Impact of Transcranial Direct Current Stimulation on Cognitive Function, Brain Functional Segregation, and Integration in Patients with Mild Cognitive Impairment According to Amyloid-Beta Deposition and APOE ε4-Allele: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. tDCS Application
2.4. fMRI Data Acquisition and Data Processing
2.5. fALFF and DC Analysis
2.6. [18 F] Flutemetamol PET-CT Image Acquisition, Assessments, and SUVR Calculations
2.7. Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinical Data
3.2. Changes in Neuropsychological Performance
3.3. Changes in Functional Segregation and Integration of the DMN: An ROI-Based Analysis
3.4. Changes in Functional Segregation and Integration Parameters: Whole Brain Voxel-Based Analysis
4. Discussion
4.1. Changes in Neuropsychological Performance
4.2. Changes in Functional Segregation and Integration of the DMN: An ROI-Based Analysis
4.3. Changes in Functional Segregation and Integration Parameters: Whole Brain Voxel-Based Analysis
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, R.C.; Negash, S. Mild Cognitive Impairment: An Overview. CNS Spectr. 2008, 13, 45–53. [Google Scholar] [CrossRef]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef]
- Jean, L.; Bergeron, M.-È.; Thivierge, S.; Simard, M. Cognitive Intervention Programs for Individuals With Mild Cognitive Impairment: Systematic Review of the Literature. Am. J. Geriatr. Psychiatry 2010, 18, 281–296. [Google Scholar] [CrossRef]
- Lautenschlager, N.T.; Cox, K.; Kurz, A.F. Physical Activity and Mild Cognitive Impairment and Alzheimer’s Disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 352–358. [Google Scholar] [CrossRef]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef]
- Coley, N.; Ngandu, T.; Lehtisalo, J.; Soininen, H.; Vellas, B.; Richard, E.; Kivipelto, M.; Andrieu, S.; Hatice, F.; Van Gool, P.; et al. Adherence to multidomain interventions for dementia prevention: Data from the FINGER and MAPT trials. Alzheimer’s Dement. 2019, 15, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Rau, A.; Gallagher, D.; Rajji, T.K.; Lanctôt, K.L.; Herrmann, N. Using transcranial direct current stimulation to treat symptoms in mild cognitive impairment and Alzheimer’s disease. Neurodegener. Dis. Manag. 2017, 7, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimula-tion in humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N. Action mechanisms of transcranial direct current stimulation in Alzheimer’s disease and memory loss. Front. Psychiatry 2012, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Mameli, F.; Guidi, I.; Mrakic-Sposta, S.; Vergari, M.; Marceglia, S.; Cogiamanian, F.; Barbieri, S.; Scarpini, E.; Priori, A. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology 2008, 71, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Khoury, L.P.; Martins, D.C.S.; Martins, O.E.M.S.; De Macedo, E.C.; Fregni, F. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J. Neurol. Neurosurg. Psychiatry 2008, 80, 444–447. [Google Scholar] [CrossRef]
- Khedr, E.M.; Gamal, N.F.; El-Fetoh, N.A.; Khalifa, H.; Ahmed, E.M.; Ali, A.M.; Noaman, M.; El-Baki, A.A.; Karim, A.A. A double-blind randomized clinical trial on the efficacy of cortical direct current stimulation for the treatment of Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 275. [Google Scholar] [CrossRef] [PubMed]
- Bystad, M.; Grønli, O.; Rasmussen, I.D.; Gundersen, N.; Nordvang, L.; Wang-Iversen, H.; Aslaksen, P.M. Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer’s disease: A randomized, placebo-controlled trial. Alzheimer’s Res. Ther. 2016, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gutchess, A.H.; Welsh, R.C.; Hedden, T.; Bangert, A.; Minear, M.; Liu, L.L.; Park, D.C. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for de-creased medial-temporal activity. J. Cogn. Neurosci. 2005, 17, 84–96. [Google Scholar] [CrossRef]
- Edin, F.; Klingberg, T.; Johansson, P.; McNab, F.; Tegner, J.; Compte, A. Mechanism for top-down control of working memory capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 6802–6807. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, M.; Lindenberg, R.; Phan, M.T.; Ulm, L.; Volk, C.; Flöel, A. Transcranial direct current stimulation in mild cognitive impairment: Behavioral effects and neural mechanisms. Alzheimer’s Dement. 2015, 11, 1032–1040. [Google Scholar] [CrossRef]
- Yun, K.; Song, I.-U.; Chung, Y.-A. Changes in cerebral glucose metabolism after 3 weeks of noninvasive electrical stimu-lation of mild cognitive impairment patients. Alzheimer’s Res. Ther. 2016, 8, 1–9. [Google Scholar]
- Sporns, O. Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 2013, 23, 162–171. [Google Scholar] [CrossRef]
- Zou, Q.-H.; Zhu, C.-Z.; Yang, Y.; Zuo, X.-N.; Long, X.-Y.; Cao, Q.-J.; Wang, Y.-F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Hou, H.; Wei, F.; Liu, J.; Chen, X. Abnormal degree centrality in Alzheimer’s disease patients with depression: A resting-state functional magnetic resonance imaging study. Exp. Gerontol. 2016, 79, 61–66. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Binnewijzend, M.A.; Schoonheim, M.M.; Sanz-Arigita, E.; Wink, A.M.; van der Flier, W.M.; Tolboom, N.; Adriaanse, S.M.; Damoiseaux, J.S.; Scheltens, P.; van Berckel, B.N.; et al. Resting-state fMRI changes in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2012, 33, 2018–2028. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, L.; Cheng, B.; Qi, G.; He, J.; Xu, Z.; Han, T.; Liu, C.; Wang, Y. Transcutaneous spinal cord direct current stimulation modulates functional activity and integration in idio-pathic restless legs syndrome. Front. Neurosci. 2020, 14, 873. [Google Scholar] [CrossRef]
- Hedden, T.; Van Dijk, K.; Becker, J.A.; Mehta, A.; Sperling, R.A.; Johnson, K.A.; Buckner, R.L. Disruption of Functional Connectivity in Clinically Normal Older Adults Harboring Amyloid Burden. J. Neurosci. 2009, 29, 12686–12694. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Ellis, K.A.; Pietrzak, R.H.; Ames, D.; Darby, D.; Harrington, K.; Martins, R.N.; Masters, C.L.; Rowe, C.; Savage, G.; et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology 2012, 79, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, A.J.; Filippini, N.; Ebmeier, K.P.; Smith, S.M.; Karpe, F.; Mackay, C.E. The effects of APOE on the functional architecture of the resting brain. NeuroImage 2012, 59, 565–572. [Google Scholar] [CrossRef]
- Christensen, H.; Batterham, P.J.; MacKinnon, A.J.; Jorm, A.F.; Mack, H.A.; Mather, K.A.; Anstey, K.J.; Sachdev, P.S.; Easteal, S. The association of APOE genotype and cognitive decline in interaction with risk factors in a 65–69 year old community sample. BMC Geriatr. 2008, 8, 14. [Google Scholar] [CrossRef]
- Kemppainen, N.; Johansson, J.; Teuho, J.; Parkkola, R.; Joutsa, J.; Ngandu, T.; Solomon, A.; Stephen, R.; Liu, Y.; Hänninen, T.; et al. Brain amyloid load and its associations with cognition and vascular risk factors in FINGER Study. Neurology 2017, 90, e206–e213. [Google Scholar] [CrossRef]
- Berkowitz, C.L.; Mosconi, L.; Rahman, A.; Scheyer, O.; Hristov, H.; Isaacson, R.S. Clinical application of APOE in Alzheimer’s prevention: A precision medicine approach. J. Prev. Alzheimer’s Dis. 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.U.; Lee, D.Y.; Kim, K.W.; Jhoo, J.H.; Kim, J.H.; Lee, K.H.; Kim, S.Y.; Han, S.H.; Woo, J.I. Development of the Korean Version of the Consortium to Establish a Registry for Alzheimer’s Disease As-sessment Packet (CERAD-K) clinical and neuropsychological assessment batteries. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2002, 57, P47–P53. [Google Scholar]
- Park, J.-H. Standardization of Korean version of the Mini-Mental State Examination (MMSE-K) for use in the elderly. Part II. Diagnostic validity. J. Korean Neuropsychiatr. Assoc. 1989, 28, 508–513. [Google Scholar]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zang, Y. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.-N.; Ehmke, R.; Mennes, M.; Imperati, D.; Castellanos, F.; Sporns, O.; Milham, M.P. Network Centrality in the Human Functional Connectome. Cereb. Cortex 2012, 22, 1862–1875. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-anatomic fractionation of the brain’s default network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef]
- Thurfjell, L.; Lilja, J.; Lundqvist, R.; Buckley, C.; Smith, A.; Vandenberghe, R.; Sherwin, P. Automated Quantification of 18F-Flutemetamol PET Activity for Categorizing Scans as Negative or Positive for Brain Amyloid: Concordance with Visual Image Reads. J. Nucl. Med. 2014, 55, 1623–1628. [Google Scholar] [CrossRef]
- Bansal, R.; Peterson, B.S. Cluster-level statistical inference in fMRI datasets: The unexpected behavior of random fields in high dimensions. Magn. Reson. Imaging 2018, 49, 101–115. [Google Scholar] [CrossRef]
- Cai, M.; Guo, Z.; Xing, G.; Peng, H.; Zhou, L.; Chen, H.; McClure, M.A.; He, L.; Xiong, L.; He, B.; et al. Transcranial Direct Current Stimulation Improves Cognitive Function in Mild to Moderate Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2019, 33, 170–178. [Google Scholar] [CrossRef]
- Jacini, F.; Sorrentino, P.; Lardone, A.; Rucco, R.; Baselice, F.; Cavaliere, C.; Aiello, M.; Orsini, M.; Iavarone, A.; Manzo, V.; et al. Amnestic Mild Cognitive Impairment Is Associated With Frequency-Specific Brain Network Alterations in Temporal Poles. Front. Aging Neurosci. 2018, 10, 400. [Google Scholar] [CrossRef]
- Xie, C.; Bai, F.; Yu, H.; Shi, Y.; Yuan, Y.; Chen, G.; Li, W.; Chen, G.; Zhang, Z.; Li, S.-J. Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. NeuroImage 2012, 63, 320–327. [Google Scholar] [CrossRef]
- Nyberg, L.; Lövdén, M.; Riklund, K.; Lindenberger, U.; Bäckman, L. Memory aging and brain maintenance. Trends Cogn. Sci. 2012, 16, 292–305. [Google Scholar] [CrossRef]
- Jones, D.T.; Machulda, M.M.; Vemuri, P.; McDade, E.M.; Zeng, G.; Senjem, M.L.; Gunter, J.L.; Przybelski, S.A.; Avula, R.T.; Knopman, D.S.; et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 2011, 77, 1524–1531. [Google Scholar] [CrossRef]
- Zott, B.; Simon, M.M.; Hong, W.; Unger, F.; Chen-Engerer, H.J.; Frosch, M.P.; Sakmann, B.; Walsh, D.M.; Konnerth, A. A vicious cycle of β amyloid–dependent neuronal hyperactivation. Science 2019, 365, 559–565. [Google Scholar] [CrossRef]
- Dillen, K.N.H.; Jacobs, H.I.L.; Kukolja, J.; Richter, N.; Von Reutern, B.; Onur, O.; Langen, K.-J.; Fink, G.R. Functional Disintegration of the Default Mode Network in Prodromal Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 59, 169–187. [Google Scholar] [CrossRef]
- Yang, L.; Yan, Y.; Wang, Y.; Hu, X.; Lu, J.; Chan, P.; Yan, T.; Han, Y. Gradual Disturbances of the Amplitude of Low-Frequency Fluctuations (ALFF) and Fractional ALFF in Alzheimer Spectrum. Front. Neurosci. 2018, 12, 975. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, B.; Wilson, G.; Kong, J.; Initiative, T.A.D.N.; Weiner, M.W.; Aisen, P.; Petersen, R.; Jack, C.R.J.; Jagust, W.; et al. New Perspective for Non-invasive Brain Stimulation Site Selection in Mild Cognitive Impairment: Based on Meta- and Functional Connectivity Analyses. Front. Aging Neurosci. 2019, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, Y.; Li, Y.; Hu, X.; Lu, J.; Chan, P.; Yan, T.; Han, Y. Frequency-dependent changes in fractional amplitude of low-frequency oscillations in Alzheimer’s disease: A resting-state fMRI study. Brain Imaging Behav. 2020, 14, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Lim, H.K.; Joo, S.-H.; Lee, N.R.; Lee, C.-U. Alterations in Intra- and Interregional Intrinsic Brain Connectivity Are Differentially Associated with Memory Performance in Amnestic Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2018, 46, 229–242. [Google Scholar] [CrossRef]
- Ashraf, A.; Fan, Z.; Brooks, D.J.; Edison, P. Cortical hypermetabolism in MCI subjects: A compensatory mechanism? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 447–458. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]


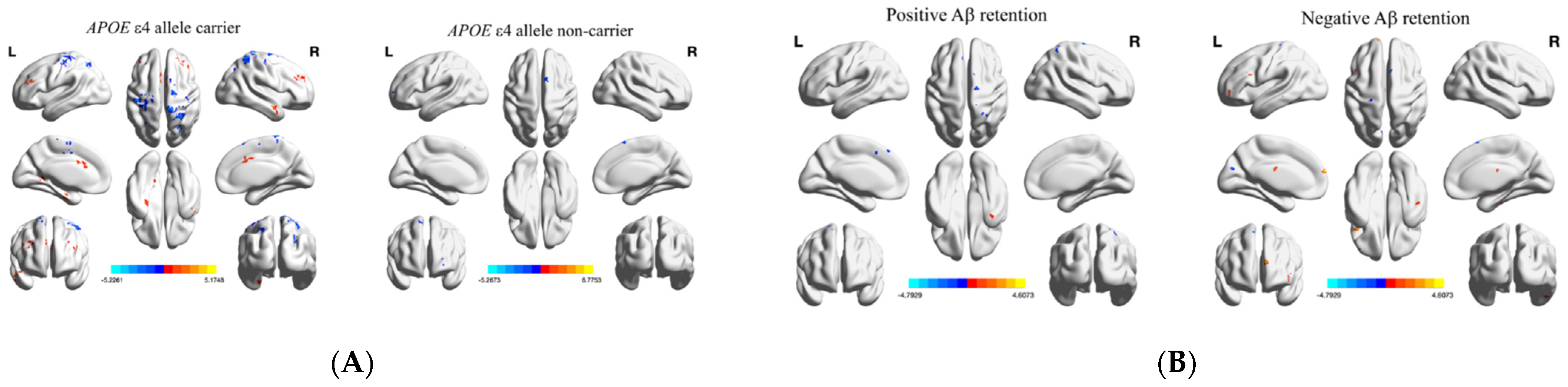

| (A) | |||
| Aβ-Deposits | Aβ-Negative | Aβ-Positive | p |
| (N = 10) | (N = 22) | ||
| Age | 77.5 ± 6.1 | 72.3 ± 7.1 | 0.054 |
| Sex | >0.999 | ||
| Male | 4 (40.0%) | 9 (40.9%) | |
| Female | 6 (60.0%) | 13 (59.1%) | |
| Years of education | 9.6 ± 4.4 | 13.5 ± 4.9 | 0.039 |
| APOE ε4 allele | 0.001 | ||
| Non-carrier | 9 (90.0%) | 4 (18.2%) | |
| Carrier | 1 (10.0%) | 18 (81.8%) | |
| Average SUVRPONS of [18 F] flutemetamol | 0.5 ± 0.1 | 0.7 ± 0.1 | <0.001 |
| (B) | |||
| APOE ε4 Allele | Non-Carrier | Carrier | p |
| (N = 13) | (N = 19) | ||
| Age (years) | 75.8 ± 6.4 | 72.6 ± 7.6 | 0.229 |
| Sex | 0.873 | ||
| Male | 6 (46.2%) | 7 (36.8%) | |
| Female | 7 (53.8%) | 12 (63.2%) | |
| Years of education | 11.1 ± 4.2 | 13.1 ± 5.5 | 0.268 |
| Aβ deposits | 0.001 | ||
| Aβ neg | 9 (69.2%) | 1 (5.3%) | |
| Aβ pos | 4 (30.8%) | 18 (94.7%) | |
| Average SUVRPONS of [18 F] flutemetamol | 0.6 ± 0.2 | 0.7 ± 0.1 | 0.003 |
| (A) | ||||||
| Region | L/R | Cluster | Peak T Value | Peak MNI Coordinates (x, y, z) | ||
| Changes in fALFF of MCI APOE ε4 Carriers | ||||||
| tDCS > baseline | ||||||
| Middle temporal gyrus | R | 98 | 3.9585 | 51 | −3 | −24 |
| Lobule III of cerebellum | L | 187 | 5.0774 | −3 | −45 | −21 |
| Parahippocampal gyrus | R | 40 | 3.2239 | 33 | −18 | −30 |
| Precuneus | L | 41 | 4.831 | −18 | −48 | 3 |
| Inferior frontal gyrus, triangular part | L | 81 | 3.4026 | −45 | 30 | 24 |
| Middle cingulate gyri | R | 62 | 3.8016 | 6 | 12 | 36 |
| Midcingulate area | L | 189 | 3.1684 | −12 | −42 | 51 |
| tDCS < baseline | ||||||
| Middle occipital gyrus | R | 45 | −5.1376 | 27 | −75 | 30 |
| Superior frontal gyrus | L | 384 | −5.2261 | −30 | −3 | 69 |
| Superior parietal gyrus | R | 250 | −4.3472 | 27 | −51 | 72 |
| Postcentral gyrus | L | 38 | −4.5553 | −42 | −21 | 57 |
| Supplementary motor area | R | 43 | −3.7324 | 3 | 18 | 63 |
| Superior frontal gyrus | R | 61 | −4.9716 | 15 | −9 | 69 |
| Changes in fALFF of MCI APOE ε4 Non-Carriers | ||||||
| tDCS > baseline | ||||||
| Inferior occipital gyrus | L | 29 | 5.1353 | −42 | −72 | −6 |
| Calcarine fissure and surrounding cortex | R | 29 | 3.8547 | 18 | −81 | 9 |
| tDCS < baseline | ||||||
| Superior frontal gyrus, orbital | L | 47 | −4.0932 | −27 | 57 | −3 |
| Supplementary motor area | L | 79 | −4.4351 | −3 | 15 | 63 |
| (B) | ||||||
| Region | L/R | Cluster | Peak T Value | Peak MNI Coordinates (x, y, z) | ||
| Changes in fALFF of MCI Patients with Aβ Deposits | ||||||
| tDCS > baseline | ||||||
| Inferior temporal gyrus | R | 61 | 4.0777 | 39 | −6 | −45 |
| Crus I of of cerebellum | L | 36 | 4.6073 | −15 | −72 | −33 |
| Lobule III of cerebellum | L | 62 | 4.374 | −3 | −45 | −21 |
| tDCS < baseline | ||||||
| Crus I of of cerebellum | R | 40 | −4.5424 | 36 | −81 | −36 |
| Supramarginal gyrus | R | 38 | −3.4884 | 60 | −30 | 33 |
| Superior parietal gyrus | R | 99 | −4.3392 | 27 | −51 | 72 |
| Superior frontal gyrus, medial | R | 146 | −3.5363 | 9 | 33 | 57 |
| Superior frontal gyrus | L | 119 | −4.3879 | −27 | −9 | 72 |
| Paracentral lobule | L | 35 | −2.7845 | −6 | −30 | 78 |
| Changes in fALFF of MCI Patients without Aβ Deposits | ||||||
| tDCS > baseline | ||||||
| Inferior temporal gyrus | R | 29 | 4.9944 | 48 | −21 | −27 |
| Middle temporal gyrus | L | 28 | 6.7989 | −57 | −36 | −6 |
| Middle frontal gyrus | L | 42 | 6.345 | −39 | 45 | −9 |
| Inferior frontal gyrus triangular part | L | 32 | 5.266 | −42 | 18 | 6 |
| Superior frontal gyrus, medial | L | 27 | 7.7112 | −9 | 63 | 21 |
| Precuneus | R | 32 | 4.4101 | 9 | −60 | 54 |
| tDCS < baseline | ||||||
| Crus I of cerebellum | R | 27 | −4.3276 | 48 | −54 | −27 |
| Cuneus | L | 36 | −4.2645 | −6 | −81 | 24 |
| Superior occipital gyrus | R | 30 | −4.1793 | 24 | −90 | 27 |
| Middle frontal gyrus | R | 29 | −5.2138 | 45 | 12 | 42 |
| Supplementary motor area | R | 27 | −6.8382 | 6 | 15 | 63 |
| Postcentral gyrus | L | 28 | −3.587 | −24 | −27 | 72 |
| (A) | ||||||
| Region | L/R | Cluster | Peak T Value | Peak MNI Coordinates (x, y, z) | ||
| Changes in DC of MCI Patients with APOE ε4 Carrier | ||||||
| tDCS > baseline | ||||||
| Lobule VIIB of cerebellar hemisphere | R | 78 | 3.7442 | 30 | −72 | −48 |
| Temporal pole: superior temporal gyrus | R | 115 | 4.3302 | 45 | 3 | −15 |
| Temporal pole: superior temporal gyrus | L | 108 | 4.3954 | −42 | 3 | −15 |
| Calcarine fissure and surrounding cortex | R | 67 | 3.7306 | 9 | −87 | 9 |
| tDCS < baseline | ||||||
| Superior parietal gyrus | R | 178 | −4.1919 | 27 | −63 | 51 |
| Superior parietal gyrus | L | 767 | −5.1001 | −24 | −69 | 48 |
| Changes in DC of MCI Patients with APOE ε4 Non-Carrier | ||||||
| tDCS > baseline | ||||||
| Inferior temporal gyrus | L | 48 | 4.5971 | −42 | −60 | −6 |
| Middle occipital gyrus | R | 60 | 4.2846 | 33 | −78 | 30 |
| Middle occipital gyrus | L | 84 | 4.7948 | −15 | −81 | 39 |
| tDCS < baseline | ||||||
| Superior frontal gyrus, medial | L | 338 | −6.6552 | 0 | 57 | 0 |
| (B) | ||||||
| Region | L/R | Cluster | Peak T Value | Peak MNI Coordinates (x, y, z) | ||
| Changes in DC of MCI Patients with Aβ Deposits | ||||||
| tDCS > baseline | ||||||
| Lobule VIII of cerebellum | R | 70 | 3.9953 | 12 | −66 | −45 |
| Calcarine fissure and surrounding cortex | R | 230 | 4.6267 | 9 | −87 | 9 |
| tDCS < baseline | ||||||
| Superior parietal gyrus | L | 76 | −3.427 | −21 | −51 | 45 |
| Changes in DC of MCI Patients without Aβ Deposits | ||||||
| tDCS > baseline | ||||||
| Superior frontal gyrus, medial orbital | R | 53 | 4.7797 | 12 | 66 | −9 |
| Middle frontal gyrus | L | 70 | 4.8997 | −48 | 42 | 15 |
| Middle temporal gyrus | R | 53 | 4.9548 | 66 | −48 | 9 |
| tDCS < baseline | ||||||
| Lingual gyrus | R | 55 | −5.8024 | 12 | −81 | −12 |
| Superior frontal gyrus, medial | L | 48 | −4.093 | 0 | 57 | 0 |
| Lenticular nucleus, Putamen | L | 48 | −4.2643 | −18 | 3 | 6 |
| Middle occipital gyrus | L | 50 | −3.9537 | −24 | −84 | 9 |
| Supplementary motor area | R | 115 | −3.9209 | 12 | 12 | 69 |
| Precentral gyrus | L | 59 | −3.5792 | −33 | −21 | 72 |
| Postcentral gyrus | R | 153 | −5.5196 | 27 | −30 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.-W.; Wang, S.-M.; Kim, T.-Y.; Kim, D.; Na, H.-R.; Kim, N.-Y.; Lee, C.-U.; Lim, H.-K. Impact of Transcranial Direct Current Stimulation on Cognitive Function, Brain Functional Segregation, and Integration in Patients with Mild Cognitive Impairment According to Amyloid-Beta Deposition and APOE ε4-Allele: A Pilot Study. Brain Sci. 2021, 11, 772. https://doi.org/10.3390/brainsci11060772
Kang D-W, Wang S-M, Kim T-Y, Kim D, Na H-R, Kim N-Y, Lee C-U, Lim H-K. Impact of Transcranial Direct Current Stimulation on Cognitive Function, Brain Functional Segregation, and Integration in Patients with Mild Cognitive Impairment According to Amyloid-Beta Deposition and APOE ε4-Allele: A Pilot Study. Brain Sciences. 2021; 11(6):772. https://doi.org/10.3390/brainsci11060772
Chicago/Turabian StyleKang, Dong-Woo, Sheng-Min Wang, Tae-Yeong Kim, Donghyeon Kim, Hae-Ran Na, Nak-Young Kim, Chang-Uk Lee, and Hyun-Kook Lim. 2021. "Impact of Transcranial Direct Current Stimulation on Cognitive Function, Brain Functional Segregation, and Integration in Patients with Mild Cognitive Impairment According to Amyloid-Beta Deposition and APOE ε4-Allele: A Pilot Study" Brain Sciences 11, no. 6: 772. https://doi.org/10.3390/brainsci11060772
APA StyleKang, D.-W., Wang, S.-M., Kim, T.-Y., Kim, D., Na, H.-R., Kim, N.-Y., Lee, C.-U., & Lim, H.-K. (2021). Impact of Transcranial Direct Current Stimulation on Cognitive Function, Brain Functional Segregation, and Integration in Patients with Mild Cognitive Impairment According to Amyloid-Beta Deposition and APOE ε4-Allele: A Pilot Study. Brain Sciences, 11(6), 772. https://doi.org/10.3390/brainsci11060772







