal in rats results in a decrease in social aggression and a loss of social dominance. Physiol. Behav. 1986, 36, 401–407. [Google Scholar] [CrossRef]
- Wöhr, M.; Borta, A.; Schwarting, R.K.W. Overt behavior and ultrasonic vocalization in a fear conditioning paradigm: A dose-response study in the rat. Neurobiol. Learn. Mem. 2005, 84, 228–240. [Google Scholar] [CrossRef]
- Sykes, L.; Haddon, J.; Lancaster, T.M.; Sykes, A.; Azzouni, K.; Ihssen, N.; Moon, A.L.; Lin, T.E.; Linden, D.E.; Owen, M.J.; et al. Genetic variation in the psychiatric risk gene CACNA1C modulates reversal learning across species. Schizophr. Bull. 2019, 45, 1024–1032. [Google Scholar] [CrossRef]
- Moon, A.L.; Brydges, N.M.; Wilkinson, L.S.; Hall, J.; Thomas, K.L. Cacna1c hemizygosity results in aberrant fear conditioning to neutral stimuli. Schizophr. Bull. 2020, 46, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Moon, A.L.; Haan, N.; Wilkinson, L.S.; Thomas, K.L.; Hall, J. CACNA1C: Association with psychiatric disorders, behavior, and neurogenesis. Schizophr. Bull. 2018, 44, 958–965. [Google Scholar] [CrossRef]
- Tigaret, C.M.; Lin, T.E.; Morrell, E.R.; Sykes, L.; Moon, A.L.; O’Donovan, M.C.; Owen, M.J.; Wilkinson, L.S.; Jones, M.W.; Thomas, K.L.; et al. Neurotrophin receptor activation rescues cognitive and synaptic abnormalities caused by hemizygosity of the psychiatric risk gene Cacna1c. Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Łopuch, S.; Popik, P. Cooperative behavior of laboratory rats (Rattus norvegicus) in an instrumental task. J. Comp. Psychol. 2011, 125, 250–253. [Google Scholar] [CrossRef]
- Wöhr, M. Measuring mania-like elevated mood through amphetamine-induced 50-kHz ultrasonic vocalizations in rats. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]

| Sender | Receiver | Sex | Age | References |
|---|---|---|---|---|
| Reduced 50-kHz USV emission rates during rough-and-tumble play | Reduced social approach behavior evoked by playback of pro-social 50-kHz USV | Male | Juvenility | [15] |
| Unchanged 50-kHz USV emission rates during rough-and-tumble play | Unchanged social approach behavior evoked by playback of pro-social 50-kHz USV 1 | Female | Juvenility | [16,17] |
| Reduced 50-kHz USV emission rates during social interactions | Female | Adulthood | [18] | |
| Reduced behavioral inhibition evoked by playback of alarm 22-kHz USV | Male | Adulthood | [19] | |
| Unchanged behavioral inhibition evoked by playback of alarm 22-kHz USV | Female | Adulthood | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wöhr, M.; Kisko, T.M.; Schwarting, R.K.W. Social Behavior and Ultrasonic Vocalizations in a Genetic Rat Model Haploinsufficient for the Cross-Disorder Risk Gene Cacna1c. Brain Sci. 2021, 11, 724. https://doi.org/10.3390/brainsci11060724
Wöhr M, Kisko TM, Schwarting RKW. Social Behavior and Ultrasonic Vocalizations in a Genetic Rat Model Haploinsufficient for the Cross-Disorder Risk Gene Cacna1c. Brain Sciences. 2021; 11(6):724. https://doi.org/10.3390/brainsci11060724
Chicago/Turabian StyleWöhr, Markus, Theresa M. Kisko, and Rainer K.W. Schwarting. 2021. "Social Behavior and Ultrasonic Vocalizations in a Genetic Rat Model Haploinsufficient for the Cross-Disorder Risk Gene Cacna1c" Brain Sciences 11, no. 6: 724. https://doi.org/10.3390/brainsci11060724
APA StyleWöhr, M., Kisko, T. M., & Schwarting, R. K. W. (2021). Social Behavior and Ultrasonic Vocalizations in a Genetic Rat Model Haploinsufficient for the Cross-Disorder Risk Gene Cacna1c. Brain Sciences, 11(6), 724. https://doi.org/10.3390/brainsci11060724