The Timecourse of Electrophysiological Brain–Heart Interaction in DoC Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Recording
2.2. Data Extraction
2.3. Data Analysis
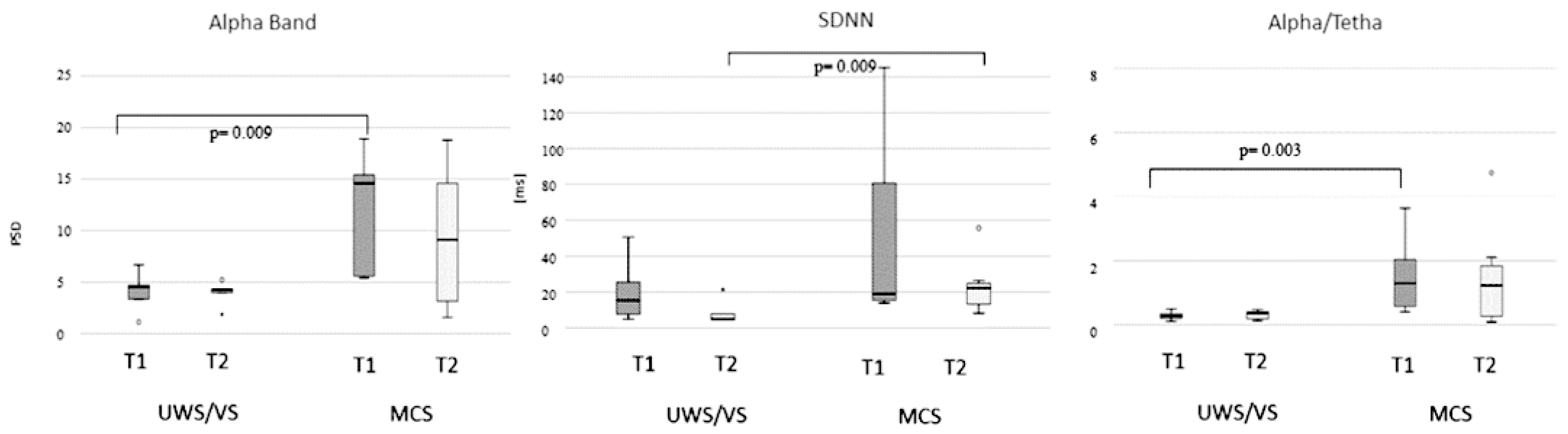
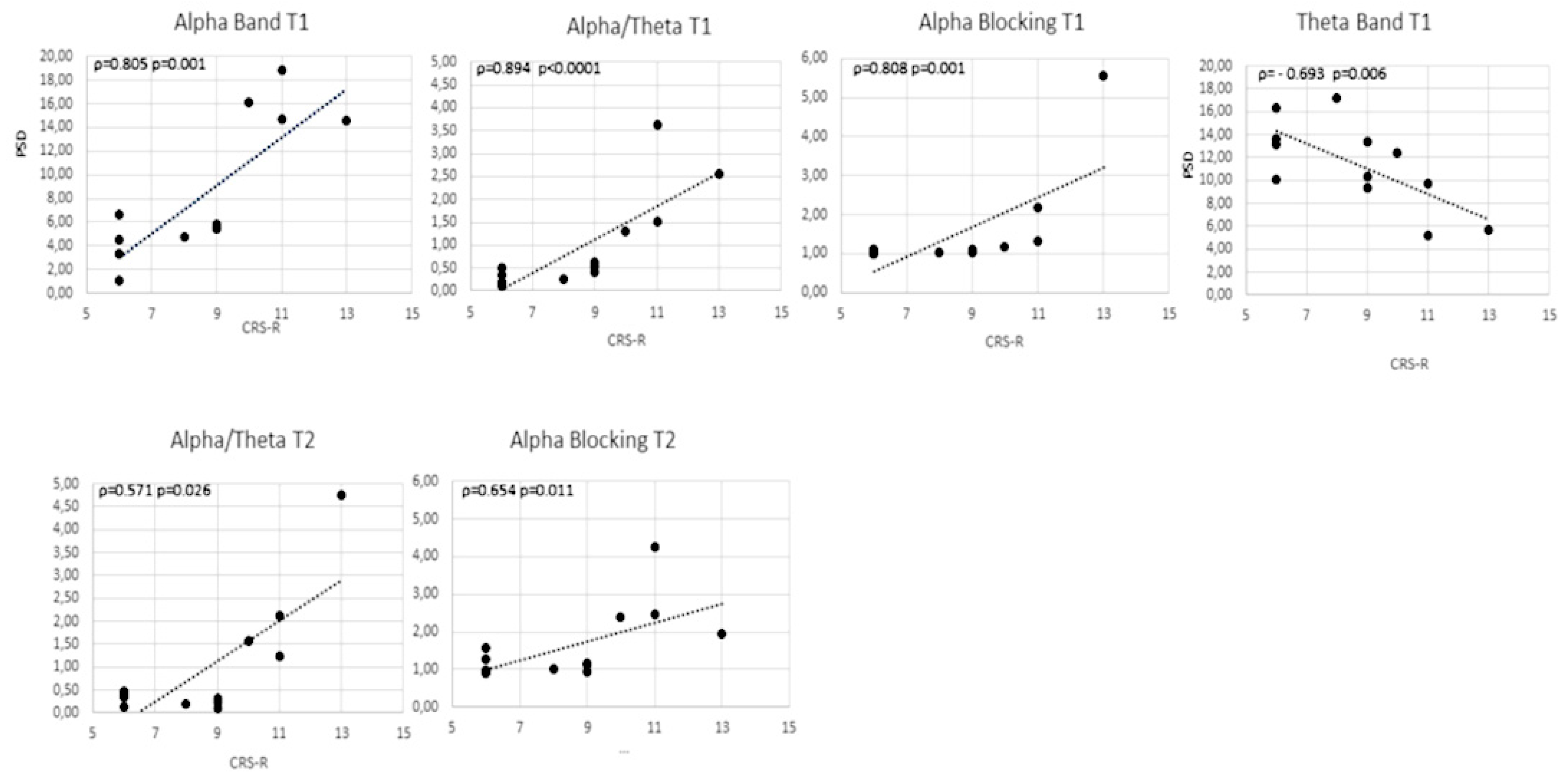
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meaney, D.F.; Morrison, B.; Dale Bass, C. The Mechanics of Traumatic Brain Injury: A Review of What We Know and What We Need to Know for Reducing Its Societal Burden. J. Biomech. Eng. 2014, 136, 021008. [Google Scholar] [CrossRef]
- Roebuck-Spencer, T.; Cernich, A. Epidemiology and Societal Impact of Traumatic Brain Injury. In Handbook on the Neuropsychology of Traumatic Brain Injury; Clinical Handbooks in Neuropsychology; Springer: New York, NY, USA, 2014; pp. 3–23. ISBN 978-1-4939-0783-0. [Google Scholar]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative Disease: Models, Mechanisms, and a New Hope. Dis. Model Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Comprehensive Systematic Review Update Summary: Disorders of Consciousness. Neurology 2018, 91, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Fins, J.J. Clinical pragmatism and the care of brain damaged patients: Toward a palliative neuroethics for disorders of consciousness. In Progress in Brain Research; Laureys, S., Ed.; The Boundaries of Consciousness: Neurobiology and Neuropathology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 150, pp. 565–582. [Google Scholar]
- Demertzi, A.; Ledoux, D.; Bruno, M.-A.; Vanhaudenhuyse, A.; Gosseries, O.; Soddu, A.; Schnakers, C.; Moonen, G.; Laureys, S. Attitudes towards End-of-Life Issues in Disorders of Consciousness: A European Survey. J. Neurol. 2011, 258, 1058–1065. [Google Scholar] [CrossRef]
- Riganello, F.; Macrì, S.; Alleva, E.; Petrini, C.; Soddu, A.; Leòn-Carriòn, J.; Dolce, G. Pain Perception in Unresponsive Wakefulness Syndrome May Challenge the Interruption of Artificial Nutrition and Hydration: Neuroethics in Action. Front. Neurol. 2016, 7. [Google Scholar] [CrossRef]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive Wakefulness Syndrome: A New Name for the Vegetative State or Apallic Syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The Minimally Conscious State Definition and Diagnostic Criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Jennett, B. The Vegetative State. J. Neurol. Neurosurg. Psychiatry 2002, 73, 355–357. [Google Scholar] [CrossRef]
- Dolce, G.; Lucca, L.F.; Candelieri, A.; Rogano, S.; Pignolo, L.; Sannita, W.G. Visual Pursuit in the Severe Disorder of Consciousness. J. Neurotrauma 2010, 28, 1149–1154. [Google Scholar] [CrossRef]
- Andrews, K.; Murphy, L.; Munday, R.; Littlewood, C. Misdiagnosis of the Vegetative State: Retrospective Study in a Rehabilitation Unit. BMJ 1996, 313, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Lancioni, G.E.; Belardinelli, M.O.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J. Vegetative State: Efforts to Curb Misdiagnosis. Cogn. Process 2010, 11, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Cruse, D.; Naci, L.; Weijer, C.; Owen, A.M. Risk, Diagnostic Error, and the Clinical Science of Consciousness. Neuroimage Clin. 2015, 7, 588–597. [Google Scholar] [CrossRef] [PubMed]
- van Erp, W.S.; Lavrijsen, J.C.M.; Vos, P.E.; Bor, H.; Laureys, S.; Koopmans, R.T.C.M. The Vegetative State: Prevalence, Misdiagnosis, and Treatment Limitations. J. Am. Med. Dir. Assoc. 2015, 16, 85.e9–85.e14. [Google Scholar] [CrossRef]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement Characteristics and Diagnostic Utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; Kalmar, K.; Pape, T.L.-B.; Zafonte, R.; et al. Assessment Scales for Disorders of Consciousness: Evidence-Based Recommendations for Clinical Practice and Research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.D.; Riganello, F.; Arcuri, F.; Pugliese, M.E.; Lucca, L.F.; Dolce, G.; Sannita, W.G. Coma Recovery Scale-r: Variability in the Disorder of Consciousness. BMC Neurol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chatelle, C.; Thibaut, A. Pain Issues in Disorders of Consciousness. Brain Inj. 2014, 28, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Leo, A.; Bramanti, P.; Calabrò, R.S. Moving Toward Conscious Pain Processing Detection in Chronic Disorders of Consciousness: Anterior Cingulate Cortex Neuromodulation. J. Pain 2015, 16, 1022–1031. [Google Scholar] [CrossRef]
- Cortese, D.; Riganello, F.; Arcuri, F.; Lucca, L.; Tonin, P.; Schnakers, C.; Laureys, S. The Trace Conditional Learning of the Noxious Stimulus in UWS Patients and Its Prognostic Value in a GSR and HRV Entropy Study. Front. Hum. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Liberati, G.; Hünefeldt, T.; Belardinelli, M.O. Questioning the Dichotomy between Vegetative State and Minimally Conscious State: A Review of the Statistical Evidence. Front. Hum. Neurosci. 2014, 8, 865. [Google Scholar] [CrossRef]
- O’Donnell, J.C.; Browne, K.D.; Kilbaugh, T.J.; Chen, H.I.; Whyte, J.; Cullen, D.K. Challenges and Demand for Modeling Disorders of Consciousness Following Traumatic Brain Injury. Neurosci. Biobehav. Rev. 2019, 98, 336–346. [Google Scholar] [CrossRef]
- Wannez, S.; Heine, L.; Thonnard, M.; Gosseries, O.; Laureys, S.; Coma Science Group collaborators. The Repetition of Behavioral Assessments in Diagnosis of Disorders of Consciousness. Ann. Neurol. 2017, 81, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Riganello, F.; Candelieri, A.; Quintieri, M.; Conforti, D.; Dolce, G. Heart Rate Variability: An Index of Brain Processing in Vegetative State? An Artificial Intelligence, Data Mining Study. Clin. Neurophysiol. 2010, 121, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, S.; Boccagni, C.; Sant’Angelo, A.; Prestandrea, C.; Mazzilli, R.; Galardi, G. EEG Predictors of Outcome in Patients with Disorders of Consciousness Admitted for Intensive Rehabilitation. Clin. Neurophysiol. 2015, 126, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J. EEG Dynamics in Patients with Alzheimer’s Disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef]
- Koenig, T.; Prichep, L.; Dierks, T.; Hubl, D.; Wahlund, L.O.; John, E.R.; Jelic, V. Decreased EEG Synchronization in Alzheimer’s Disease and Mild Cognitive Impairment. Neurobiol. Aging 2005, 26, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Binetti, G.; Cassetta, E.; Dal Forno, G.; Del Percio, C.; Ferreri, F.; Ferri, R.; Frisoni, G.; Hirata, K.; Lanuzza, B.; et al. Sources of Cortical Rhythms Change as a Function of Cognitive Impairment in Pathological Aging: A Multicenter Study. Clin. Neurophysiol. 2006, 117, 252–268. [Google Scholar] [CrossRef]
- Rossini, P.M.; Rossi, S.; Babiloni, C.; Polich, J. Clinical Neurophysiology of Aging Brain: From Normal Aging to Neurodegeneration. Prog. Neurobiol. 2007, 83, 375–400. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric fields of the brain: The neurophysics of EEG; Oxford University Press: New York, NY, USA, 2006; ISBN 978-0-19-505038-7. [Google Scholar]
- Klimesch, W. Event-Related Band Power Changes and Memory Performance. Event-Related Desynchronization and Related Oscillatory Phenomena of the Brain. Handb. Electroencephalogr. Clin. Neurophysiol. 1999, 6, 151–178. [Google Scholar]
- Klimesch, W.; Doppelmayr, M.; Stadler, W.; Pöllhuber, D.; Sauseng, P.; Röhm, D. Episodic Retrieval Is Reflected by a Process Specific Increase in Human Electroencephalographic Theta Activity. Neurosci. Lett. 2001, 302, 49–52. [Google Scholar] [CrossRef]
- Klimesch, W.; Schack, B.; Schabus, M.; Doppelmayr, M.; Gruber, W.; Sauseng, P. Phase-Locked Alpha and Theta Oscillations Generate the P1-N1 Complex and Are Related to Memory Performance. Brain Res. Cogn. Brain Res. 2004, 19, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Gruber, W.; Doppelmayr, M.; Stadler, W.; Schabus, M. The Interplay between Theta and Alpha Oscillations in the Human Electroencephalogram Reflects the Transfer of Information between Memory Systems. Neurosci. Lett. 2002, 324, 121–124. [Google Scholar] [CrossRef]
- Schiff, N.D. Recovery of Consciousness after Brain Injury: A Mesocircuit Hypothesis. Trends Neurosci. 2010, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Kanda, P.; Basile, L.; da Silva Lopes, H.F.; Baratho, R.; Demario, J.; Jorge, M.; Nardi, A.; Machado, S.; Ianof, J.N.; et al. Index of Alpha/Theta Ratio of the Electroencephalogram: A New Marker for Alzheimer’s Disease. Front. Aging Neurosci. 2013, 5. [Google Scholar] [CrossRef]
- Bousleiman, H.; Chaturvedi, M.; Gschwandtner, U.; Hatz, F.; Schindler, C.; Zimmermann, R.; Fuhr, P. P122. Alpha1/Theta Ratio from Quantitative EEG (QEEG) as a Reliable Marker for Mild Cognitive Impairment (MCI) in Patients with Parkinson’s Disease (PD). Clin. Neurophysiol. 2015, 126, e150–e151. [Google Scholar] [CrossRef]
- Penttila, M.; Partanen, J.V.; Soininen, H.; Riekkinen, P.J. Quantitative analysis of occipital eeg in different stages of alzheimer’s disease. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 1–6. [Google Scholar] [CrossRef]
- Benarroch, E.E. The autonomic nervous system: Basic anatomy and physiology. Contin. Lifelong Learn. Neurol. 2007, 13, 13–32. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the Heart–Brain Connection: Further Elaboration of a Model of Neurovisceral Integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Riganello, F. Responsiveness and the Autonomic Control–CNS Two-Way Interaction in Disorders of Consciousness. In Brain Function and Responsiveness in Disorders of Consciousness; Monti, M.M., Sannita, W.G., Eds.; Springer International Publishing: Cham, Swizerland, 2016; pp. 145–155. ISBN 978-3-319-21424-5. [Google Scholar]
- Lane, R.; Mcrae, K.; Reiman, E.; Chen, K.; Ahern, G.; Thayer, J. Neural Correlates of Heart Rate Variability during Emotion. NeuroImage 2009, 44, 213–222. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5. [Google Scholar] [CrossRef]
- Riganello, F.; Larroque, S.K.; Di Perri, C.; Prada, V.; Sannita, W.G.; Laureys, S. Measures of CNS-Autonomic Interaction and Responsiveness in Disorder of Consciousness. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A Meta-Analysis of Heart Rate Variability and Neuroimaging Studies: Implications for Heart Rate Variability as a Marker of Stress and Health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Ernst, G. Heart-Rate Variability—More than Heart Beats? Front. Public Health 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E.; Huikuri, H.V.; Sacha, J.; Trimmel, K. An Introduction to Heart Rate Variability: Methodological Considerations and Clinical Applications. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef]
- Riganello, F.; Larroque, S.K.; Bahri, M.A.; Heine, L.; Martial, C.; Carrière, M.; Charland-Verville, V.; Aubinet, C.; Vanhaudenhuyse, A.; Chatelle, C.; et al. A Heartbeat Away From Consciousness: Heart Rate Variability Entropy Can Discriminate Disorders of Consciousness and Is Correlated With Resting-State FMRI Brain Connectivity of the Central Autonomic Network. Front. Neurol. 2018, 9, 769. [Google Scholar] [CrossRef]
- Fusheng, Y.; Bo, H.; Qingyu, T. Approximate Entropy and Its Application to Biosignal Analysis. In Nonlinear Biomedical Signal Processing; Akay, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 72–91. ISBN 978-0-470-54537-9. [Google Scholar]
- Delgado-Bonal, A.; Marshak, A. Approximate Entropy and Sample Entropy: A Comprehensive Tutorial. Entropy 2019, 21, 541. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A. Generalized Multiscale Entropy Analysis: Application to Quantifying the Complex Volatility of Human Heartbeat Time Series. Entropy 2015, 17, 1197–1203. [Google Scholar] [CrossRef]
- Schibler, U.; Ripperger, J.; Brown, S.A. Peripheral Circadian Oscillators in Mammals: Time and Food. J. Biol. Rhythm. 2003, 18, 250–260. [Google Scholar] [CrossRef]
- Ivanov, P.C.; Hu, K.; Hilton, M.F.; Shea, S.A.; Stanley, H.E. Endogenous Circadian Rhythm in Human Motor Activity Uncoupled from Circadian Influences on Cardiac Dynamics. PNAS 2007, 104, 20702–20707. [Google Scholar] [CrossRef] [PubMed]
- Riganello, F.; Prada, V.; Soddu, A.; di Perri, C.; Sannita, W.G. Circadian Rhythms and Measures of CNS/Autonomic Interaction. IJERPH 2019, 16, 2336. [Google Scholar] [CrossRef]
- Candelieri, A.; Cortese, M.D.; Dolce, G.; Riganello, F.; Sannita, W.G. Visual Pursuit: Within-Day Variability in the Severe Disorder of Consciousness. J. Neurotrauma 2011, 28, 2013–2017. [Google Scholar] [CrossRef]
- Cologan, V.; Drouot, X.; Parapatics, S.; Delorme, A.; Gruber, G.; Moonen, G.; Laureys, S. Sleep in the Unresponsive Wakefulness Syndrome and Minimally Conscious State. J. Neurotrauma 2013, 30, 339–346. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–Heart Rate Variability Analysis Software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Gibbons, J.D.; Chakraborti, S. Nonparametric Statistical Inference. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 977–979. ISBN 978-3-642-04897-5. [Google Scholar]
- Rosenthal, R. Effect Sizes: Pearson’s Correlation, Its Display via the BESD, and Alternative Indices. Am. Psychol. 1991, 46, 1086–1087. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice Guideline Update Recommendations Summary: Disorders of Consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch Phys. Med. Rehabil. 2018, 99, 1699–1709. [Google Scholar] [CrossRef]
- Lehembre, R.; Bruno, M.-A.; Vanhaudenhuyse, A.; Chatelle, C.; Cologan, V.; Leclercq, Y.; Soddu, A.; Macq, B.; Laureys, S.; Noirhomme, Q. Resting-State EEG Study of Comatose Patients: A Connectivity and Frequency Analysis to Find Differences between Vegetative and Minimally Conscious States. Funct. Neurol. 2012, 27, 41–47. [Google Scholar] [PubMed]
- Lechinger, J.; Bothe, K.; Pichler, G.; Michitsch, G.; Donis, J.; Klimesch, W.; Schabus, M. CRS-R Score in Disorders of Consciousness Is Strongly Related to Spectral EEG at Rest. J. Neurol. 2013, 260, 2348–2356. [Google Scholar] [CrossRef]
- Piarulli, A.; Bergamasco, M.; Thibaut, A.; Cologan, V.; Gosseries, O.; Laureys, S. EEG Ultradian Rhythmicity Differences in Disorders of Consciousness during Wakefulness. J. Neurol. 2016, 263, 1746–1760. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Sarà, M.; Vecchio, F.; Pistoia, F.; Sebastiano, F.; Onorati, P.; Albertini, G.; Pasqualetti, P.; Cibelli, G.; Buffo, P.; et al. Cortical Sources of Resting-State Alpha Rhythms Are Abnormal in Persistent Vegetative State Patients. Clin. Neurophysiol. 2009, 120, 719–729. [Google Scholar] [CrossRef]
- Danze, F.; Brule, J.F.; Haddad, K. Chronic Vegetative State after Severe Head Injury: Clinical Study; Electrophysiological Investigations and CT Scan in 15 Cases. Neurosurg. Rev. 1989, 12, 477–499. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.S.; Schomer, D.L.; Niedermeyer, E. Normal EEG and Sleep: Adults and Elderly. In Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 183–214. [Google Scholar]
- Vázquez Marrufo, M.; Vaquero, E.; Cardoso, M.J.; Gómez, C.M. Temporal Evolution of Alpha and Beta Bands during Visual Spatial Attention. Brain Res. Cogn. Brain Res. 2001, 12, 315–320. [Google Scholar] [CrossRef]
- Thut, G.; Nietzel, A.; Brandt, S.A.; Pascual-Leone, A. Alpha-Band Electroencephalographic Activity over Occipital Cortex Indexes Visuospatial Attention Bias and Predicts Visual Target Detection. J. Neurosci. 2006, 26, 9494–9502. [Google Scholar] [CrossRef]
- Vijayan, S.; Ching, S.; Purdon, P.L.; Brown, E.N.; Kopell, N.J. Thalamocortical Mechanisms for the Anteriorization of α Rhythms during Propofol-Induced Unconsciousness. J. Neurosci. 2013, 33, 11070–11075. [Google Scholar] [CrossRef]
- Abeysuriya, R.G.; Rennie, C.J.; Robinson, P.A. Physiologically Based Arousal State Estimation and Dynamics. J. Neurosci. Methods 2015, 253, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Elul, R. The Genesis of the Eeg. In International Review of Neurobiology; Pfeiffer, C.C., Smythies, J.R., Eds.; Academic Press: Cambridge, MA, USA, 1972; Volume 15, pp. 227–272. [Google Scholar]
- Aeschbach, D.; Matthews, J.R.; Postolache, T.T.; Jackson, M.A.; Giesen, H.A.; Wehr, T.A. Two Circadian Rhythms in the Human Electroencephalogram during Wakefulness. Am. J. Physiol. 1999, 277, R1771–R1779. [Google Scholar] [CrossRef]
- Mertel, I.; Pavlov, Y.G.; Barner, C.; Müller, F.; Diekelmann, S.; Kotchoubey, B. Sleep in Disorders of Consciousness: Behavioral and Polysomnographic Recording. BMC Med. 2020, 18, 350. [Google Scholar] [CrossRef] [PubMed]
- Riganello, F.; Napoletano, G.; Cortese, M.D.; Arcuri, F.; Solano, A.; Lucca, L.F.; Tonin, P.; Soddu, A. What Impact Can Hospitalization Environment Produce on the ANS Functioning in Patients with Unresponsive Wakefulness Syndrome?—24-Hour Monitoring. Brain Inj. 2019, 33, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Broughton, R. Biorhythmic Variations in Consciousness and Psychological Functions. Can. Psychol. Rev. Psychol. Can. 1975, 16, 217–239. [Google Scholar] [CrossRef]
- Bekinschtein, T.A.; Golombek, D.A.; Simonetta, S.H.; Coleman, M.R.; Manes, F.F. Circadian Rhythms in the Vegetative State. Brain Inj. 2009, 23, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, P.; Sancisi, E.; Morgia, C.L.; Calandra-Buonaura, G.; Carelli, V.; Cameli, O.; Battistini, A.; Cortelli, P.; Piperno, R. Nocturnal Melatonin Regulation in Post-Traumatic Vegetative State: A Possible Role for Melatonin Supplementation? Chronobiol. Int. 2014, 31, 741–745. [Google Scholar] [CrossRef]
- Blume, C.; Angerer, M.; Raml, M.; del Giudice, R.; Santhi, N.; Pichler, G.; Kunz, A.B.; Scarpatetti, M.; Trinka, E.; Schabus, M. Healthier Rhythm, Healthier Brain? Integrity of Circadian Melatonin and Temperature Rhythms Relates to the Clinical State of Brain-Injured Patients. Eur. J. Neurol. 2019, 26, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.T. Minsize2: A Computer Program for Determining Effect Size and Minimum Sample Size for Statistical Significance for Univariate, Multivariate, and Nonparametric Tests. Educ. Psychol. Meas. 1999, 59, 518–531. [Google Scholar] [CrossRef]

| SUBJECT | GENDER | AGE | FROM EVENT TO HOSPITALIZATION (DAYS) | ETIOLOGY | DIAGNOSIS | CRS |
|---|---|---|---|---|---|---|
| 1 | M | 72 | 40 | HEM | MCS | 11 |
| 2 | F | 71 | 26 | HEM | MCS | 9 |
| 3 | F | 72 | 36 | HEM | MCS | 9 |
| 4 | M | 71 | 22 | TBI | UWS | 6 |
| 5 | F | 65 | 14 | HEM | MCS | 9 |
| 6 | F | 33 | 35 | TBI | UWS | 6 |
| 7 | M | 40 | 39 | HEM | UWS | 8 |
| 8 | F | 63 | 53 | ISC | UWS | 6 |
| 9 | M | 73 | 49 | ISC | UWS | 6 |
| 10 | M | 45 | 26 | ISC | MCS | 13 |
| 11 | F | 34 | 34 | HEM | MCS | 10 |
| 12 | M | 48 | 34 | HEM | MCS | 11 |
| 13 | M | 48 | HC | |||
| 14 | M | 40 | HC | |||
| 15 | F | 36 | HC | |||
| 16 | F | 38 | HC | |||
| 17 | M | 51 | HC | |||
| 18 | F | 40 | HC | |||
| 19 | F | 51 | HC | |||
| 20 | F | 45 | HC | |||
| 21 | M | 40 | HC | |||
| 22 | M | 55 | HC | |||
| 23 | F | 49 | HC | |||
| 24 | F | 40 | HC | |||
| 25 | F | 46 | HC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riganello, F.; Vatrano, M.; Carozzo, S.; Russo, M.; Lucca, L.F.; Ursino, M.; Ruggiero, V.; Cerasa, A.; Porcaro, C. The Timecourse of Electrophysiological Brain–Heart Interaction in DoC Patients. Brain Sci. 2021, 11, 750. https://doi.org/10.3390/brainsci11060750
Riganello F, Vatrano M, Carozzo S, Russo M, Lucca LF, Ursino M, Ruggiero V, Cerasa A, Porcaro C. The Timecourse of Electrophysiological Brain–Heart Interaction in DoC Patients. Brain Sciences. 2021; 11(6):750. https://doi.org/10.3390/brainsci11060750
Chicago/Turabian StyleRiganello, Francesco, Martina Vatrano, Simone Carozzo, Miriam Russo, Lucia Francesca Lucca, Maria Ursino, Valentina Ruggiero, Antonio Cerasa, and Camillo Porcaro. 2021. "The Timecourse of Electrophysiological Brain–Heart Interaction in DoC Patients" Brain Sciences 11, no. 6: 750. https://doi.org/10.3390/brainsci11060750
APA StyleRiganello, F., Vatrano, M., Carozzo, S., Russo, M., Lucca, L. F., Ursino, M., Ruggiero, V., Cerasa, A., & Porcaro, C. (2021). The Timecourse of Electrophysiological Brain–Heart Interaction in DoC Patients. Brain Sciences, 11(6), 750. https://doi.org/10.3390/brainsci11060750









