Personal Protective Equipment Alters Leg Muscle Fatigability Independent of Transcranial Direct Current Stimulation: A Comparison with Pre-COVID-19 Pandemic Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Isokinetic/Isometric Strength Testing
2.4. Isokinetic Fatigue Task (FT)
2.5. Electromyography (EMG)
2.6. Transcranial Direct Current Stimulation (tDCS)
2.7. Data Analysis
2.8. Statistical Analysis
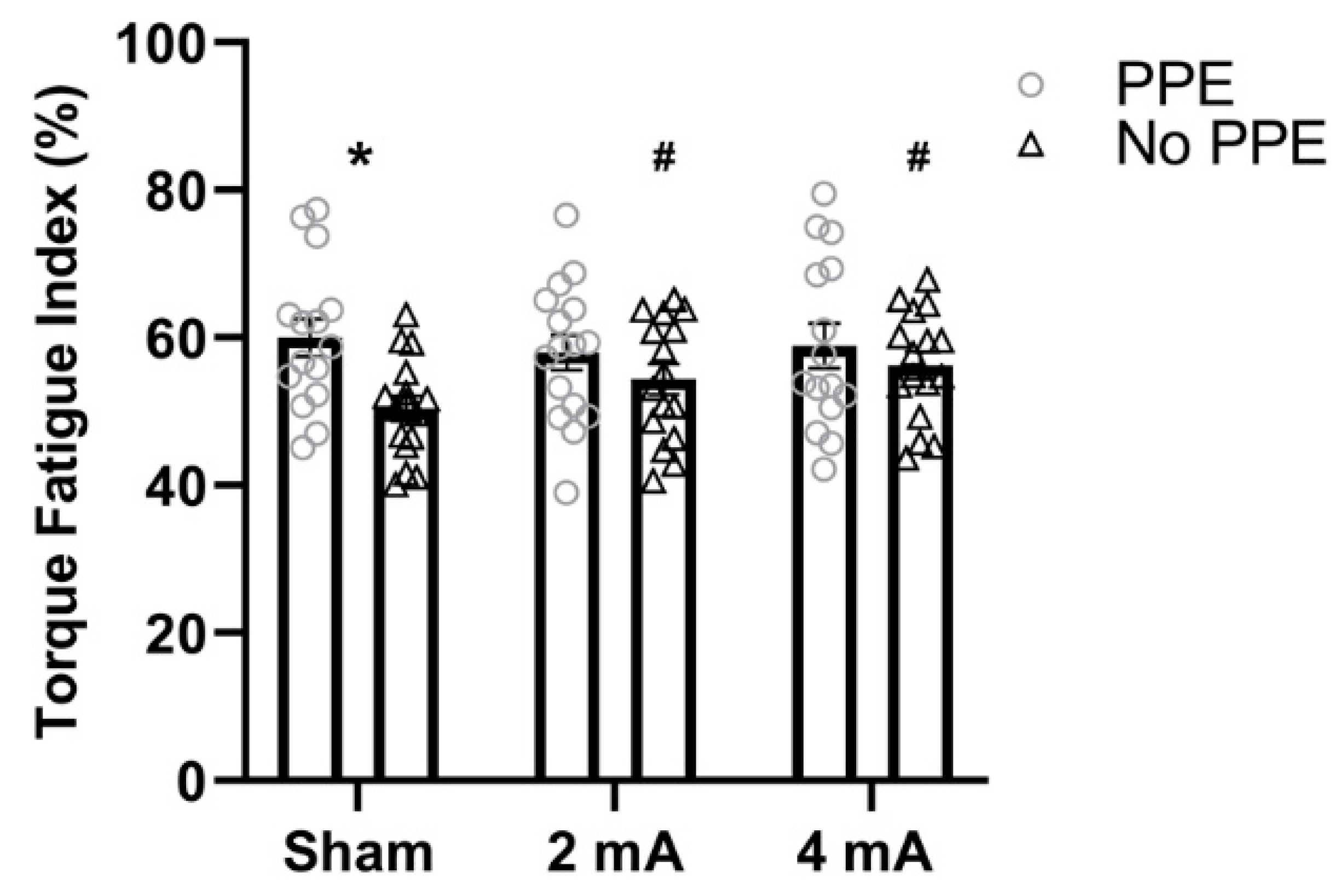
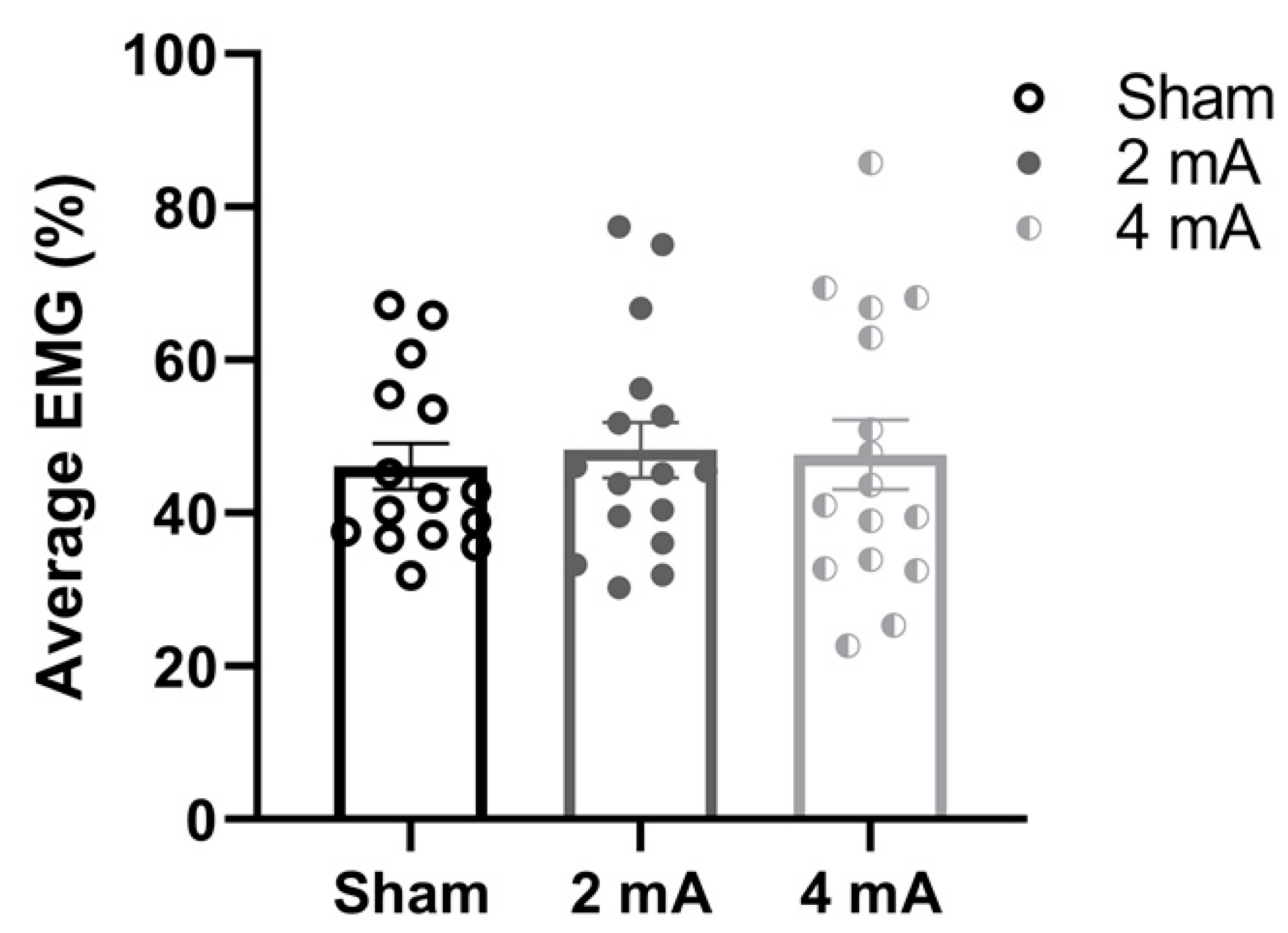
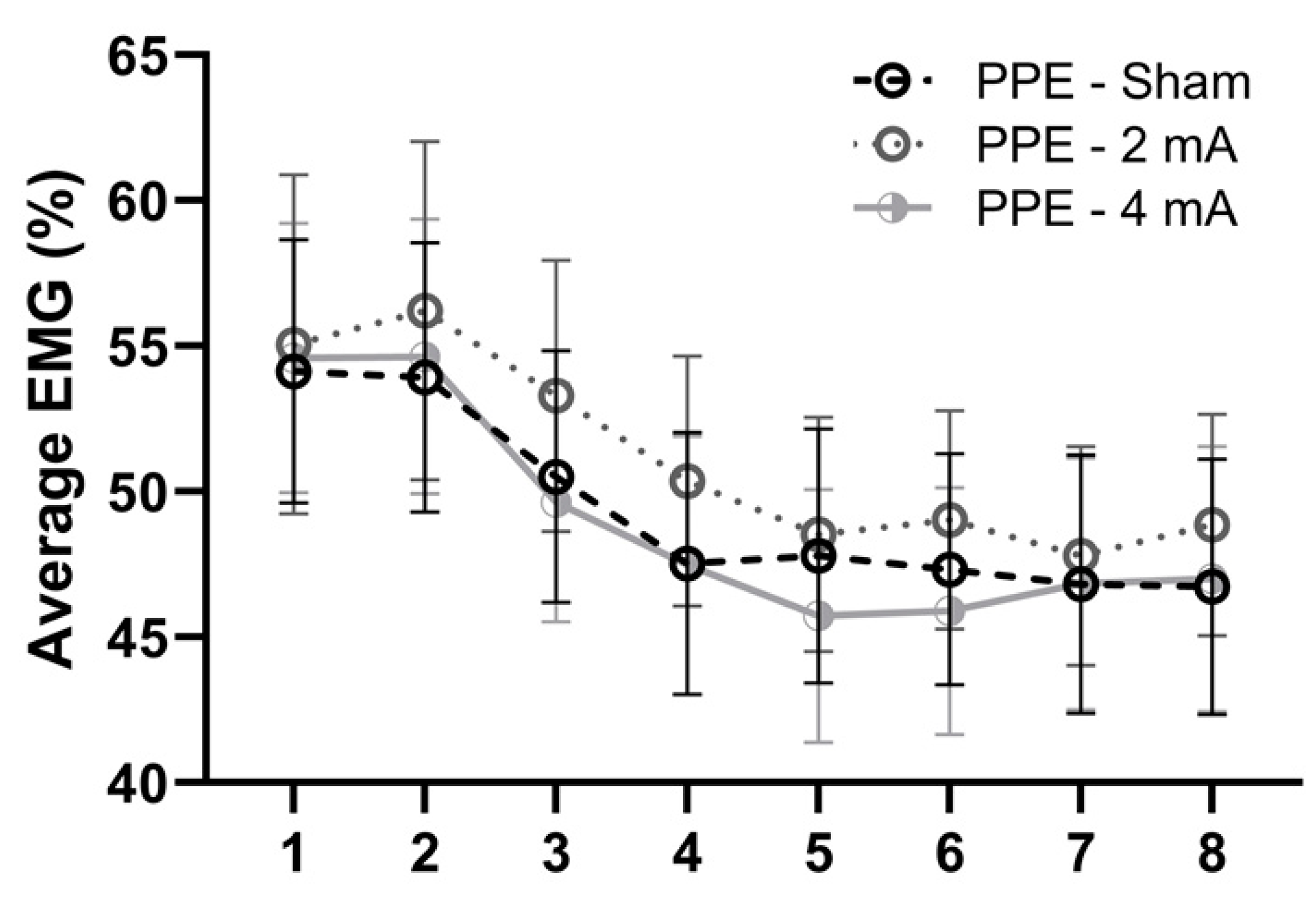
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jefferson, T.; Del Mar, C.B.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; Bawazeer, G.A.; van Driel, M.L.; Nair, S.; Jones, M.A.; Thorning, S.; et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekaran, B.; Fernandes, S. Exercise with facemask; are we handling a devil’s sword? A physiological hypothesis. Med. Hypotheses 2020, 144, 110002. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Kindred, J.H.; Ketelhut, N.B. Fatigue in multiple sclerosis: Misconceptions and future research directions. Front. Neurol. 2016, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Rudroff, T. Different effects of 2 mA and 4 mA transcranial direct current stimulation on muscle activity and torque in a maximal isokinetic fatigue task. Front. Hum. Neurosci. 2020, 14, 240. [Google Scholar] [CrossRef]
- Workman, C.D.; Kamholz, J.; Rudroff, T. Increased leg muscle fatigability during 2 mA and 4 mA transcranial direct current stimulation over the left motor cortex. Exp. Brain Res. 2020, 238, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.D.; Kamholz, J.; Rudroff, T. The tolerability and efficacy of 4 mA transcranial direct current stimulation on leg muscle fatigability. Brain Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Workman, C.D.; Fietsam, A.C.; Rudroff, T. Transcranial direct current stimulation at 4 mA induces greater leg muscle fatigability in women compared to men. Brain Sci. 2020, 10, 244. [Google Scholar] [CrossRef] [Green Version]
- Angius, L.; Mauger, A.R.; Hopker, J.; Pascual-Leone, A.; Santarnecchi, E.; Marcora, S.M. Bilateral extracephalic transcranial direct current stimulation improves endurance performance in healthy individuals. Brain Stimul. 2018, 11, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, R.; Vergari, M.; Cogiamanian, F.; Bocci, T.; Ciocca, M.; Tomasini, E.; De Riz, M.; Scarpini, E.; Priori, A. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. Neuro Rehabil. 2014, 34, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Tecchio, F.; Cancelli, A.; Cottone, C.; Zito, G.; Pasqualetti, P.; Ghazaryan, A.; Rossini, P.M.; Filippi, M.M. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J. Neurol. 2014, 261, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Chalah, M.A.; Mhalla, A.; Palm, U.; Ayache, S.S.; Mylius, V. The treatment of fatigue by non-invasive brain stimulation. Neurophysiol. Clin. 2017, 47, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Fietsam, A.C.; Workman, C.D.; Ponto, L.L.B.; Kamholz, J.; Rudroff, T. Different effects of transcranial direct current stimulation on leg muscle glucose uptake asymmetry in two women with multiple sclerosis. Brain Sci. 2020, 10, 549. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Rudroff, T. Associations of lower limb joint asymmetry with fatigue and disability in people with multiple sclerosis. Clin. Biomech. 2020, 75, 104989. [Google Scholar] [CrossRef]
- Workman, C.D.; Kamholz, J.; Rudroff, T. Transcranial direct current stimulation (tDCS) for the treatment of a multiple sclerosis symptom cluster. Brain Stimul. 2020, 13, 263–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workman, C.D.; Kamholz, J.; Rudroff, T. Transcranial direct current stimulation (tDCS) to improve gait in multiple sclerosis: A timing window comparison. Front. Hum. Neurosci. 2019, 13, 420. [Google Scholar] [CrossRef] [Green Version]
- Cancelli, A.; Cottone, C.; Giordani, A.; Migliore, S.; Lupoi, D.; Porcaro, C.; Mirabella, M.; Rossini, P.M.; Filippi, M.M.; Tecchio, F. Personalized, bilateral whole-body somatosensory cortex stimulation to relieve fatigue in multiple sclerosis. Mult. Scler. J. 2018, 24, 1366–1374. [Google Scholar] [CrossRef]
- Alix-Fages, C.; Romero-Arenas, S.; Castro-Alonso, M.; Colomer-Poveda, D.; Rio-Rodriguez, D.; Jerez-Martinez, A.; Fernandez-Del-Olmo, M.; Marquez, G. Short-term effects of anodal transcranial direct current stimulation on endurance and maximal force production. A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 536. [Google Scholar] [CrossRef] [Green Version]
- Angius, L.; Pageaux, B.; Hopker, J.; Marcora, S.M.; Mauger, A.R. Transcranial direct current stimulation improves isometric time to exhaustion of the knee extensors. Neuroscience 2016, 339, 363–375. [Google Scholar] [CrossRef]
- Radel, R.; Tempest, G.; Denis, G.; Besson, P.; Zory, R. Extending the limits of force endurance: Stimulation of the motor or the frontal cortex? Cortex 2017, 97, 96–108. [Google Scholar] [CrossRef]
- Krishnan, C.; Ranganathan, R.; Kantak, S.S.; Dhaher, Y.Y.; Rymer, W.Z. Anodal transcranial direct current stimulation alters elbow flexor muscle recruitment strategies. Brain Stimul. 2014, 7, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Flood, A.; Waddington, G.; Keegan, R.J.; Thompson, K.G.; Cathcart, S. The effects of elevated pain inhibition on endurance exercise performance. PeerJ 2017, 5, e3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthalib, M.; Kan, B.; Nosaka, K.; Perrey, S. Effects of transcranial direct current stimulation of the motor cortex on prefrontal cortex activation during a neuromuscular fatigue task: An fnirs study. Adv. Exp. Med. Biol. 2013, 789, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Epstein, D.; Korytny, A.; Isenberg, Y.; Marcusohn, E.; Zukermann, R.; Bishop, B.; Minha, S.; Raz, A.; Miller, A. Return to training in the COVID-19 era: The physiological effects of face masks during exercise. Scand. J. Med. Sci. Sports 2021, 31, 70–75. [Google Scholar] [CrossRef]
- Roberge, R.J.; Coca, A.; Williams, W.J.; Powell, J.B.; Palmiero, A.J. Physiological impact of the n95 filtering facepiece respirator on healthcare workers. Respir. Care 2010, 55, 569–577. [Google Scholar]
- Person, E.; Lemercier, C.; Royer, A.; Reychler, G. Effect of a surgical mask on six minute walking distance. Rev. Mal. Respir. 2018, 35, 264–268. [Google Scholar] [CrossRef]
- Amann, M.; Eldridge, M.W.; Lovering, A.T.; Stickland, M.K.; Pegelow, D.F.; Dempsey, J.A. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J. Physiol. 2006, 575, 937–952. [Google Scholar] [CrossRef]
- Amann, M.; Romer, L.M.; Subudhi, A.W.; Pegelow, D.F.; Dempsey, J.A. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J. Physiol. 2007, 581, 389–403. [Google Scholar] [CrossRef]
- Baig, A.S.; Knapp, C.; Eagan, A.E.; Radonovich, L.J., Jr. Health care workers’ views about respirator use and features that should be included in the next generation of respirators. Am. J. Infect. Control 2010, 38, 18–25. [Google Scholar] [CrossRef]
- Johnson, A.T. Respirator masks protect health but impact performance: A review. J. Biol. Eng. 2016, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Perna, G.; Cuniberti, F.; Dacco, S.; Nobile, M.; Caldirola, D. Impact of respiratory protective devices on respiration: Implications for panic vulnerability during the COVID-19 pandemic. J. Affect. Disord. 2020, 277, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Fikenzer, S.; Uhe, T.; Lavall, D.; Rudolph, U.; Falz, R.; Busse, M.; Hepp, P.; Laufs, U. Effects of surgical and ffp2/n95 face masks on cardiopulmonary exercise capacity. Clin. Res. Cardiol. 2020, 109, 1522–1530. [Google Scholar] [CrossRef]
- Shaw, K.; Butcher, S.; Ko, J.; Zello, G.A.; Chilibeck, P.D. Wearing of cloth or disposable surgical face masks has no effect on vigorous exercise performance in healthy individuals. Int. J. Environ. Res. Public Health 2020, 17, 8110. [Google Scholar] [CrossRef]
- Coronavirus in the U.S.: Latest Map and Case Count. Available online: https://www.nytimes.com/interactive/2021/us/covid-cases.html (accessed on 6 January 2021).
- Jang, H.; Lee, J.Y.; Lee, K.I.; Park, K.M. Are there differences in brain morphology according to handedness? Brain Behav. 2017, 7, e00730. [Google Scholar] [CrossRef]
- Foerster, A.S.; Rezaee, Z.; Paulus, W.; Nitsche, M.A.; Dutta, A. Effects of cathode location and the size of anode on anodal transcranial direct current stimulation over the leg motor area in healthy humans. Front. Neurosci. 2018, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, A.B.; Deckert, J.A.; Schlabs, C.R.; Tilden, M.J.; Herda, T.J.; Gallagher, P.M.; Weir, J.P. Transcranial direct current stimulation of the temporal lobe does not affect high-intensity work capacity. J. Strength Cond. Res. 2019, 33, 2074–2086. [Google Scholar] [CrossRef]
- Martin, D.M.; Liu, R.; Alonzo, A.; Green, M.; Loo, C.K. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: Effect of timing of stimulation. Exp. Brain Res. 2014, 232, 3345–3351. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.D.; Fietsam, A.C.; Rudroff, T. Tolerability and blinding of transcranial direct current stimulation in people with parkinson’s disease: A critical review. Brain Sci. 2020, 10, 467. [Google Scholar] [CrossRef]
- Thorstensson, A.; Karlsson, J. Fatiguability and fibre composition of human skeletal muscle. Acta Physiol. Scand. 1976, 98, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.P.; Archer, R.L.; Evans, W.J. Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Med. Sci. Sports Exerc. 2001, 33, 1613–1619. [Google Scholar] [CrossRef]
- Hameau, S.; Bensmail, D.; Roche, N.; Zory, R. Adaptations of fatigue and fatigability after a short intensive, combined rehabilitation program in patients with multiple sclerosis. J. Rehabil. Med. 2018, 50, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.A.; Onyskiw, J.E.; Prasad, N.G. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung 1998, 27, 387–408. [Google Scholar] [CrossRef]
- Kelly, A.M.; McAlpine, R.; Kyle, E. How accurate are pulse oximeters in patients with acute exacerbations of chronic obstructive airways disease? Respir. Med. 2001, 95, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef] [Green Version]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: An update. J. Appl. Physiol. 2014, 117, 1215–1230. [Google Scholar] [CrossRef] [Green Version]
- Del Vecchio, A.; Negro, F.; Felici, F.; Farina, D. Associations between motor unit action potential parameters and surface EMG features. J. Appl. Physiol. 2017, 123, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Pageaux, B.; Angius, L.; Hopker, J.G.; Lepers, R.; Marcora, S.M. Central alterations of neuromuscular function and feedback from group iii-iv muscle afferents following exhaustive high-intensity one-leg dynamic exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R1008–R1020. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S. The Psychology of Pandemics: Preparing for the Next Global Outbreak of Infectious Disease; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019. [Google Scholar]
- Stein, D.J.; Fernandes Medeiros, L.; Caumo, W.; Torres, I.L. Transcranial direct current stimulation in patients with anxiety: Current perspectives. Neuropsychiatr. Dis. Treat. 2020, 16, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.; Cooper, N.R.; Paulmann, S.; Fitzgerald, P.B.; Russo, R. Perceived comfort and blinding efficacy in randomised sham-controlled transcranial direct current stimulation (tDCS) trials at 2 mA in young and older healthy adults. PLoS ONE 2016, 11, e0149703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, N.E.; Cossar, J.; Marston, L.; Wand, B.M.; Bunce, D.; Moseley, G.L.; De Souza, L.H. Rethinking clinical trials of transcranial direct current stimulation: Participant and assessor blinding is inadequate at intensities of 2ma. PLoS ONE 2012, 7, e47514. [Google Scholar] [CrossRef] [Green Version]
- Fertonani, A.; Ferrari, C.; Miniussi, C. What do you feel if i apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. 2015, 126, 2181–2188. [Google Scholar] [CrossRef]
- Farnad, L.; Ghasemian-Shirvan, E.; Mosayebi-Samani, M.; Kuo, M.F.; Nitsche, M.A. Exploring and optimizing the neuroplastic effects of anodal transcranial direct current stimulation over the primary motor cortex of older humans. Brain Stimul. 2021, 14, 622–634. [Google Scholar] [CrossRef]
- McFadden, J.L.; Borckardt, J.J.; George, M.S.; Beam, W. Reducing procedural pain and discomfort associated with transcranial direct current stimulation. Brain Stimul. 2011, 4, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.; Jackson, C.; Learmonth, G. Is the “end-of-study guess” a valid measure of sham blinding during transcranial direct current stimulation? Eur. J. Neurosci. 2021, 53, 1592–1604. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fietsam, A.C.; Deters, J.R.; Workman, C.D.; Rudroff, T. Personal Protective Equipment Alters Leg Muscle Fatigability Independent of Transcranial Direct Current Stimulation: A Comparison with Pre-COVID-19 Pandemic Results. Brain Sci. 2021, 11, 962. https://doi.org/10.3390/brainsci11080962
Fietsam AC, Deters JR, Workman CD, Rudroff T. Personal Protective Equipment Alters Leg Muscle Fatigability Independent of Transcranial Direct Current Stimulation: A Comparison with Pre-COVID-19 Pandemic Results. Brain Sciences. 2021; 11(8):962. https://doi.org/10.3390/brainsci11080962
Chicago/Turabian StyleFietsam, Alexandra C., Justin R. Deters, Craig D. Workman, and Thorsten Rudroff. 2021. "Personal Protective Equipment Alters Leg Muscle Fatigability Independent of Transcranial Direct Current Stimulation: A Comparison with Pre-COVID-19 Pandemic Results" Brain Sciences 11, no. 8: 962. https://doi.org/10.3390/brainsci11080962
APA StyleFietsam, A. C., Deters, J. R., Workman, C. D., & Rudroff, T. (2021). Personal Protective Equipment Alters Leg Muscle Fatigability Independent of Transcranial Direct Current Stimulation: A Comparison with Pre-COVID-19 Pandemic Results. Brain Sciences, 11(8), 962. https://doi.org/10.3390/brainsci11080962








