Changes in Sensorimotor Cortical Activation in Children Using Prostheses and Prosthetic Simulators
Abstract
:1. Introduction
1.1. Incidence of Limb Reduction
1.2. Body Schema and Phantom Sensations
1.3. Neuronal Group Selection Theory and Regions of Interest
1.4. Prosthetic Simulators
1.5. Functional Near-Infrared Spectroscopy
1.6. Aims and Hypothesis
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Prostheses and Prosthetic Simulators
2.4. Functional Near-Infrared Spectroscopy
2.5. Statistical Analysis
3. Results
3.1. Box and Block Scores
3.2. Contralateral Activations in the ULR Group Compared to the TD Group
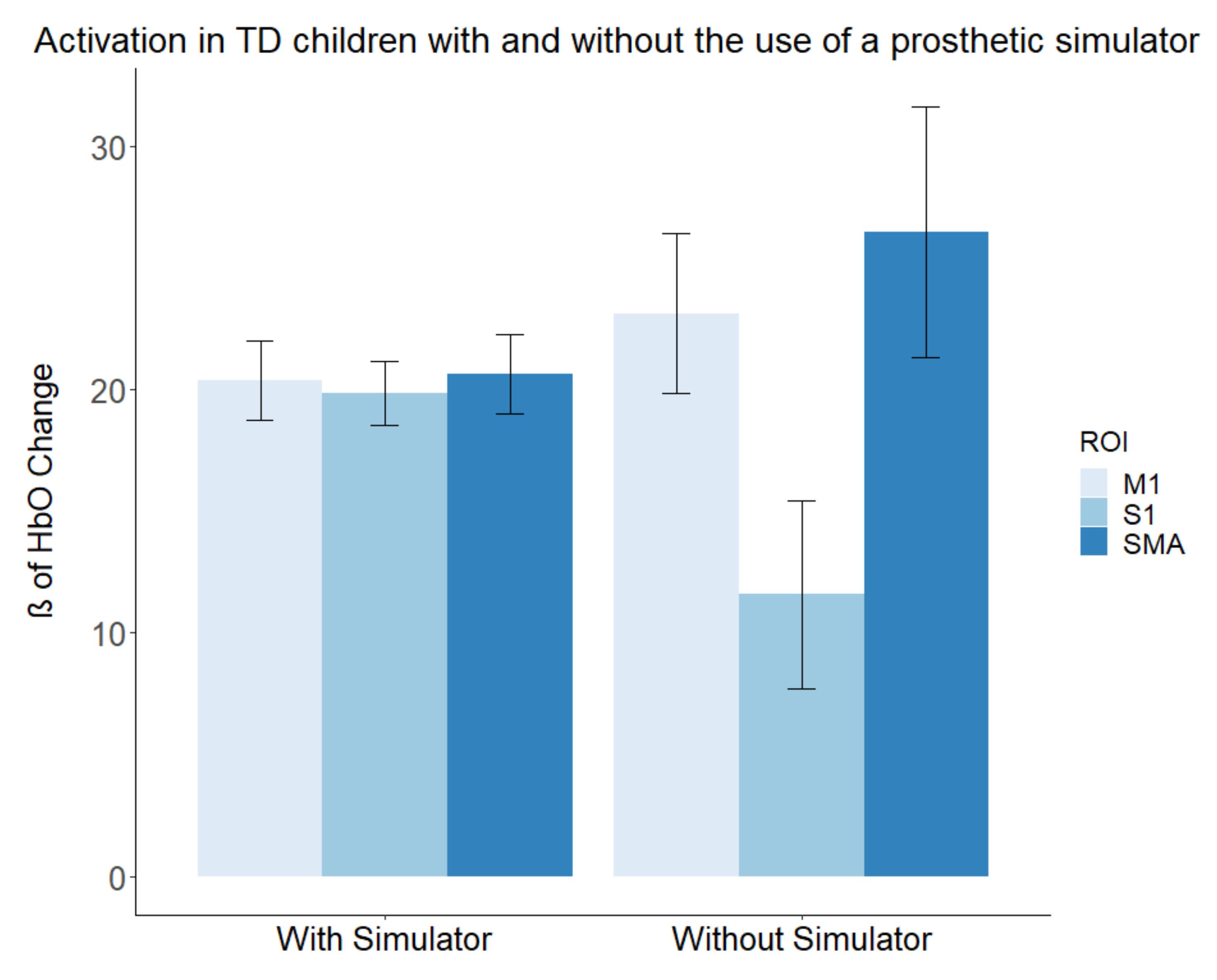
3.3. Contralateral Activations in the TD Group with and without a Simulator
4. Discussion
4.1. Group Differences between Prosthesis and Prosthetic Simulator Use
4.2. Differences in Activation Produced by Prosthetic Simulator Use
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.T.; Isenburg, J.L.; Canfield, M.A.; Meyer, R.E.; Correa, A.; Alverson, C.J.; Lupo, P.J.; Riehle-Colarusso, T.; Cho, S.J.; Aggarwal, D.; et al. National Population-Based Estimates for Major Birth Defects, 2010–2014. Birth Defects Res. 2019, 111, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Krebs, D.E.; Edelstein, J.E.; Thornby, M.A. Prosthetic Management of Children with Limb Deficiencies. Phys. Ther. 1991, 71, 920–934. [Google Scholar] [CrossRef]
- CDC. Facts about Upper and Lower Limb Reduction Defects. Available online: https://www.cdc.gov/ncbddd/birthdefects/ul-limbreductiondefects.html (accessed on 14 November 2019).
- James, M.A.; Bagley, A.M.; Brasington, K.; Lutz, C.; Mcconnell, S.; Molitor, F. Impact of Prostheses on Function and Quality of Life for Children with Unilateral Congenital Below-the-Elbow Deficiency. J. Bone Jt. Surg. 2006, 88, 2356–2365. [Google Scholar] [CrossRef] [Green Version]
- Weeks, D.L.; Wallace, S.A.; Anderson, D.I. Training with an Upper-Limb Prosthetic Simulator to Enhance Transfer of Skill across Limbs. Arch. Phys. Med. Rehabil. 2003, 84 (Suppl. 1), 437–443. [Google Scholar] [CrossRef] [PubMed]
- Huinink, L.H.B.; Bouwsema, H.; Plettenburg, D.H.; Van der Sluis, C.K.; Bongers, R.M. Learning to Use a Body-Powered Prosthesis: Changes in Functionality and Kinematics. J. Neuroeng. Rehabil. 2016, 13, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melzack, R.; Israel, R.; Lacroix, R.; Schultz, G. Phantom Limbs in People with Congenital Limb Deficiency or Amputation in Early Childhood. Brain 1997, 120, 1603–1620. [Google Scholar] [CrossRef] [Green Version]
- Reilly, K.T.; Sirigu, A. Motor Cortex Representation of the Upper-Limb in Individuals Born without a Hand. PLoS ONE 2011, 6, e18100. [Google Scholar] [CrossRef] [Green Version]
- Elbert, T.; Flor, H.; Birbaumer, N.; Knecht, S.; Hampson, S.; Larbig, W.; Taub, E. Extensive Reorganization of the Somatosensory Cortex in Adult Humans after Nervous System Injury. Neuroreport 1994, 5, 2593–2597. [Google Scholar] [CrossRef] [Green Version]
- de Vignemont, F. Body Schema and Body Image-Pros and Cons. Neuropsychologia 2010, 48, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, K.L.; McGrath, P.J.; Finley, G.A.; Katz, J. Phantom Limb Sensations and Phantom Limb Pain in Child and Adolescent Amputees. Pain 1998, 78, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Edelman, G.M. Neural Darwinism: The Theory of Neuronal Group Selection; Basic Books: New York, NY, USA, 1987. [Google Scholar]
- Sporns, O.; Edelman, G.M. Solving Bernstein’s Problem: A Proposal for the Development of Coordinated Movement by Selection. Child Dev. 1993, 64, 960–981. [Google Scholar] [CrossRef]
- Huizing, K.; Reinders-Messelink, H.; Maathuis, C.; Hadders-Algra, M.; Van Der Sluis, C.K. Age at First Prosthetic Fitting and Later Functional Outcome in Children and Young Adults with Unilateral Congenital Below-Elbow Deficiency: A Cross-Sectional Study. Prosthet. Orthot. Int. 2010, 34, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meurs, M.; Maathuis, C.G.B.; Lucas, C.; Hadders-Algra, M.; van der Sluis, C.K. Prescription of the First Prosthesis and Later Use in Children with Congenital Unilateral Upper Limb Deficiency: A Systematic Review. Prosthet. Orthot. Int. 2006, 30, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penfield, W.; Theodore, R. The Cerebral Cortex of Man a Clinical Study of Localization of Function; The MacMillan Company: New York, NY, USA, 1950. [Google Scholar]
- Penfield, W.; Boldrey, E. Somatic Motor and Sensory Representation in the Cerebral Cortex of Man as Studied by Electrical Stimulation. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Tanji, J. The Supplementary Motor Area in the Cerebral Cortex. Neurosci. Res. 1994, 19, 251–268. [Google Scholar] [CrossRef]
- Ruddy, K.L.; Carson, R.G. Neural Pathways Mediating Cross Education of Motor Function. Front. Hum. Neurosci. 2013, 7, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheaton, L.A. Neurorehabilitation in Upper Limb Amputation: Understanding How Neurophysiological Changes Can Affect Functional Rehabilitation. J. NeuroEng. Rehabil. 2017, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Weeks, D.L.; Anderson, D.I.; Wallace, S.A. The Role of Variability in Practice Structure When Learning. JPO J. Prosthet. Orthot. 2003, 15, 84–92. [Google Scholar] [CrossRef]
- de Boer, E.; Romkema, S.; Cutti, A.G.; Brouwers, M.A.; Bongers, R.M.; van der Sluis, C.K. Intermanual Transfer Effects in Below-Elbow Myoelectric Prosthesis Users. Arch. Phys. Med. Rehabil. 2016, 97, 1924–1930. [Google Scholar] [CrossRef]
- Romkema, S.; Bongers, R.M.; van der Sluis, C.K. Intermanual Transfer Effect in Young Children after Training in a Complex Skill: Mechanistic, Pseudorandomized, Pretest-Posttest Study. Phys. Ther. 2015, 95, 730–739. [Google Scholar] [CrossRef]
- Romkema, S.; Bongers, R.M.; van der Sluis, C.K. Intermanual Transfer in Training with an Upper-Limb Myoelectric Prosthesis Simulator: A Mechanistic, Randomized, Pretest-Posttest Study. Phys. Ther. 2013, 93, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-Infrared Spectroscopy (FNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2018, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A Review on Continuous Wave Functional Near-Infrared Spectroscopy and Imaging Instrumentation and Methodology. Neuroimage 2014, 85, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.B.; Luna, B.; Hein, T.C.; Huppert, T.J. FNIRS Evidence of Prefrontal Regulation of Frustration in Early Childhood. Neuroimage 2014, 85, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Nishiyori, R. FNIRS: An Emergent Method to Document Functional Cortical Activity during Infant Movements. Front. Psychol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuniga, J.M.; Pierce, J.E.; Copeland, C.; Cortes-Reyes, C.; Salazar, D.; Wang, Y.Y.; Arun, K.M.; Huppert, T. Brain Lateralization in Children with Upper-Limb Reduction Deficiency. J. Neuroeng. Rehabil. 2021, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, J.; Bravo, G.; Hébert, R.; Dutil, E.M.L. Validation of the Box and Block Test as a Meansure of Dexterity of Erderlt Peple: Reliability, Validity, and Norms Studies. Arch. Phys. Med. Rehabil. 1994, 75, 751–755. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult Norms for the Box and Block Test of Manual Dexterity. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 1985, 39, 386–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathiowetz, V.; Federman, S.; Wiemer, D. Box and Block Test of Manual Dexterity: Norms for 6–19 Year Olds. Can. J. Occup. Ther. 1985, 52, 241–245. [Google Scholar] [CrossRef]
- Zuniga, J.M.; Young, K.J.; Peck, J.L.; Srivastava, R.; Pierce, J.E.; Dudley, D.R.; Salazar, D.A.; Bergmann, J. Remote Fitting Procedures for Upper Limb 3d Printed Prostheses. Expert Rev. Med. Devices 2019, 16, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The Ten-Twenty Electrode System of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar] [PubMed]
- Yücel, M.A.; Selb, J.; Aasted, C.M.; Lin, P.-Y.; Borsook, D.; Becerra, L.; Boas, D.A. Mayer Waves Reduce the Accuracy of Estimated Hemodynamic Response Functions in Functional Near-Infrared Spectroscopy. Biomed. Opt. Express 2016, 7, 3078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinti, P.; Scholkmann, F.; Hamilton, A.; Burgess, P.; Tachtsidis, I. Current Status and Issues Regarding Pre-Processing of FNIRS Neuroimaging Data: An Investigation of Diverse Signal Filtering Methods within a General Linear Model Framework. Front. Hum. Neurosci. 2019, 12, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppes, C.W.; Huppert, T.J.; Whitney, S.L.; Dunlap, P.M.; Disalvio, N.L.; Alshebber, K.M.; Furman, J.M.; Kwon, Y.H.; Rosso, A.L. Changes in Cortical Activation during Dual-Task Walking in Individuals with and without Visual Vertigo. J. Neurol. Phys. Ther. 2020, 44, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Strangman, G.; Franceschini, M.A.; Boas, D.A. Factors Affecting the Accuracy of Near-Infrared Spectroscopy Concentration Calculations for Focal Changes in Oxygenation Parameters. Neuroimage 2003, 18, 865–879. [Google Scholar] [CrossRef]
- Santosa, H.; Zhai, X.; Fishburn, F.; Huppert, T. The NIRS Brain AnalyzIR Toolbox. Algorithms 2018, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Barker, J.W.; Aarabi, A.; Huppert, T.J. Autoregressive Model Based Algorithm for Correcting Motion and Serially Correlated Errors in FNIRS. Biomed. Opt. Express 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huppert, T.J. Commentary on the Statistical Properties of Noise and Its Implication on General Linear Models in Functional Near-Infrared Spectroscopy. Neurophotonics 2016, 3, 010401. [Google Scholar] [CrossRef] [Green Version]
- Holmes, C.J.; Hoge, R.; Collins, L.; Woods, R.; Toga, A.W.; Evans, A.C. Enhancement of MR Images Using Registration for Signal Averaging. J. Comput. Assist. Tomogr. 1998, 22, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Huppert, T.J.; Karim, H.; Lin, C.C.; Alqahtani, B.A.; Greenspan, S.L.; Sparto, P.J. Functional Imaging of Cognition in an Old-Old Population: A Case for Portable Functional near-Infrared Spectroscopy. PLoS ONE 2017, 12, e0184918. [Google Scholar] [CrossRef] [PubMed]
- Team R Development Core. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; 2020. Available online: https://www.R-project.org (accessed on 17 January 2021).
- Wesselink, D.B.; Van Den Heiligenberg, F.M.Z.; Ejaz, N. Obtaining and Maintaining Cortical Hand Representation as Evidenced from Acquired and Congenital Handlessness. Elife 2019, 8, e37227. [Google Scholar] [CrossRef] [PubMed]
- Nico, D.; Daprati, E.; Rigal, F.; Parsons, L.; Sirigu, A. Left and Right Hand Recognition in Upper Limb Amputees. Brain 2004, 127, 120–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaser, C.; Nenadic, I.; Weiss, T.; Miltner, W.; Sauer, H. Gray Matter Volume Loss after Upper Limb Amputation. Klin. Neurophysiol. 2004, 35, 69. [Google Scholar] [CrossRef]
- Preißler, S.; Feiler, J.; Dietrich, C.; Hofmann, G.O.; Miltner, W.H.R.; Weiss, T. Gray Matter Changes Following Limb Amputation with High and Low Intensities of Phantom Limb Pain. Cereb. Cortex 2013, 23, 1038–1048. [Google Scholar] [CrossRef] [Green Version]
- Reinkingh, M.; Reinders-Messelink, H.A.; Dijkstra, P.U.; Maathuis, K.G.B.; Van Der Sluis, C.K. Stump Sensibility in Children with Upper Limb Reduction Deficiency. J. Rehabil. Med. 2014, 46, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polovina, S.; Polovina, T.S.; Polovina, A.; Polovina-Proloscić, T. Intensive Rehabilitation in Children with Cerebral Palsy: Our View on the Neuronal Group Selection Theory. Coll. Antropol. 2010, 34, 981–988. [Google Scholar] [PubMed]
- Hadders-Algra, M. The Neuronal Group Selection Theory: Promising Principles for Understanding and Treating Developmental Motor Disorders. Dev. Med. Child Neurol. 2000, 42, 707–715. [Google Scholar] [CrossRef]
- Abd Razak, N.A.; Osman, N.A.A.; Gholizadeh, H.; Ali, S. Biomechanics Principle of Elbow Joint for Transhumeral Prostheses: Comparison of Normal Hand, Body-Powered, Myoelectric & Air Splint Prostheses. Biomed. Eng. Online 2014, 13, 1–12. [Google Scholar]

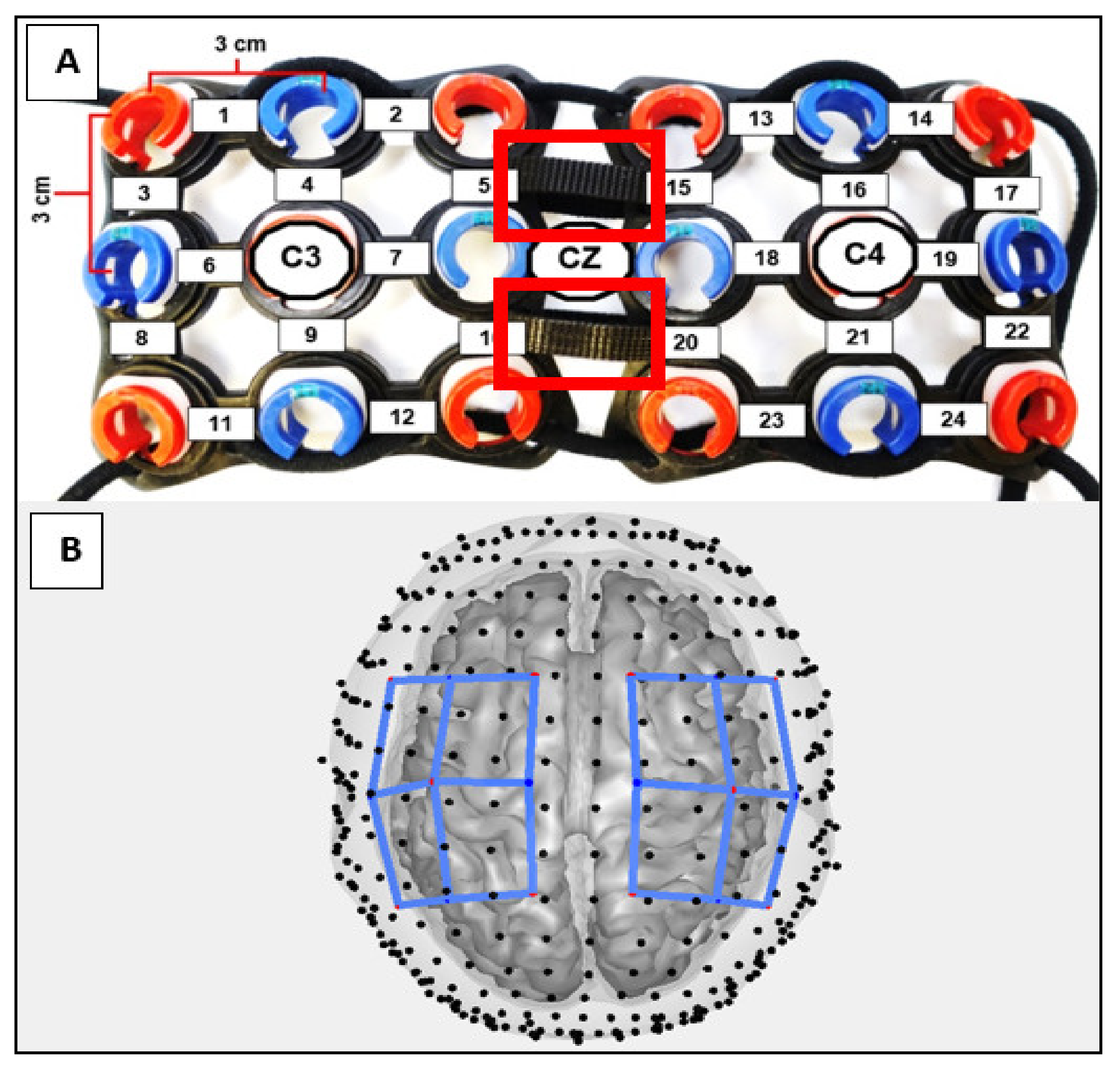


| Experimental Group (Congenital Upper Limb Reduction) | ||||||
|---|---|---|---|---|---|---|
| ID | Sex | Age (Years) | Preferred Side | Reduction Level | Affected Side | Ability to Pinch |
| 1 | Female | 6.2 | Right | Partial Hand | Left | No |
| 2 | Female | 8.2 | Right | Trans-Radial | Left | No |
| 3 | Male | 11.1 | Right | Partial Hand | Left | No |
| 4 | Male | 5.1 | Right | Trans-Radial | Left | No |
| 5 | Male | 13.2 | Right | Partial Hand | Left | No |
| M ± SD | 8.76 ± 3.37 | |||||
| TD Group (Typically Developing) | ||||||
| 1 | Female | 6.4 | Right | None | None | Yes |
| 2 | Female | 8.3 | Right | None | None | Yes |
| 3 | Male | 11.3 | Right | None | None | Yes |
| 4 | Male | 5.6 | Right | None | None | Yes |
| 5 | Male | 13.2 | Right | None | None | Yes |
| M ± SD | 8.96 ± 3.23 | |||||
| Box and Block Score | |||||
|---|---|---|---|---|---|
| ULR Group | TD Group | ||||
| Participant | Preferred Limb | Prosthesis/Simulator | Participant | Non-Preferred Limb | Prosthesis/Simulator |
| 1 | 37 | 3 | 1 | 37 | 6 |
| 2 | 31 | 3 | 2 | 30 | 6 |
| 3 | 50 | 7 | 3 | 65 | 17 |
| 4 | 39 | 7 | 4 | 62 | 9 |
| 5 | 42 | 10 | 5 | 67 | 7 |
| Mean | 39.80 | 6 | Mean | 52.2 | 9 |
| SD | 6.98 | 3 | SD | 17.34 | 4.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copeland, C.; Mukherjee, M.; Wang, Y.; Fraser, K.; Zuniga, J.M. Changes in Sensorimotor Cortical Activation in Children Using Prostheses and Prosthetic Simulators. Brain Sci. 2021, 11, 991. https://doi.org/10.3390/brainsci11080991
Copeland C, Mukherjee M, Wang Y, Fraser K, Zuniga JM. Changes in Sensorimotor Cortical Activation in Children Using Prostheses and Prosthetic Simulators. Brain Sciences. 2021; 11(8):991. https://doi.org/10.3390/brainsci11080991
Chicago/Turabian StyleCopeland, Christopher, Mukul Mukherjee, Yingying Wang, Kaitlin Fraser, and Jorge M. Zuniga. 2021. "Changes in Sensorimotor Cortical Activation in Children Using Prostheses and Prosthetic Simulators" Brain Sciences 11, no. 8: 991. https://doi.org/10.3390/brainsci11080991






