Influence of Intervertebral Fixation and Segmental Thrust Level on Immediate Post-Spinal Manipulation Trunk Muscle Spindle Response in an Animal Model
Abstract
:1. Introduction
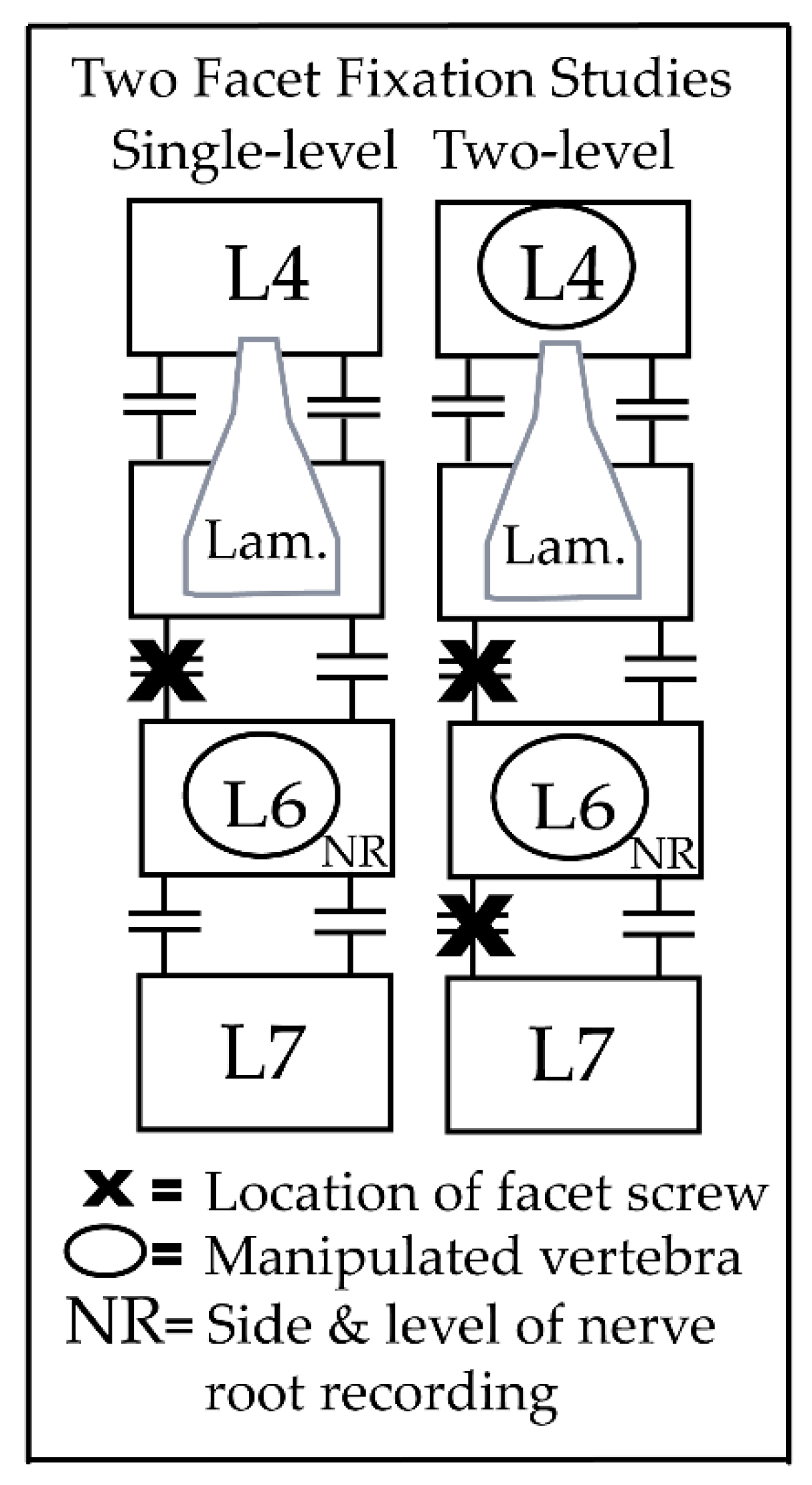
2. Materials and Methods
2.1. HVLA-SM
2.2. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef] [Green Version]
- Meier, M.L.; Vrana, A.; Schweinhardt, P. Low Back Pain: The Potential Contribution of Supraspinal Motor Control and Proprioception. Neuroscience 2019, 25, 583–596. [Google Scholar] [CrossRef]
- MacDonald, D.; Moseley, L.; Hodges, P. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. Pain 2009, 142, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.; Breen, A. Uneven intervertebral motion sharing is related to disc degeneration and is greater in patients with chronic, non-specific low back pain: An in vivo, cross-sectional cohort comparison of intervertebral dynamics using quantitative fluoroscopy. Eur. Spine J. 2018, 27, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Breen, A.; Mellor, F.; Breen, A. Aberrant intervertebral motion in patients with treatment-resistant nonspecific low back pain: A retrospective cohort study and control comparison. Eur. Spine J. 2018, 27, 2831–2839. [Google Scholar] [CrossRef] [Green Version]
- Macefield, V.G.; Knellwolf, T.P. Functional properties of human muscle spindles. J. Neurophysiol. 2018, 120, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Allen, T. The neural basis of the senses of effort, force and heaviness. Exp. Brain Res. 2019, 237, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Kröger, S.; Watkins, B. Muscle spindle function in healthy and diseased muscle. Skelet. Muscle 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Johansson, H.; Sojka, P. Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: A hypothesis. Med. Hypotheses 1991, 35, 196–203. [Google Scholar] [CrossRef]
- Jovanovic, K.; Anastasijevic, R.; Vuco, J. Reflex effects on γ fusimotor neurones of chemically induced discharges in small-diameter muscle afferents in decerebrate cats. Brain Res. 1990, 521, 89–94. [Google Scholar] [CrossRef]
- Johansson, H.; Djupsjöbacka, M.; Sjölander, P. Influences on the γ-muscle spindle system from muscle afferents stimulated by KCl and lactic acid. Neurosci. Res. 1993, 16, 49–57. [Google Scholar] [CrossRef]
- Thunberg, J.; Hellström, F.; Sjölander, P.; Bergenheim, M.; Wenngren, B.-I.; Johansson, H. Influences on the fusimotor-muscle spindle system from chemosensitive nerve endings in cervical facet joints in the cat: Possible implications for whiplash induced disorders. Pain 2001, 91, 15–22. [Google Scholar] [CrossRef]
- Thunberg, J.; Ljubisavljevic, M.; Djupsjöbacka, M.; Johansson, H. Effects on the fusimotor-muscle spindle system induced by intramuscular injections of hypertonic saline. Exp. Brain Res. 2002, 142, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.R.; Sahu, P.K.; Martins, D.F.; Reed, W.R. The Neurophysiological Impact of Experimentally-Induced Pain on Direct Muscle Spindle Afferent Response: A Scoping Review. Front. Cell. Neurosci. 2021, 15, 649529. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.R.; Long, C.R.; Pickar, J.G. Effects of unilateral facet fixation and facetectomy on muscle spindle responsiveness during simulated spinal manipulation in an animal model. J. Manip. Physiol. Ther. 2013, 36, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, W.R.; Pickar, J.G. Paraspinal Muscle Spindle Response to Intervertebral Fixation and Segmental Thrust Level During Spinal Manipulation in an Animal Model. Spine 2015, 40, E752–E759. [Google Scholar] [CrossRef] [Green Version]
- Reed, W.R.; Liebschner, M.A.; Sozio, R.S.; Pickar, J.G.; Gudavalli, M.R. Neural Response During a Mechanically Assisted Spinal Manipulation in an Animal Model: A Pilot Study. J. Nov. Physiother. Phys. Rehabil. 2015, 2, 020–027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, W.R.; Cao, D.-Y.; Long, C.R.; Kawchuk, G.N.; Pickar, J.G. Relationship between Biomechanical Characteristics of Spinal Manipulation and Neural Responses in an Animal Model: Effect of Linear Control of Thrust Displacement versus Force, Thrust Amplitude, Thrust Duration, and Thrust Rate. Evid.-Based Complement. Altern. Med. 2013, 2013, 492039. [Google Scholar] [CrossRef]
- Cao, D.-Y.; Reed, W.R.; Long, C.R.; Kawchuk, G.N.; Pickar, J.G. Effects of thrust amplitude and duration of high-velocity, low-amplitude spinal manipulation on lumbar muscle spindle responses to vertebral position and movement. J. Manip. Physiol. Ther. 2013, 36, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Reed, W.R.; Long, C.R.; Kawchuk, G.N.; Pickar, J.G. Neural responses to the mechanical characteristics of high velocity, low amplitude spinal manipulation: Effect of specific contact site. Man. Ther. 2015, 20, 797–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, C.R.; Sozio, R.S.; Law, A.C.; Nelson, A.J.; Singh, H.; Hurt, C.P.; Li, P.; Reed, W.R. Effects of Thrust Magnitude and Duration on Immediate Postspinal Manipulation Trunk Muscle Spindle Responses. J. Manip. Physiol. Ther. 2021, in press. [Google Scholar]
- Cleland, J.A.; Fritz, J.M.; Kulig, K.; Davenport, T.E.; Eberhart, S.; Magel, J.; Childs, J.D. Comparison of the effectiveness of three manual physical therapy techniques in a subgroup of patients with low back pain who satisfy a clinical prediction rule: A randomized clinical trial. Spine 2009, 34, 2720–2729. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Groupp, E.; Panzer, D.; Partna, L.; Lumsden, S.; Aickin, M. Efficacy of Cervical Endplay Assessment as an Indicator for Spinal Manipulation. Spine 2003, 28, 1091–1096. [Google Scholar] [CrossRef]
- Korr, I.M. Neurochemical and neurotrophic consequences of nerve deformation: Clinical implications in relation to spinal manipulation. J. Am. Osteopat. Assoc. 1975, 75, 409–414. [Google Scholar]
- Pickar, J.G. Neurophysiological effects of spinal manipulation. Spine J. 2002, 2, 357–371. [Google Scholar] [CrossRef]
- Pickar, J.; Bolton, P. Spinal manipulative therapy and somatosensory activation. J. Electromyogr. Kinesiol. 2012, 22, 785–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Outcomes | Thrust Magnitude (%BW) | Thrust Duration (ms) | # of Screws | n | Mean | SD | p | FDR |
|---|---|---|---|---|---|---|---|---|
| Time to 1st AP (s) | 0% | 0 | 1 | 18 | 0.035 | 0.020 | 0.0481 * | 0.5115 |
| 2 | 21 | 0.023 | 0.013 | |||||
| 55% | 75 | 1 | 18 | 0.089 | 0.065 | 0.4433 | 0.5115 | |
| 2 | 21 | 0.105 | 0.070 | |||||
| 55% | 100 | 1 | 18 | 0.097 | 0.065 | 0.3103 | 0.5115 | |
| 2 | 21 | 0.118 | 0.074 | |||||
| 55% | 150 | 1 | 18 | 0.106 | 0.079 | 0.3956 | 0.5115 | |
| 2 | 21 | 0.123 | 0.084 | |||||
| 55% | 250 | 1 | 18 | 0.129 | 0.103 | 0.7058 | 0.7562 | |
| 2 | 21 | 0.155 | 0.162 | |||||
| 1s post-thrust (Hz) | 0% | 0 | 1 | 18 | 34.00 | 9.28 | 0.2606 | 0.5115 |
| 2 | 21 | 31.10 | 13.54 | |||||
| 55% | 75 | 1 | 18 | 28.44 | 11.46 | 0.3365 | 0.5115 | |
| 2 | 21 | 25.14 | 13.37 | |||||
| 55% | 100 | 1 | 18 | 27.44 | 10.73 | 0.1707 | 0.5115 | |
| 2 | 21 | 24.24 | 13.36 | |||||
| 55% | 150 | 1 | 18 | 27.00 | 11.08 | 0.3506 | 0.5115 | |
| 2 | 21 | 24.38 | 13.04 | |||||
| 55% | 250 | 1 | 18 | 24.89 | 11.98 | 0.7582 | 0.7582 | |
| 2 | 21 | 23.57 | 13.33 | |||||
| 2 s post-thrust (Hz) | 0% | 0 | 1 | 18 | 68.89 | 18.51 | 0.2847 | 0.5115 |
| 2 | 21 | 62.67 | 26.66 | |||||
| 55% | 75 | 1 | 18 | 59.94 | 21.59 | 0.2608 | 0.5115 | |
| 2 | 21 | 53.10 | 26.42 | |||||
| 55% | 100 | 1 | 18 | 58.11 | 20.30 | 0.2495 | 0.5115 | |
| 2 | 21 | 52.24 | 27.00 | |||||
| 55% | 150 | 1 | 18 | 58.83 | 20.82 | 0.3581 | 0.5115 | |
| 2 | 21 | 53.38 | 26.34 | |||||
| 55% | 250 | 1 | 18 | 55.28 | 21.92 | 0.4348 | 0.5115 | |
| 2 | 21 | 51.10 | 26.81 |
| Outcomes | Thrust Magnitude (%BW) | Thrust Duration (ms) | # of Screws | Vertebra | n | Mean | SD | p |
|---|---|---|---|---|---|---|---|---|
| Time to 1st AP (s) | 0% | 0 | 2 | L4 | 20 | 0.020 | 0.013 | 0.4462 |
| L6 | 21 | 0.023 | 0.013 | |||||
| 55% | 75 | 2 | L4 | 20 | 0.081 | 0.065 | 0.2323 | |
| L6 | 21 | 0.105 | 0.070 | |||||
| 55% | 100 | 2 | L4 | 20 | 0.090 | 0.061 | 0.2226 | |
| L6 | 21 | 0.118 | 0.074 | |||||
| 55% | 150 | 2 | L4 | 19 | 0.105 | 0.068 | 0.6097 | |
| L6 | 21 | 0.123 | 0.084 | |||||
| 55% | 250 | 2 | L4 | 19 | 0.116 | 0.086 | 0.5728 | |
| L6 | 21 | 0.155 | 0.162 | |||||
| 1 s post-thrust (Hz) | 0% | 0 | 2 | L4 | 20 | 30.80 | 11.55 | 0.9483 |
| L6 | 21 | 31.10 | 13.54 | |||||
| 55% | 75 | 2 | L4 | 20 | 26.70 | 11.96 | 0.6595 | |
| L6 | 21 | 25.14 | 13.37 | |||||
| 55% | 100 | 2 | L4 | 20 | 26.00 | 11.78 | 0.4454 | |
| L6 | 21 | 24.24 | 13.36 | |||||
| 55% | 150 | 2 | L4 | 20 | 25.80 | 12.80 | 0.5775 | |
| L6 | 21 | 24.38 | 13.04 | |||||
| 55% | 250 | 2 | L4 | 20 | 24.95 | 11.90 | 0.5952 | |
| L6 | 21 | 23.57 | 13.33 | |||||
| 2 s post-thrust (Hz) | 0% | 0 | 2 | L4 | 20 | 61.95 | 23.02 | 0.9999 |
| L6 | 21 | 62.67 | 26.66 | |||||
| 55% | 75 | 2 | L4 | 20 | 55.20 | 22.94 | 0.6503 | |
| L6 | 21 | 53.10 | 26.42 | |||||
| 55% | 100 | 2 | L4 | 20 | 55.20 | 22.98 | 0.4851 | |
| L6 | 21 | 52.24 | 27.00 | |||||
| 55% | 150 | 2 | L4 | 20 | 54.05 | 23.88 | 0.7557 | |
| L6 | 21 | 53.38 | 26.34 | |||||
| 55% | 250 | 2 | L4 | 20 | 52.60 | 23.15 | 0.6690 | |
| L6 | 21 | 51.10 | 26.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, C.R.; Martins, D.F.; Avatapally, S.; Cho, M.; Li, P.; Reed, W.R. Influence of Intervertebral Fixation and Segmental Thrust Level on Immediate Post-Spinal Manipulation Trunk Muscle Spindle Response in an Animal Model. Brain Sci. 2021, 11, 1022. https://doi.org/10.3390/brainsci11081022
Lima CR, Martins DF, Avatapally S, Cho M, Li P, Reed WR. Influence of Intervertebral Fixation and Segmental Thrust Level on Immediate Post-Spinal Manipulation Trunk Muscle Spindle Response in an Animal Model. Brain Sciences. 2021; 11(8):1022. https://doi.org/10.3390/brainsci11081022
Chicago/Turabian StyleLima, Carla R., Daniel F. Martins, Snigdhasree Avatapally, Minjung Cho, Peng Li, and William R. Reed. 2021. "Influence of Intervertebral Fixation and Segmental Thrust Level on Immediate Post-Spinal Manipulation Trunk Muscle Spindle Response in an Animal Model" Brain Sciences 11, no. 8: 1022. https://doi.org/10.3390/brainsci11081022






