Comparing Different Filter-Parameter for Pre-Processing of Brain-Stimulation Evoked Motor Potentials
Abstract
:1. Introduction
2. Considerations for Signal Processing
2.1. Signal Stationarity
2.2. Ergodic Theorem
3. Methods
3.1. Subjects
3.2. Study Design
3.3. MEP Release and Recording
4. Statistics
5. Results
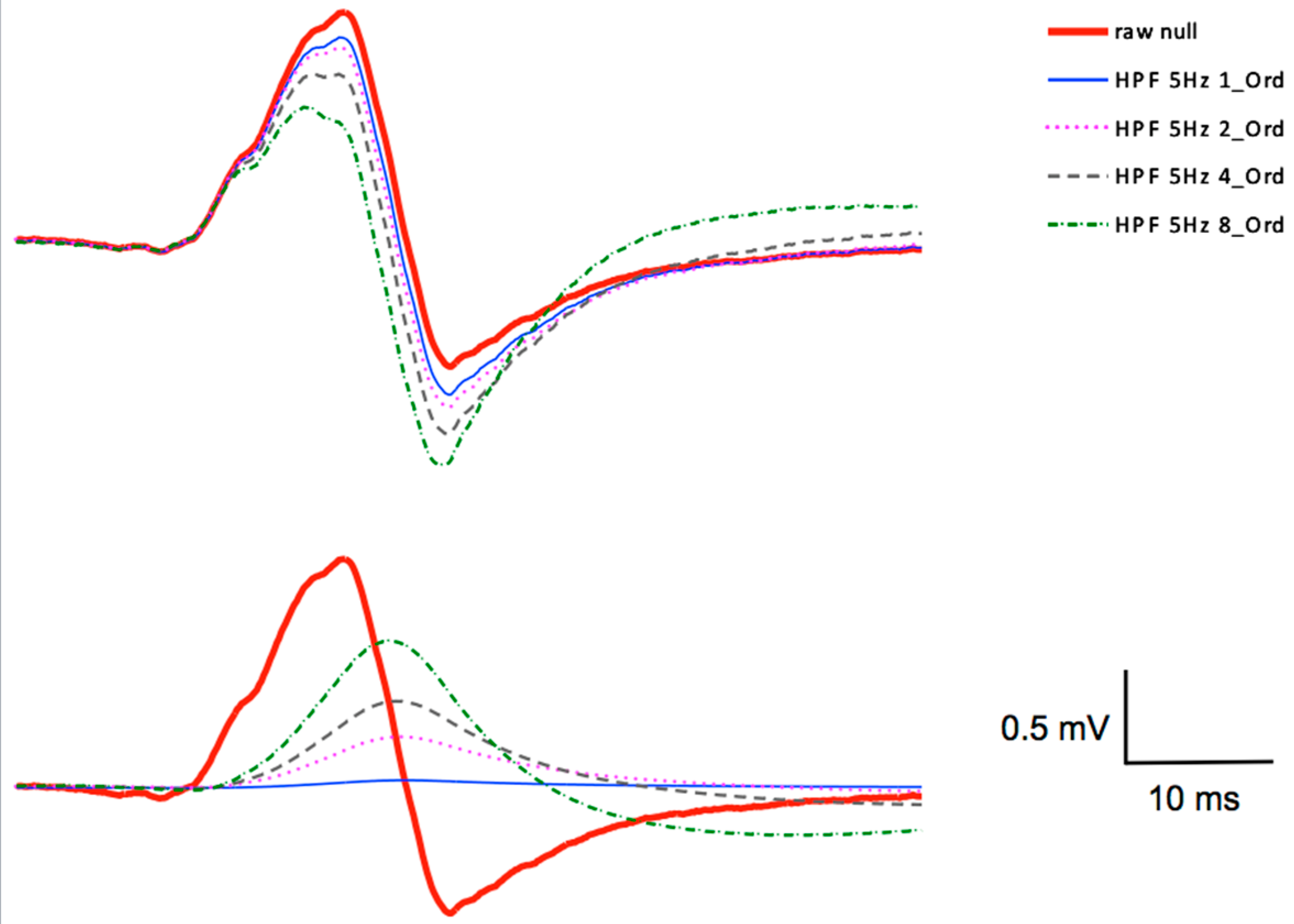
5.1. Variation of the Cut-Off Frequency
5.2. Variation of the Filter-Order
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baratta, R.; Solomonow, M.; Zhou, B.-H.; Zhu, M. Methods to reduce the variability of emg power spectrum estimates. J. Electromyogr. Kinesiol. 1998, 8, 279–285. [Google Scholar] [CrossRef]
- Mewett, D.T.; Nazeran, H.; Reynolds, K.J. Removing power line noise from recorded emg, Engineering in Medicine and Biology Society, 2001. In Proceedings of the 23rd Annual International Conference of the IEEE, Istanbul, Turkey, 25–28 October 2001; pp. 2190–2193. [Google Scholar]
- Levkov, C.; Mihov, G.; Ivanov, R.; Daskalov, I.; Christov, I.; Dotsinsky, I. Removal of power-line interference from the ecg: A review of the subtraction procedure. BioMed. Eng. OnLine 2005, 4, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, C.J.; Gilmore, L.D.; Kuznetsov, M.; Roy, S.H. Filtering the surface emg signal: Movement artifact and baseline noise contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Redfern, M.; Hughes, R.; Chaffin, D. High-pass filtering to remove electrocardiographic interference from torso emg recordings. Clin. Biomech. 1993, 8, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Willigenburg, N.W.; Daffertshofer, A.; Kingma, I.; van Dieën, J.H. Removing ecg contamination from emg recordings: A comparison of ica-based and other filtering procedures. J. Electromyogr. Kinesiol. 2012, 22, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Carreno, I.; Malanda-Trigueros, A.; Gila-Useros, L.; Navallas-Irujo, J.; Rodriguez-Falces, J. Filter design for cancellation of baseline-fluctuation in needle emg recordings. Comput. Methods Programs Biomed. 2006, 81, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Law, L.F.; Krishnan, C.; Avin, K. Modeling nonlinear errors in surface electromyography due to baseline noise: A new methodology. J. Biomech. 2011, 44, 202–205. [Google Scholar] [PubMed] [Green Version]
- Zschorlich, V. Digital filtering of emg-signals. Electromyogr. Clin. Neurophysiol. 1989, 29, 81–86. [Google Scholar] [PubMed]
- Brownell, A.A.; Bromberg, M.B. Effects of high-pass filtering on muap metrics. Muscle Nerve 2009, 40, 1008–1011. [Google Scholar] [CrossRef]
- Acunzo, D.J.; MacKenzie, G.; van Rossum, M.C. Systematic biases in early erp and erf components as a result of high-pass filtering. J. Neurosci. Methods 2012, 209, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Rousselet, G.A. Does filtering preclude us from studying erp time-courses? Front. Psychol. 2012, 3, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, D.; Morgan-Short, K.; Luck, S.J. How inappropriate high-pass filters can produce artifactual effects and incorrect conclusions in erp studies of language and cognition. Psychophysiology 2015, 52, 997–1009. [Google Scholar] [CrossRef] [Green Version]
- Tanner, D.; Norton, J.J.; Morgan-Short, K.; Luck, S.J. On high-pass filter artifacts (they’re real) and baseline correction (it’sa good idea) in erp/ermf analysis. J. Neurosci. Methods 2016, 266, 166–170. [Google Scholar] [CrossRef]
- Bendat, J.S.; Piersol, A.G. Random Data: Analysis and Measurement Procedures; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 729. [Google Scholar]
- Merletti, R.; Di Torino, P. Standards for reporting emg data. J. Electromyogr. Kinesiol. 1999, 9, 3–4. [Google Scholar]
- Merletti, R.; Parker, P.A. Electromyography: Physiology, Engineering, and Non-Invasive Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 11. [Google Scholar]
- Stegeman, D.; Hermens, H. Standards for surface Electromyography: The European Project Surface Emg for Non-Invasive Assessment of Muscles; SENIAM: Enschede, The Netherlands, 2007; pp. 108–112. [Google Scholar]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Group, S.o.T.C. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [Green Version]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.; George, M. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an ifcn committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Stearns, S.D.; Hush, D.R. Digital Signal Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 1990. [Google Scholar]
- Geddes, L.; Baker, L. The specific resistance of biological material—A compendium of data for the biomedical engineer and physiologist. Med. Biol. Eng. 1967, 5, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Harriss, D.; Atkinson, G. Ethical standards in sport and exercise science research: 2014 update·. Int. J. Sports Med. 2013, 34, 1025–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastani, A.; Jaberzadeh, S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis. Clin. Neurophysiol. 2012, 123, 644–657. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Fountain, S.; Daskalakis, Z.J. A comprehensive review of the effects of rtms on motor cortical excitability and inhibition. Clin. Neurophysiol. 2006, 117, 2584–2596. [Google Scholar] [CrossRef]
- Cavaleri, R.; Schabrun, S.M.; Chipchase, L.S. Determining the number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (tms): A protocol for a systematic review and meta-analysis. Syst. Rev. 2015, 4, 107. [Google Scholar] [CrossRef] [Green Version]
- Cavaleri, R.; Schabrun, S.M.; Chipchase, L.S. The number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (tms): A systematic review and meta-analysis. Syst. Rev. 2017, 6, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadiga, L.; Fogassi, L.; Pavesi, G.; Rizzolatti, G. Motor facilitation during action observation: A magnetic stimulation study. J. Neurophysiol. 1995, 73, 2608–2611. [Google Scholar] [CrossRef] [PubMed]
- Walsh, V.; Rushworth, M. A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia 1999, 37, 125–136. [Google Scholar] [PubMed]
- Widmann, A.; Schröger, E. Filter effects and filter artifacts in the analysis of electrophysiological data. Front. Psychol. 2012, 3, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widmann, A.; Schröger, E.; Maess, B. Digital filter design for electrophysiological data–A practical approach. J. Neurosci. Methods 2015, 250, 34–46. [Google Scholar] [CrossRef] [Green Version]
- De Cheveigné, A.; Nelken, I. Filters: When, why, and how (not) to use them. Neuron 2019, 102, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Stearns, S. Digital Signal Analysis; Hayden Book Company, Inc.: Rochelle Park, NJ, USA, 1975. [Google Scholar]
- Chicos, D.; Otto, M.; Zölsch, S.; Clausnitzer, S.; Vetter, D.; Pfeiffer, G.; de Vogel, F.; Schaar, O. Crash Analysis-Criteria Description-Workgroup Data Processing Vehicle Safety; Bundesanstalt für Straßenwesen: Bergisch Gladbach, Germany, 2017. [Google Scholar]
- Nikolov, P.; Hassan, S.S.; Schnitzler, A.; Groiss, S.J. Influence of high pass filter settings on motor evoked potentials. Front. Neurosci. 2021, 15, 485. [Google Scholar] [CrossRef]

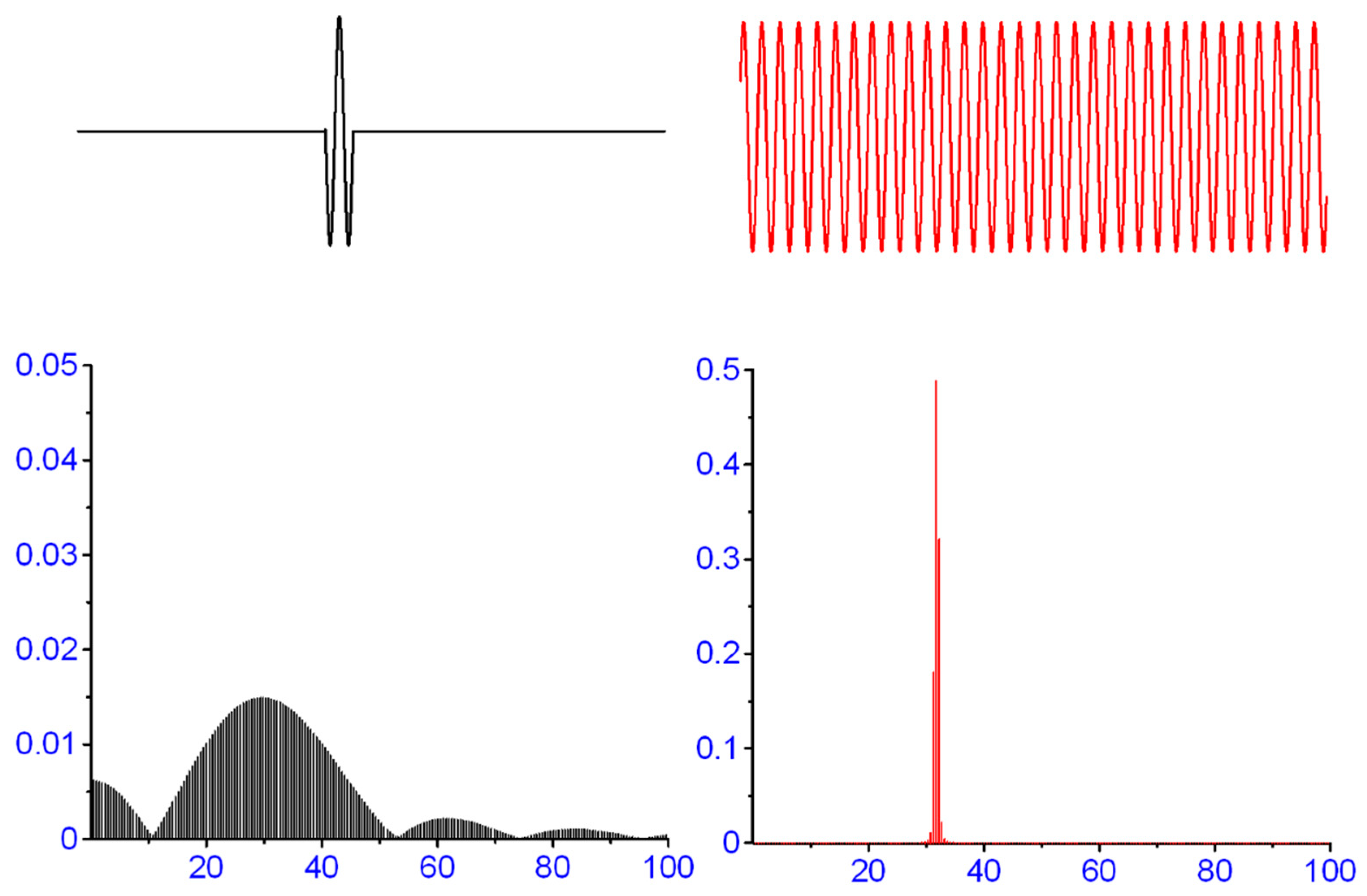
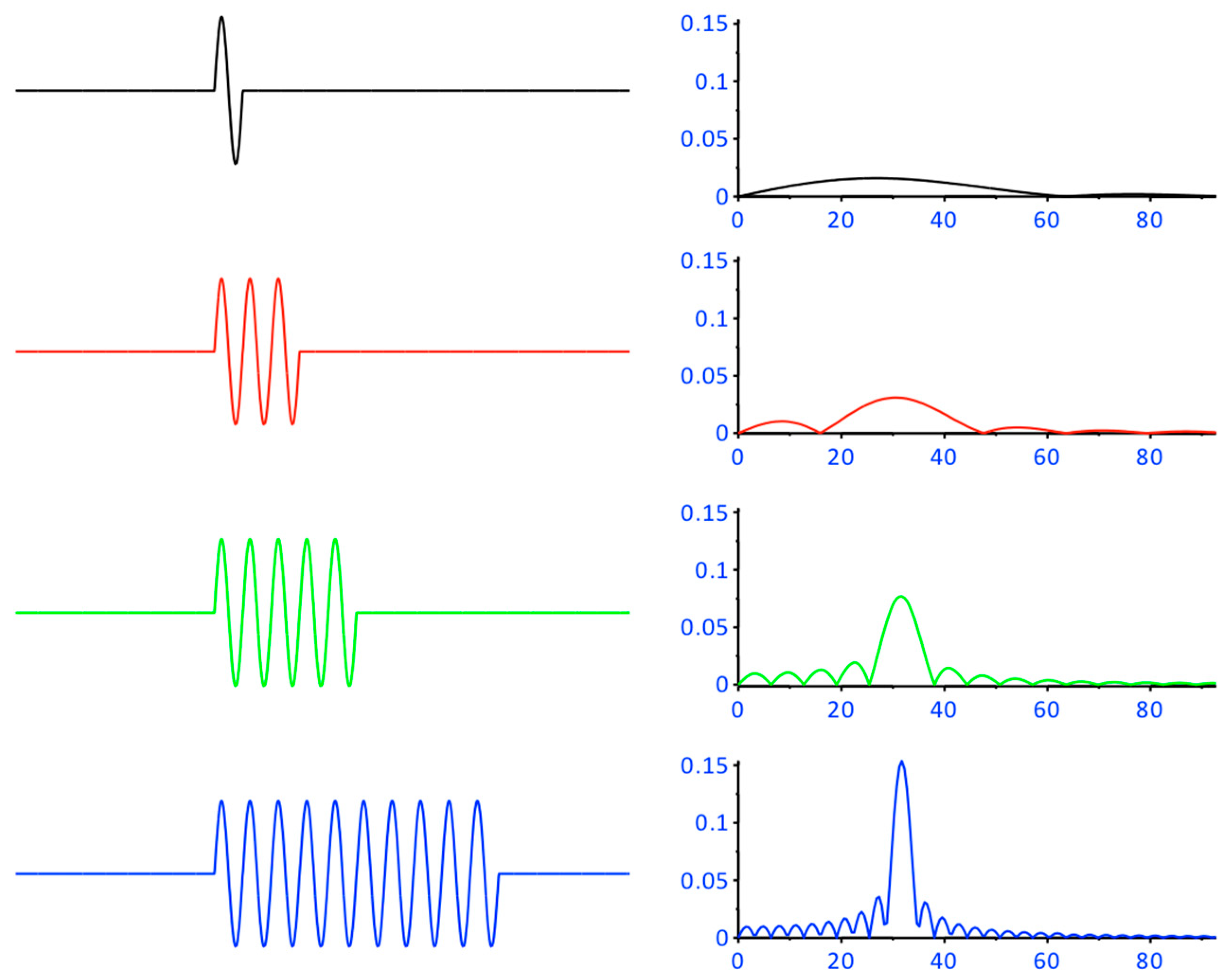
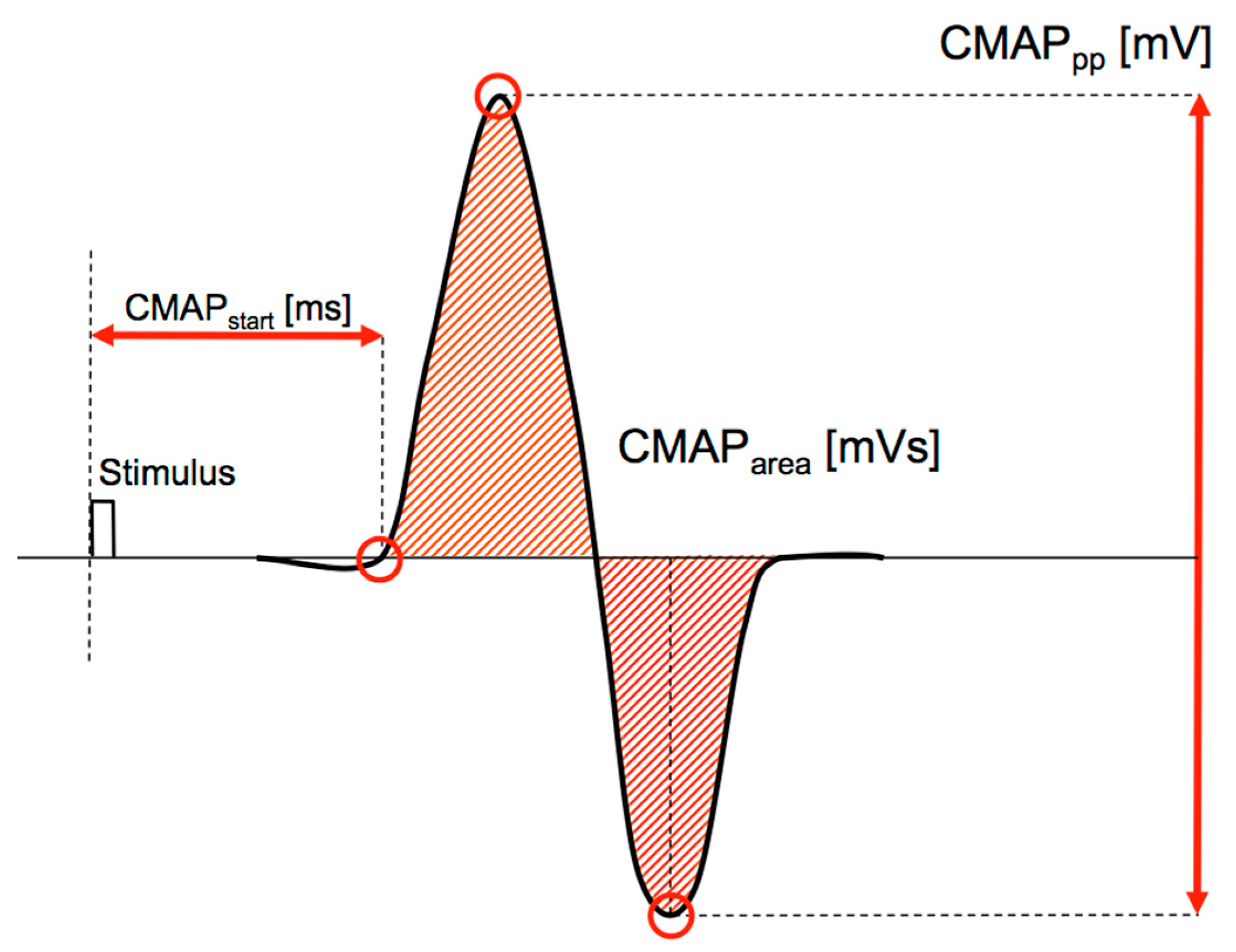



| Filter | MEParea | MEPpp | MEPstart |
|---|---|---|---|
| HPF 1 Hz—1. Ord | *** −1.94 | *** −0.53 | ns 0.80 |
| HPF 20 Hz—1. Ord | *** 17.24 | ns 3.10 | ns −3.81 |
| HPF 40 Hz—1. Ord | *** 32.23 | *** 15.09 | ns −3.88 |
| HPF 80 Hz—1. Ord | *** 51.25 | *** 35.51 | ns −7.87 |
| HPF 5 Hz—1. Ord | ns −0.50 | ** −1.46 | ns −4.26 |
| HPF 5 Hz—2. Ord | ns −2.65 | ** −2.58 | ns −1.73 |
| HPF 5 Hz—4. Ord | ns −1.34 | * −3.17 | ns −1.63 |
| HPF 5 Hz—8. Ord | ** −7.40 | ns −1.10 | ns −4.19 |
| High-Pass | Filter 1.Ord | ||||
|---|---|---|---|---|---|
| Parameter | Raw Data | 1 Hz | 20 Hz | 40 Hz | 80 Hz |
| MEPstart (ms) | 14.40 ± 2.62 | 14.29 ± 2.82 | 14.95 ± 2.73 | 14.96 ± 2.72 | 15.54 ± 2.21 |
| MEPpp (mV) | 1.80 ± 0.51 | 1.81 ± 0.52 | 1.74 ± 0.50 | 1.52 ± 0.47 | 1.16 ± 0.38 |
| MEParea (mVs) | 10.35 ± 2.70 | 10.55 ± 2.69 | 8.56 ± 2.13 | 7.01 ± 1.75 | 5.04 ± 1.31 |
| 5 Hz High-Pass Filter | |||||
| Parameter | Raw Data | 1.Order | 2.Order | 4.Order | 8.Order |
| MEPstart (ms) | 14.40 ± 2.62 | 15.02 ± 2.45 | 14.95 ± 2.73 | 14.64± 2.78 | 15.51 ± 2.71 |
| MEPpp (mV) | 1.80 ± 0.51 | 1.82 ± 0.51 | 1.84 ± 0.51 | 1.85 ± 0.52 | 1.82 ± 0.53 |
| MEParea (mVs) | 10.35 ± 2.70 | 10.40 ± 2.58 | 10.62 ± 2.64 | 10.49 ± 2.62 | 11.12 ± 2.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zschorlich, V.R.; Qi, F.; Wolff, N. Comparing Different Filter-Parameter for Pre-Processing of Brain-Stimulation Evoked Motor Potentials. Brain Sci. 2021, 11, 1118. https://doi.org/10.3390/brainsci11091118
Zschorlich VR, Qi F, Wolff N. Comparing Different Filter-Parameter for Pre-Processing of Brain-Stimulation Evoked Motor Potentials. Brain Sciences. 2021; 11(9):1118. https://doi.org/10.3390/brainsci11091118
Chicago/Turabian StyleZschorlich, Volker R., Fengxue Qi, and Norbert Wolff. 2021. "Comparing Different Filter-Parameter for Pre-Processing of Brain-Stimulation Evoked Motor Potentials" Brain Sciences 11, no. 9: 1118. https://doi.org/10.3390/brainsci11091118







