Acid Precipitation and the Prevalence of Parkinson’s Disease: An Ecologic Study in U.S. States
Abstract
1. Introduction
2. Materials and Methods
2.1. PD Prevalence
2.2. Exposure Variables
2.2.1. Annual Precipitation
2.2.2. pH of Precipitation
2.2.3. Acid Precipitation Index
2.2.4. Well Water Use
2.2.5. Sulfuric Acid
2.2.6. Ultraviolet Index
2.2.7. Smoking
2.3. Statistical Analysis
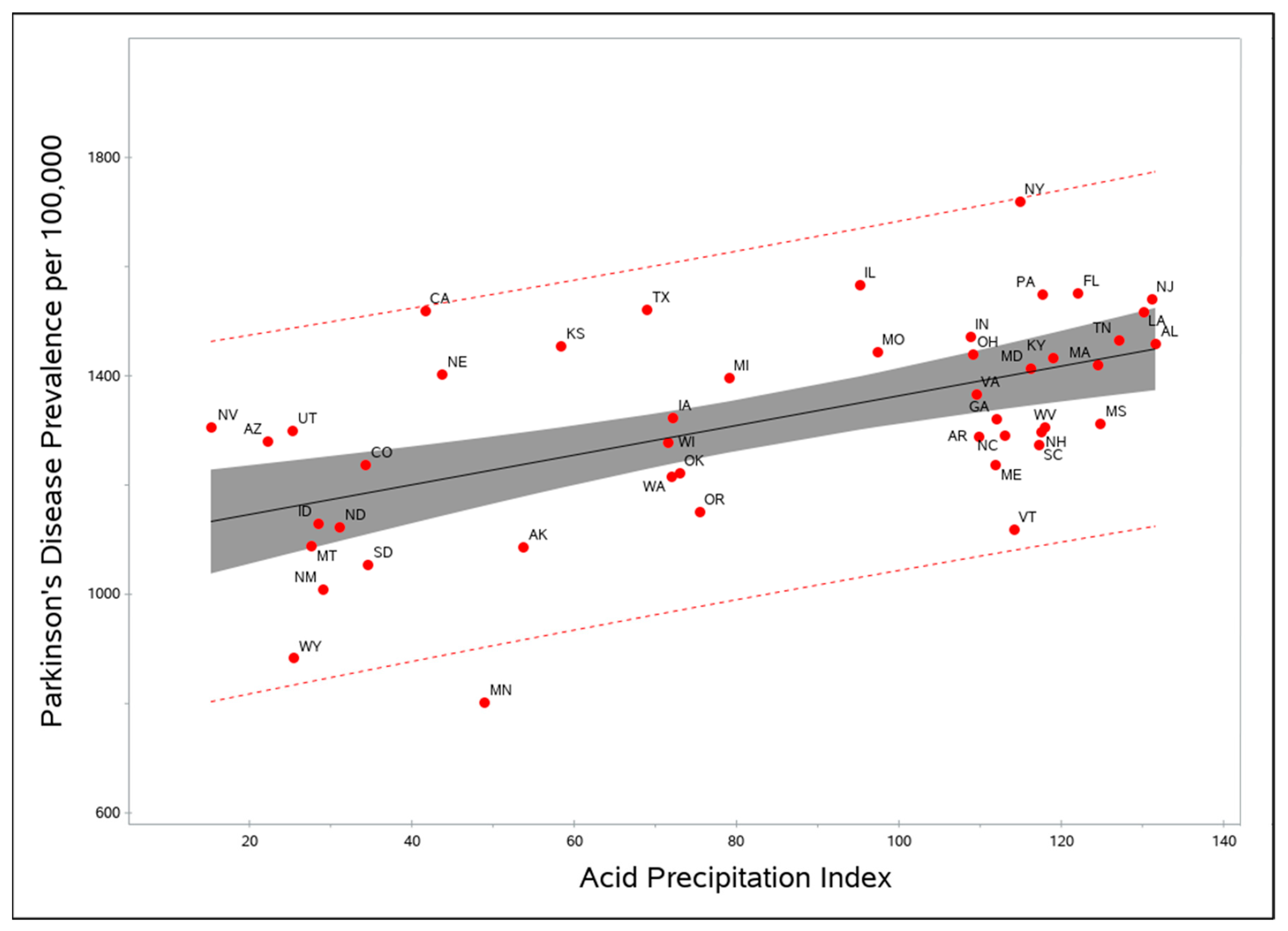
3. Results
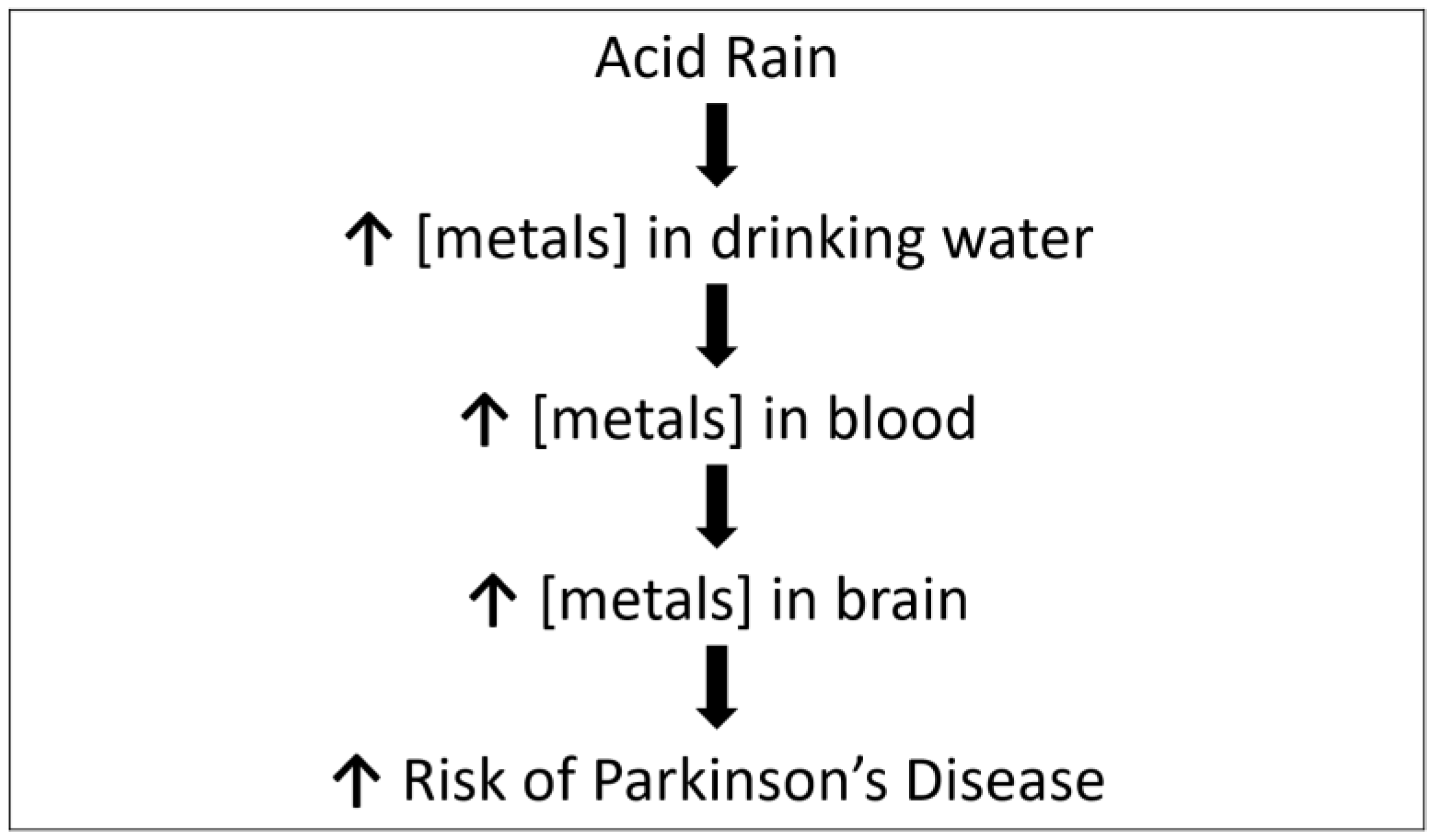
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The emerging evidence of the Parkinson pandemic. J. Parkinson Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Balestrino, R.; Shapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W. The MPTP story. J. Parkinsons’s Dis. 2017, 7 (Suppl. 1), 511–519. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.M. Environmental toxins and Parkinson’s Disease. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Wright-Willis, A.; Evanoff, B.A.; Lian, M.; Criswell, S.R.; Racette, B.A. Geographic and ethnic variation in Parkinson Disease: A population-based study of US Medicare beneficiaries. Neuroepidemiology 2010, 34, 143–151. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geologic Survey, pH of Rainfall in the USA, 2002. 2002. Available online: https://www.usgs.gov/media/images/ph-rainfall-usa-2002 (accessed on 1 April 2021).
- Goyer, R.A.; Bachmann, J.; Clarkson, T.W.; Ferris, B.G., Jr.; Graham, J.; Mushak, P.; Perl, D.P.; Rall, D.P.; Schlesinger, R.; Sharpe, W.; et al. Potential human health effects of acid rain: Report of a workshop. Environ. Health Perspect. 1985, 60, 355–368. [Google Scholar] [CrossRef]
- Acid Rain and Water. Available online: https://www.usgs.gov/special-topic/water-science-school/science/acid-rain-and-water?qt-science_center_objects=0#qt-science_center_objects (accessed on 1 April 2021).
- Bensyrd, I.; Rylander, L.; Högstedt, B.; Aprea, P.; Bratt, I.; Fåhraéus, C.; Holmén, A.; Karlsson, A.; Nilsson, A.; Svensson, B.-L.; et al. Effect of acid precipitation on retention and excretion of elements in man. Sci. Total Environ. 1994, 145, 81–102. [Google Scholar] [CrossRef]
- Mantri, S.; Fullard, M.E.; Beck, J.; Willis, A.W. State-level prevalence, health service use, and spending vary widely among Medicare beneficiaries with Parkinson disease. NPJ Parkinson’s Dis. 2019, 5, 1. [Google Scholar] [CrossRef]
- U.S. Average Precipitation State Rank. Available online: http://www.usa.com/rank/us--average-precipitation--state-rank.htm (accessed on 15 April 2021).
- National Atmospheric Deposition Program. Available online: https://nadp.slh.wisc.edu/data/NTN/ntnAllsites.aspx (accessed on 15 April 2021).
- Kenny, J.F.; Barber, N.L.; Hutson, S.S.; Linsey, K.S.; Lovelace, J.K.; Maupin, M.A. Estimated Use of Water in the United States in 2005. Circular 1344; U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 2009.
- Schwartz, G.G.; Klug, M.G. Motor neuron disease mortality rates in U.S. states are associated with well water use. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 528–534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EPA TRI Explorer. Available online: https://enviro.epa.gov/triexplorer/tri_release.chemical (accessed on 19 April 2021).
- Zhou, Z.; Zhou, R.; Zhang, Z.; Li, K. The association between vitamin D status, vitamin D supplementation, sunlight exposure, and Parkinson’s Disease: A systematic review and meta-analysis. Med. Sci. Monit. 2019, 25, 666–674. [Google Scholar] [CrossRef]
- Elwood, J.M.; Lee, J.A.; Walter, S.D.; Mo, T.; Green, A.E. Relationship of melanoma and other skin cancer mortality to latitude and ultraviolet radiation in the United States and Canada. Int. J. Epidemiol. 1974, 3, 325–352. [Google Scholar] [CrossRef]
- CDC. State-specific prevalence of current cigarette smoking among adults, and policies and attitudes about secondhand smoke, United States, 2000. MMWR 2001, 50, 1101–1106. [Google Scholar]
- Hawles, C.H.; Tredici, K.D.; Braak, H. A timeline for Parkinson’s disease. Parkinsonism Relat. Disord. 2010, 16, 79–84. [Google Scholar]
- Pezzoli, G.; Careda, E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 2013, 80, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, C.B.; Berry, C.; Chang, E.T.; Sielken, R.L., Jr.; Mandel, J.S. Association between Parkinson’s disease and cigarette smoking, rural living, well-water consumption, farming, and pesticide use: Systematic review and meta-analysis. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Silver, M.R.; Racette, B.A.; Dube, U.; Faust, I.M.; Nielsen, S.S. Well water and Parkinson Disease in Medicare beneficiaries: A nationwide case-control study. J. Parkinsons Dis. 2020, 10, 693–705. [Google Scholar] [CrossRef]
- EPA. Acid Rain, Students Site. Available online: https://www3.epa.gov/acidrain/education/site_students/phscale.html#:~:text=Normal%2C%20clean%20rain%20has%20a,a%20pH%20value%20of%204.0 (accessed on 1 April 2021).
- Driscoll, C.T.; Lawrence, G.B.; Bulger, A.J.; Butler, T.J.; Cronan, C.S.; Eager, C.; Lambert, K.F.; Likens, G.E.; Stoddard, J.L.; Weathers, K.C. Acidic deposition in the Northeastern United States: Sources and inputs, ecosystem effects, and management strategies. BioScience 2001, 51, 180–198. [Google Scholar] [CrossRef]
- Montgomery, E.B., Jr. Heavy metals and the etiology of Parkinson’s disease and other movement disorders. Toxicology 1995, 97, 3–9. [Google Scholar] [CrossRef]
- Willis, A.W.; Evanoff, B.A.; Galarza, A.; Wegrzyn, A.; Shootman, M.; Racette, B.A. Metal emissions and urban incident Parkinson disease: A community health study of Medicare beneficiaries by using Geographic Information Systems. Am. J. Epidemiol. 2010, 172, 1357–1363. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Goyer, R.A.; Clarkson, T.W. Impact of effects of acid precipitation on toxicity of metals. Environ. Health Perspect. 1985, 62, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lei, I.-L.; Ng, D.-Q.; Sable, S.S.; Lin, Y.-P. Evaluation of lead release potential of new premise plumbing materials. Environ. Sci. Pollut. Res. 2018, 25, 27972–27981. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.E. Acid deposition and drinking water. Environ. Sci. Technol. 1985, 10, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, D.A.; Brown, R.A.; Via, S. National survey of lead service line occurrence. J. Am. Water Works Assoc. 2016, 108, E182–E191. [Google Scholar] [CrossRef]
- Moore, M.R. Influence of acid rain upon water plumbosolvency. Environ. Health Perspect. 1985, 63, 121–126. [Google Scholar] [CrossRef]
- Weisskopf, M.G.; Weuve, J.; Nie, H.; Sanit-Hilaire, M.-H.; Sudarsky, L.; Simon, D.K.; Hersh, B.; Schwartz, J.; Wright, R.O.; Hu, H. Association of cumulative lead exposure with Parkinson’s disease. Environ. Health Perspect. 2010, 118, 1609–1613. [Google Scholar] [CrossRef]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorerlli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and neurodegenerative diseases. A systematic review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- An, H.; Zeng, X.; Niu, T.; Li, G.; Yang, J.; Zheng, L. Quantifying iron deposition within the substantia nigra of Parkinson’s disease by quantitative susceptibility mapping. J. Neurol. Sci. 2018, 386, 46–52. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef] [PubMed]
- Nandipati, S.; Litvan, I. Environmental exposures and Parkinson’s disease. Int. J. Environ. Res. Public Health 2016, 13, 881. [Google Scholar] [CrossRef] [PubMed]
- US Geological Survey National Water-Quality Assessment (NAWQA) Project. Available online: https://water.usgs.gov/nawqa/pnsp/usage/maps/ (accessed on 1 April 2021).
- Nishida, Y. Elucidation of endemic neurodegenerative diseases—A commentary. Z. Naturforsch C. 2003, 58, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Perl, D.P. Relationship of aluminum to Alzheimer’s disease. Environ. Health Perspect. 1985, 63, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Stejskal, V.; Urbina, M.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s disease: Mechanisms and biochemical processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef]
- Rajput, A.H.; Uitti, R.J.; O’Donnell, D.; O’Donnell, K. Metal concentrations in drinking water in Parkinson’s disease and controls. Can. J. Neurol. Sci. 1986, 13, 185. [Google Scholar]
- Rajput, A.H.; Ryan, J.; Uitti, W.; Stern, W.; Laverty, W.; O’Donnell, K.; O’Donnell, D.; Yuen, W.K.; Dua, A. Geography, drinking water chemistry, pesticides and herbicides and the etiology of Parkinson’s disease. Can. J. Neurol. Sci. 1987, 14, 414–418. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean (SD) | Range (State) |
|---|---|---|
| Parkinson’s disease prevalence rates | 1325.0 (185.4) | 802.9 (MN)–1719.8 (NY) |
| Sulfuric acid release amount | 846,954.5 (1,299,945.3) | 250 (SD)–7,609,755 (TN) |
| pH value | 4.81 (0.4) | 5.43 (SD)–4.22 (OH) |
| Precipitation amount | 37.5 (13.6) | 9.46 (NV)–59.15 (LA) |
| Acid Precipiation Index (API) | 83.12 (38.5) | 15.23 (NV)–131.6 (AL) |
| Well-water usage rate | 17.3% (9.9) | 3% (UT)–44% (ME) |
| Smoking prevalence rate | 22.9% (3.0) | 12.9% (UT)–30.5% (KY) |
| UV Index | 25.1 (6.3) | 14.63 (WA)–39.72 (FL) |
| Model | Predictor | β-Coefficient | t-Value | p-Value | AIC 1 |
|---|---|---|---|---|---|
| pH alone | Mean pH | −255.7 | −4.01 | 0.0002 | 469.90 |
| Precipitation alone | Mean Precipitation | 7.0 | 4.14 | 0.0001 | 509.95 |
| Well water alone | Well-water use | −6.2 | −2.42 | 0.0193 | 519.48 |
| UV alone | UV Index | 9.6 | 2.32 | 0.0249 | 499.23 |
| Smoking alone | Smoking prevalence | 6.0 | 0.69 | 0.4965 | 524.75 |
| Sulfuric acid alone | Log sulfuric acid | 24.3 | 2.13 | 0.0381 | 520.71 |
| API alone | API | 2.7 | 4.48 | <0.0001 | 466.95 |
| Full (API + well water + UV + smoking + sulfuric acid) | API | 2.6 | 3.9 | 0.0004 | 447.85 |
| Well-water use | −7.7 | −2.8 | 0.0078 | ||
| UV Index | 1.9 | 0.5 | 0.6532 | ||
| Smoking prevalence | 0.01 | 0 | 0.9989 | ||
| Log sulfuric acid | 15.8 | 1.3 | 0.1996 | ||
| Final (API + well water + sulfuric acid) | API | 2.6 | 4.43 | <0.0001 | 452.79 |
| Well-water use | −8.4 | 0.0001 | |||
| Log sulfuric acid | 18.0 | 0.0968 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, G.G.; Williamson, M.R. Acid Precipitation and the Prevalence of Parkinson’s Disease: An Ecologic Study in U.S. States. Brain Sci. 2021, 11, 779. https://doi.org/10.3390/brainsci11060779
Schwartz GG, Williamson MR. Acid Precipitation and the Prevalence of Parkinson’s Disease: An Ecologic Study in U.S. States. Brain Sciences. 2021; 11(6):779. https://doi.org/10.3390/brainsci11060779
Chicago/Turabian StyleSchwartz, Gary G., and Mark R. Williamson. 2021. "Acid Precipitation and the Prevalence of Parkinson’s Disease: An Ecologic Study in U.S. States" Brain Sciences 11, no. 6: 779. https://doi.org/10.3390/brainsci11060779
APA StyleSchwartz, G. G., & Williamson, M. R. (2021). Acid Precipitation and the Prevalence of Parkinson’s Disease: An Ecologic Study in U.S. States. Brain Sciences, 11(6), 779. https://doi.org/10.3390/brainsci11060779







