Mirror Visual Feedback Induces M1 Excitability by Disengaging Functional Connections of Perceptuo-Motor-Attentional Processes during Asynchronous Bimanual Movement: A Magnetoencephalographic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimulation
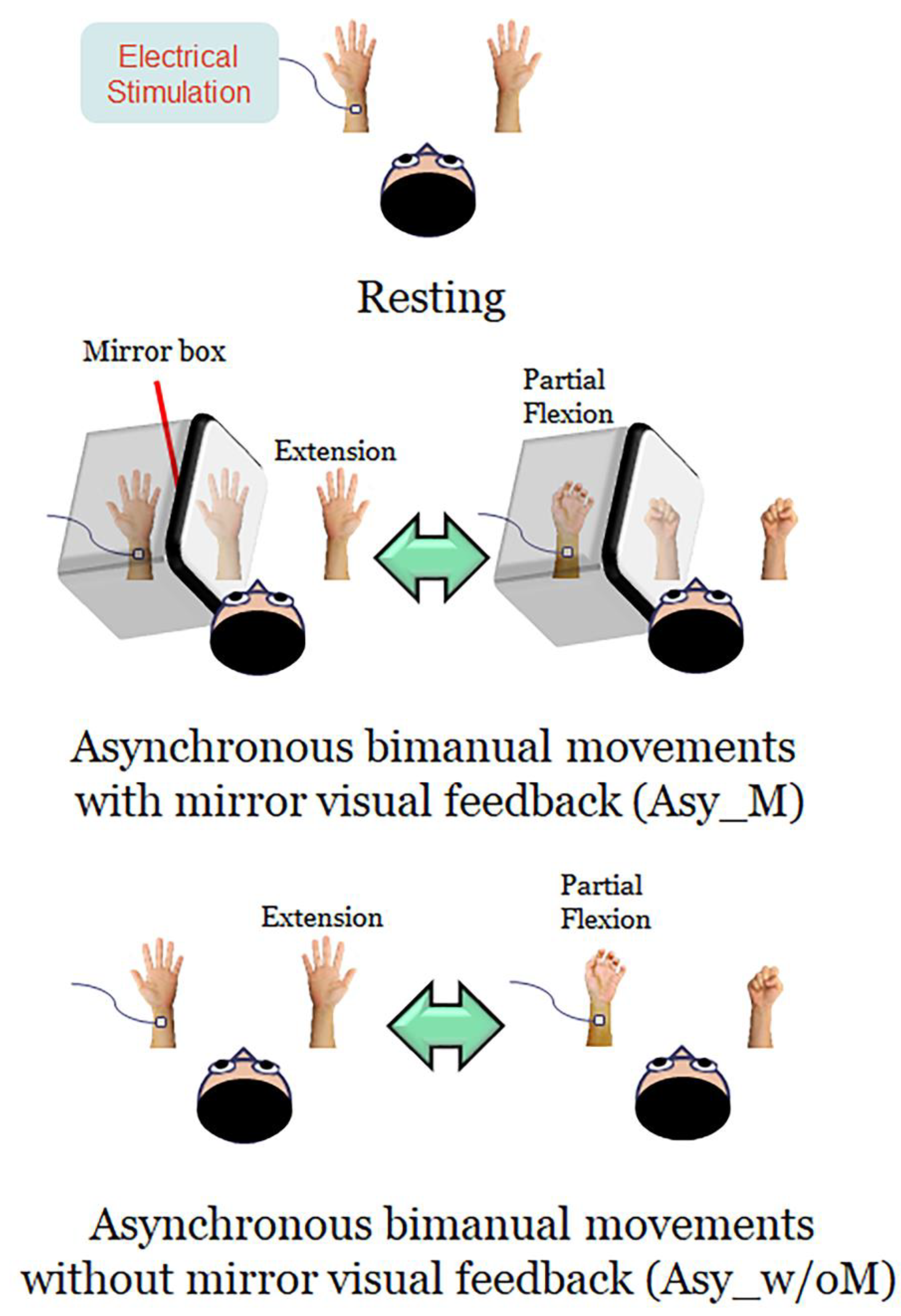
2.3. Experimental Design and Procedures
- (1)
- Resting: Both hands were kept stationary with the forearms supinated, and the participants were instructed to look at both stationary hands.
- (2)
- Asynchronous bimanual movement with MVF (Asy_M): The participant’s left hand was masked by a mirror box and the participant was instructed to look at both the unmasked right hand and the mirror reflection of the right hand. Participants were instructed to perform bimanual in-phase fingers flexion/extension repetitively with a frequency of approximately 1 Hz, with their masked left hand performing partial range of movement and their right hand performing full range of movement. The mirror reflection of the right hand is as if the left hand performed the movement the same as the right hand. In this condition, participants performed asynchronous bimanual movement while receiving the visual feedback of synchronous bimanual movement.
- (3)
- Asynchronous bimanual movement without MVF (Asy_w/oM): All settings were similar to the Asy_M condition, except that the mirror box was removed in this condition. Participants were instructed to look at this asynchronous bimanual movement. In this case, participants performed asynchronous bimanual movement and received the visual feedback of asynchronous bimanual movement.
2.4. MEG Recordings
2.5. Analysis of M1 Beta Rebound Oscillation
2.6. Analysis of Functional Connectivity
2.7. Statistical Analysis
3. Results
3.1. Analysis of M1 Beta Rebound Oscillation
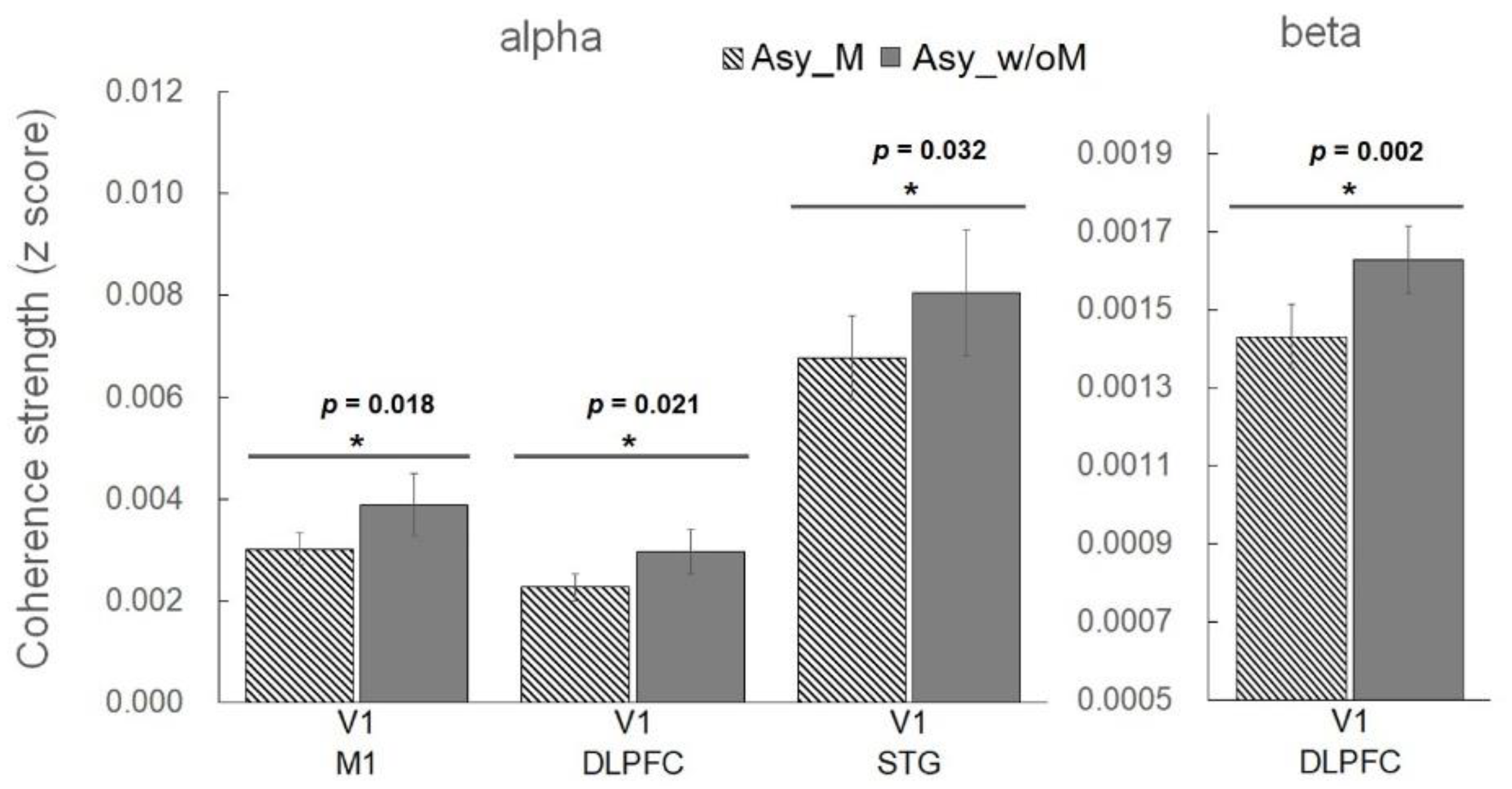
3.2. Analysis of Functional Connectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nojima, I.; Mima, T.; Koganemaru, S.; Thabit, M.N.; Fukuyama, H.; Kawamata, T. Human Motor Plasticity Induced by Mirror Visual Feedback. J. Neurosci. 2012, 32, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Ezendam, D.; Bongers, R.M.; Jannink, M.J.A. Systematic review of the effectiveness of mirror therapy in upper extremity function. Disabil. Rehabil. 2009, 31, 2135–2149. [Google Scholar] [CrossRef]
- Rothgangel, A.S.; Braun, S.M.; Beurskens, A.J.H.M.; Seitz, R.J.; Wade, D.T. The clinical aspects of mirror therapy in rehabilitation. Int. J. Rehabil. Res. 2011, 34, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thieme, H.; Morkisch, N.; Mehrholz, J.; Pohl, M.; Behrens, J.; Borgetto, B.; Dohle, C. Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2018, 2018, CD008449. [Google Scholar] [CrossRef]
- Winstein, C.J.; Wolf, S.L.; Dromerick, A.W.; Lane, C.J.; Nelsen, M.A.; Lewthwaite, R.; Cen, S.Y.; Azen, S.P.; Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE) Investigative Team. Effect of a Task-Oriented Rehabilitation Program on Upper Extremity Recovery Following Motor Stroke. JAMA 2016, 315, 571–581. [Google Scholar] [CrossRef]
- Fukumura, K.; Sugawara, K.; Tanabe, S.; Ushiba, J.; Tomita, Y. Influence of Mirror Therapy on Human Motor Cortex. Int. J. Neurosci. 2007, 117, 1039–1048. [Google Scholar] [CrossRef]
- Garry, M.; Loftus, A.; Summers, J.J. Mirror, mirror on the wall: Viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp. Brain Res. 2005, 163, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, W.; Matsubayashi, J.; Deguchi, Y.; Minami, C.; Kinai, T.; Nakamura, M.; Nagamine, T.; Matsuhashi, M.; Mima, T.; Fukuyama, H.; et al. A mirror reflection of a hand modulates stimulus-induced 20-Hz activity. NeuroImage 2009, 46, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, W.; Matsubayashi, J.; Furuya, M.; Matsuhashi, M.; Mima, T.; Fukuyama, H.; Mitani, A. Asymmetric Activation of the Primary Motor Cortex during Observation of a Mirror Reflection of a Hand. PLoS ONE 2011, 6, e28226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Fritzsch, C.; Bernarding, J.; Holtze, S.; Mauritz, K.; Brunetti, M.; Dohle, C. A comparison of neural mechanisms in mirror therapy and movement observation therapy. J. Rehabil. Med. 2013, 45, 410–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohle, C.; Kleiser, R.; Seitz, R.J.; Freund, H.-J. Body Scheme Gates Visual Processing. J. Neurophysiol. 2004, 91, 2376–2379. [Google Scholar] [CrossRef] [Green Version]
- Matthys, K.; Smits, M.; Van der Geest, J.N.; Van der Lugt, A.; Seurinck, R.; Stam, H.J.; Selles, R.W. Mirror-Induced Visual Illusion of Hand Movements: A Functional Magnetic Resonance Imaging Study. Arch. Phys. Med. Rehabil. 2009, 90, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.R.; Marshall, J.C.; Halligan, P.W.; Frith, C.; Driver, J.; Frackowiak, R.; Dolan, R. The neural consequences of conflict between intention and the senses. Brain 1999, 122, 497–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Z.; Fong, K.N.K.; Zhang, J.; Hu, Z. Cortical mapping of mirror visual feedback training for unilateral upper extremity: A functional near-infrared spectroscopy study. Brain Behav. 2019, 10, e01489. [Google Scholar] [CrossRef] [PubMed]
- Rjosk, V.; Lepsien, J.; Kaminski, E.; Hoff, M.; Sehm, B.; Steele, C.J.; Villringer, A.; Ragert, P. Neural Correlates of Mirror Visual Feedback-Induced Performance Improvements: A Resting-State fMRI Study. Front. Hum. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deconinck, F.J.A.; Smorenburg, A.R.P.; Benham, A.; Ledebt, A.; Feltham, M.G.; Savelsbergh, G.J.P. Reflections on Mirror Therapy. Neurorehabilit. Neural Repair 2015, 29, 349–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.-H.; Belliveau, J.W.; Dale, A.M.; Hämäläinen, M.S. Distributed current estimates using cortical orientation constraints. Hum. Brain Mapp. 2006, 27, 1–13. [Google Scholar] [CrossRef]
- Lin, F.-H.; Witzel, T.; Ahlfors, S.; Stufflebeam, S.M.; Belliveau, J.W.; Hämäläinen, M.S. Assessing and improving the spatial accuracy in MEG source localization by depth-weighted minimum-norm estimates. NeuroImage 2006, 31, 160–171. [Google Scholar] [CrossRef]
- Zhang, J.J.; Fong, K.N.K.; Welage, N.; Liu, K.P.Y. The Activation of the Mirror Neuron System during Action Observation and Action Execution with Mirror Visual Feedback in Stroke: A Systematic Review. Neural Plast. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Wasaka, T.; Kakigi, R. Conflict caused by visual feedback modulates activation in somatosensory areas during movement execution. NeuroImage 2012, 59, 1501–1507. [Google Scholar] [CrossRef]
- Wasaka, T.; Kakigi, R. The effect of unpredicted visual feedback on activation in the secondary somatosensory cortex during movement execution. BMC Neurosci. 2012, 13, 138. [Google Scholar] [CrossRef] [Green Version]
- Pfurtscheller, G.; da Silva, F.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- van der Vliet, R.; Ribbers, G.M.; Vandermeeren, Y.; Frens, M.A.; Selles, R.W. BDNF Val66Met but not transcranial direct current stimulation affects motor learning after stroke. Brain Stimul. 2017, 10, 882–892. [Google Scholar] [CrossRef]
- Cheyne, D.; Gaetz, W.; Garnero, L.; Lachaux, J.-P.; Ducorps, A.; Schwartz, D.; Varela, F.J. Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Cogn. Brain Res. 2003, 17, 599–611. [Google Scholar] [CrossRef]
- Anderson, K.; Ding, M. Attentional modulation of the somatosensory mu rhythm. Neuroscience 2011, 180, 165–180. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Lebar, N.; Danna, J.; Moré, S.; Mouchnino, L.; Blouin, J. On the neural basis of sensory weighting: Alpha, beta and gamma modulations during complex movements. NeuroImage 2017, 150, 200–212. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lin, M.-Y.; Yang, S.-H. Age Effect on Automatic Inhibitory Function of the Somatosensory and Motor Cortex: An MEG Study. Front. Aging Neurosci. 2018, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.-H.; Sun, H.-H.; Weng, J.-Q.; Tseng, Y.-J. Differential motor cortex excitability during observation of normal and abnormal goal-directed movement patterns. Neurosci. Res. 2017, 123, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Tsai, S.-Y.; Liu, C.-Y.; Niddam, D.M. Automatic inhibitory function in the human somatosensory and motor cortices: An MEG-MRS study. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Uusitalo, M.A.; Ilmoniemi, R.J. Signal-space projection method for separating MEG or EEG into components. Med. Biol. Eng. Comput. 1997, 35, 135–140. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 13. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.X.; Mosher, J.C.; Leahy, R.M. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys. Med. Biol. 1999, 44, 423–440. [Google Scholar] [CrossRef]
- Cheng, C.; Tseng, Y.; Chen, R.; Lin, Y. Reduced functional connectivity of somatosensory network in writer’s cramp patients. Brain Behav. 2016, 6, e00433. [Google Scholar] [CrossRef]
- Michielsen, M.E.; Smits, M.; Ribbers, G.; Stam, H.J.; Van Der Geest, J.N.; Bussmann, J.B.J.; Selles, R.W. The neuronal correlates of mirror therapy: An fMRI study on mirror induced visual illusions in patients with stroke. J. Neurol. Neurosurg. Psychiatry 2010, 82, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doesburg, S.M.; Bedo, N.; Ward, L.M. Top-down alpha oscillatory network interactions during visuospatial attention orienting. NeuroImage 2016, 132, 512–519. [Google Scholar] [CrossRef]
- Ossmy, O.; Mukamel, R. Behavioral and neural effects of congruency of visual feedback during short-term motor learning. NeuroImage 2018, 172, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [Green Version]
- Leech, R.; Braga, R.; Sharp, D.J. Echoes of the Brain within the Posterior Cingulate Cortex. J. Neurosci. 2012, 32, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Hari, R.; Forss, N.; Avikainen, S.; Kirveskari, E.; Salenius, S.; Rizzolatti, G. Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc. Natl. Acad. Sci. USA 1998, 95, 15061–15065. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, A.; Yamamoto, H.; Ono, I.; Matsubayashi, J.; Nagamine, T.; Fukuyama, H.; Mitani, A. Stimulus-related 20-Hz activity of human cortex modulated by the way of presenting hand actions. Neurosci. Res. 2007, 58, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Järveläinen, J.; Schürmann, M.; Hari, R. Activation of the human primary motor cortex during observation of tool use. NeuroImage 2004, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaraswamy, S.; Myers, J.; Wilson, S.; Nutt, D.; Lingford-Hughes, A.; Singh, K.; Hamandi, K. The effects of elevated endogenous GABA levels on movement-related network oscillations. NeuroImage 2013, 66, 36–41. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-H.; Cheng, C.-H.; Wu, C.-Y.; Liu, C.-T.; Chen, C.-L.; Hsieh, Y.-W. Mirror Visual Feedback Induces M1 Excitability by Disengaging Functional Connections of Perceptuo-Motor-Attentional Processes during Asynchronous Bimanual Movement: A Magnetoencephalographic Study. Brain Sci. 2021, 11, 1092. https://doi.org/10.3390/brainsci11081092
Lin S-H, Cheng C-H, Wu C-Y, Liu C-T, Chen C-L, Hsieh Y-W. Mirror Visual Feedback Induces M1 Excitability by Disengaging Functional Connections of Perceptuo-Motor-Attentional Processes during Asynchronous Bimanual Movement: A Magnetoencephalographic Study. Brain Sciences. 2021; 11(8):1092. https://doi.org/10.3390/brainsci11081092
Chicago/Turabian StyleLin, Szu-Hung, Chia-Hsiung Cheng, Ching-Yi Wu, Chien-Ting Liu, Chia-Ling Chen, and Yu-Wei Hsieh. 2021. "Mirror Visual Feedback Induces M1 Excitability by Disengaging Functional Connections of Perceptuo-Motor-Attentional Processes during Asynchronous Bimanual Movement: A Magnetoencephalographic Study" Brain Sciences 11, no. 8: 1092. https://doi.org/10.3390/brainsci11081092
APA StyleLin, S.-H., Cheng, C.-H., Wu, C.-Y., Liu, C.-T., Chen, C.-L., & Hsieh, Y.-W. (2021). Mirror Visual Feedback Induces M1 Excitability by Disengaging Functional Connections of Perceptuo-Motor-Attentional Processes during Asynchronous Bimanual Movement: A Magnetoencephalographic Study. Brain Sciences, 11(8), 1092. https://doi.org/10.3390/brainsci11081092






