Serotonin Transporter Gene Polymorphisms and Maternal Overprotection Regulate Adult Social Expectations on Close Relationships
Abstract
:1. Introduction
1.1. Parental Bonding and Adult Attachment across Human Development
1.2. Genotype 5HTTLPR and Attachment: Effects on Human Development
1.3. Aim and Hypothesis
2. Methods
2.1. Participants
2.2. Data Collection
2.3. Parental Bonding
2.4. Close Relationship
2.5. Genetic Assessment
2.6. Statistical Analysis
3. Results
3.1. Preliminary Analysis: Age and Sex
3.2. Correlational Analysis: PBI and ECR-R Subscales
3.3. Univariate and Multivariate Analysis: Genotype-by-Environment Interactions on ECR-R Subscales
3.4. Post-Hoc Analysis: Effects of Genotype and Maternal Overprotection on Avoidance
4. Discussion
5. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HTTLPR | Serotonin Transporter linked polymorphic region |
| ECR-R | Experience in Close Relationship-Revised |
| MMLM | Multivariate Mixed Linear Model |
| PBI | Parental Bonding Instrument |
References
- Parke, R.D.; Roisman, G.I.; Rose, A.J. Social Development; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Morris, T.L. Social development. In Anxiety Disorders in Children and Adolescents; Cambridge University Press: Cambridge, UK, 2004; pp. 59–70. [Google Scholar]
- Feldman, R. Parent–infant synchrony: A biobehavioral model of mutual influences in the formation of affiliative bonds. Monogr. Soc. Res. Child Dev. 2012, 77, 42–51. [Google Scholar] [CrossRef]
- Ebstein, R.P.; Israel, S.; Chew, S.H.; Zhong, S.; Knafo, A. Genetics of human social behavior. Neuron 2010, 65, 831–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, J.E.; Lorberbaum, J.P.; Kose, S.; Strathearn, L. Brain basis of early parent–infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. J. Child Psychol. Psychiatry 2007, 48, 262–287. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.S. Biological perspectives on social attachment and bonding. In Attachment and Bonding: A New Synthesis; MIT Press: Cambridge, MA, USA, 2005; pp. 85–100. [Google Scholar]
- Bowlby, J. Attachment and loss: Volume II: Separation, anxiety and anger. In Attachment and Loss: Volume II: Separation, Anxiety and Anger; The Hogarth Press and the Institute of Psycho-Analysis: London, UK, 1973; pp. 1–429. [Google Scholar]
- Parker, G.; Tupling, H.; Brown, L.B. A parental bonding instrument. Br. J. Med. Psychol. 1979, 52, 1–10. [Google Scholar] [CrossRef]
- Hazan, C.; Shaver, P. Romantic love conceptualized as an attachment process. J. Personal. Soc. Psychol. 1987, 52, 511. [Google Scholar] [CrossRef]
- Shaver, P.R.; Mikulincer, M. Attachment-related psychodynamics. Attach. Hum. Dev. 2002, 4, 133–161. [Google Scholar] [CrossRef]
- Mikulincer, M.; Shaver, P.R. Adult attachment and affect regulation. In Handbook of Attachment: Theory, Research, and Clinical Applications; Guilford Press: New York, NY, USA, 2008; pp. 503–531. [Google Scholar]
- De Wolff, M.S.; Van Ijzendoorn, M.H. Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Dev. 1997, 68, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, I.; Azhari, A.; Lepri, B.; Esposito, G. Oxytocin receptors (OXTR) and early parental care: An interaction that modulates psychiatric disorders. Res. Dev. Disabil. 2018, 82, 27–38. [Google Scholar] [CrossRef]
- Mikulincer, M.; Shaver, P.R.; Pereg, D. Attachment theory and affect regulation: The dynamics, development, and cognitive consequences of attachment-related strategies. Motiv. Emot. 2003, 27, 77–102. [Google Scholar] [CrossRef]
- Brumariu, L.E. Parent–child attachment and emotion regulation. New Dir. Child Adolesc. Dev. 2015, 2015, 31–45. [Google Scholar] [CrossRef]
- Shaver, P.R.; Mikulincer, M. Adult attachment and emotion regulation. In Handbook of Emotion Regulation; Guilford Press: New York, NY, USA, 2014; pp. 237–250. [Google Scholar]
- Cataldo, I.; Neoh, M.J.Y.; Chew, W.F.; Foo, J.N.; Lepri, B.; Esposito, G. Oxytocin receptor gene and parental bonding modulate prefrontal responses to cries: A nirs study. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rork, K.E.; Morris, T.L. Influence of parenting factors on childhood social anxiety: Direct observation of parental warmth and control. Child Fam. Behav. Ther. 2009, 31, 220–235. [Google Scholar] [CrossRef]
- Zafiropoulou, M.; Avagianou, P.A.; Vassiliadou, S. Parental bonding and early maladaptive schemas. J. Psychol. Abnorm. Child. 2014, 3, 1000110. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, I.; Bonassi, A.; Lepri, B.; Foo, J.N.; Setoh, P.; Esposito, G. Recalled parental bonding interacts with oxytocin receptor gene polymorphism in modulating anxiety and avoidance in adult relationships. Brain Sci. 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Riskind, J.H.; Williams, N.L.; Altman, M.D.; Black, D.O.; Balaban, M.S.; Gessner, T.L. Developmental antecedents of the looming maladaptive style: Parental bonding and parental attachment insecurity. J. Cogn. Psychother. 2004, 18, 43–52. [Google Scholar] [CrossRef]
- Sun, Q.W.; Ng, K.M.; Guo, L. The link between parental bonding and adult attachment in Chinese graduate students: Gender differences. Fam. J. 2010, 18, 386–394. [Google Scholar] [CrossRef]
- Canli, T.; Lesch, K.P. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007, 10, 1103–1109. [Google Scholar] [CrossRef]
- Kobiella, A.; Reimold, M.; Ulshöfer, D.; Ikonomidou, V.; Vollmert, C.; Vollstädt-Klein, S.; Rietschel, M.; Reischl, G.; Heinz, A.; Smolka, M. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: The roles of in vivo serotonin transporter expression and amygdala structure. Transl. Psychiatry 2011, 1, e37. [Google Scholar] [CrossRef] [Green Version]
- Senese, V.P.; Shinohara, K.; Esposito, G.; Doi, H.; Venuti, P.; Bornstein, M.H. Implicit association to infant faces: Genetics, early care experiences, and cultural factors influence caregiving propensities. Behav. Brain Res. 2017, 325, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Hariri, A.R.; Mattay, V.S.; Tessitore, A.; Kolachana, B.; Fera, F.; Goldman, D.; Egan, M.F.; Weinberger, D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science 2002, 297, 400–403. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, R.W.; Tomko, R.L.; Trull, T.J.; Boomsma, D.I. Gene-environment studies and borderline personality disorder: A review. Curr. Psychiatry Rep. 2013, 15, 336. [Google Scholar] [CrossRef] [Green Version]
- Beevers, C.G.; Scott, W.D.; McGeary, C.; McGeary, J.E. Negative cognitive response to a sad mood induction: Associations with polymorphisms of the serotonin transporter (5-HTTLPR) gene. Cogn. Emot. 2009, 23, 726–738. [Google Scholar] [CrossRef]
- Mileva-Seitz, V.; Kennedy, J.; Atkinson, L.; Steiner, M.; Levitan, R.; Matthews, S.G.; Meaney, M.J.; Sokolowski, M.B.; Fleming, A.S. Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants. Genes Brain Behav. 2011, 10, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cheon, B.K.; Livingston, R.W.; Hong, Y.Y.; Chiao, J.Y. Gene × environment interaction on intergroup bias: The role of 5-HTTLPR and perceived outgroup threat. Soc. Cogn. Affect. Neurosci. 2014, 9, 1268–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senese, V.P.; Azhari, A.; Shinohara, K.; Doi, H.; Venuti, P.; Bornstein, M.H.; Esposito, G. Implicit associations to infant cry: Genetics and early care experiences influence caregiving propensities. Horm. Behav. 2019, 108, 1–9. [Google Scholar] [CrossRef]
- Truzzi, A.; Bornstein, M.H.; Senese, V.P.; Shinohara, K.; Setoh, P.; Esposito, G. Serotonin transporter gene polymorphisms and early parent-infant interactions are related to adult male heart rate response to female crying. Front. Physiol. 2017, 8, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonassi, A.; Cataldo, I.; Gabrieli, G.; Tandiono, M.; Foo, J.N.; Lepri, B.; Esposito, G. The interaction between serotonin transporter allelic variation and maternal care modulates sociability on Instagram. PsyArXiv 2020. [Google Scholar] [CrossRef]
- Fraley, R.C.; Shaver, P.R. Adult romantic attachment: Theoretical developments, emerging controversies, and unanswered questions. Rev. Gen. Psychol. 2000, 4, 132–154. [Google Scholar] [CrossRef]
- Dalsant, A.; Truzzi, A.; Setoh, P.; Esposito, G. Maternal bonding in childhood moderates autonomic responses to distress stimuli in adult males. Behav. Brain Res. 2015, 292, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Truzzi, A.; Setoh, P.; Putnick, D.L.; Shinohara, K.; Bornstein, M.H. Genetic predispositions and parental bonding interact to shape adults’ physiological responses to social distress. Behav. Brain Res. 2017, 325, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Bonassi, A.; Ghilardi, T.; Truzzi, A.; Cataldo, I.; Azhari, A.; Setoh, P.; Shinohara, K.; Esposito, G. Dataset on genetic and physiological adults’ responses to social distress. Data Brief 2017, 13, 742–748. [Google Scholar] [CrossRef]
- Truzzi, A.; Poquérusse, J.; Setoh, P.; Shinohara, K.; Bornstein, M.H.; Esposito, G. Oxytocin receptor gene polymorphisms (rs53576) and early paternal care sensitize males to distressing female vocalizations. Dev. Psychobiol. 2018, 60, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Parker, G. Parental Overprotection: A Risk Factor in Psychosocial Development; Grune & Stratton, Incorporated: Philadelphia, PA, USA, 1983. [Google Scholar]
- Manassis, K.; Owens, M.; Adam, K.S.; West, M.; Sheldon-Keller, A.E. Assessing attachment: Convergent validity of the adult attachment interview and the parental bonding instrument. Aust. N. Z. J. Psychiatry 1999, 33, 559–567. [Google Scholar] [CrossRef]
- Fraley, R.C.; Waller, N.G.; Brennan, K.A. An item response theory analysis of self-report measures of adult attachment. J. Personal. Soc. Psychol. 2000, 78, 350. [Google Scholar] [CrossRef]
- Sibley, C.G.; Fischer, R.; Liu, J.H. Reliability and validity of the revised experiences in close relationships (ECR-R) self-report measure of adult romantic attachment. Personal. Soc. Psychol. Bull. 2005, 31, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Dimitriadou, E.; Hornik, K.; Weingessel, A.; Leisch, F.; Chang, C.C.; Lin, C.C.; Meyer, M.D. Package ‘e1071’. R J. 2019. Available online: sunsite2.icm.edu.pl/pub/unix/math/cran/web/packages/e1071/e1071.pdf (accessed on 19 August 2021).
- Revelle, W.; Revelle, M.W. Package ‘psych’. Compr. R Arch. Netw. 2015, 337, 338. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S.; Ullman, J.B. Using Multivariate Statistics; Pearson: Boston, MA, USA, 2007; Volume 5. [Google Scholar]
- Harrell, F.E., Jr.; Harrell, M.F.E., Jr. Package ‘hmisc’. CRAN2018 2019, 2019, 235–236. [Google Scholar]
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Bovy, G.; Ellison, S.; Firth, D.; Friendly, M.; Gorjanc, G.; Graves, S.; et al. Package ‘Car’; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: Berlin/Heidelberg, Germany, 2013; Volume 112. [Google Scholar]
- Bruce, P.; Bruce, A.; Gedeck, P. Practical Statistics for Data Scientists: 50+ Essential Concepts Using R and Python; O’Reilly Media: Newton, MA, USA, 2020. [Google Scholar]
- Fox, J.; Friendly, M.; Weisberg, S. Hypothesis tests for multivariate linear models using the car package. R J. 2013, 5, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Rao, C.R.; Rao, C.R.; Statistiker, M.; Rao, C.R.; Rao, C.R. Linear Statistical Inference and Its Applications; Wiley: New York, NY, USA, 1973; Volume 2. [Google Scholar]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Creat. Elegant Data Vis. Using Gramm. Graph. Vers. 2016, 2, 1–189. [Google Scholar]
- Kelley, K. Methods for the behavioral, educational, and social sciences: An R package. Behav. Res. Methods 2020, 39, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, J.; Friendly, M.; Monette, G. Visualizing hypothesis tests in multivariate linear models: The heplots package for R. Comput. Stat. 2009, 24, 233–246. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Waters, E.; Hamilton, C.E.; Weinfield, N.S. The stability of attachment security from infancy to adolescence and early adulthood: General introduction. Child Dev. 2000, 71, 678–683. [Google Scholar] [CrossRef]
- Lee Raby, K.; Cicchetti, D.; Carlson, E.A.; Egeland, B.; Andrew Collins, W. Genetic contributions to continuity and change in attachment security: A prospective, longitudinal investigation from infancy to young adulthood. J. Child Psychol. Psychiatry 2013, 54, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Waters, T.E.; Raby, K.L.; Ruiz, S.K.; Martin, J.; Roisman, G.I. Adult attachment representations and the quality of romantic and parent–child relationships: An examination of the contributions of coherence of discourse and secure base script knowledge. Dev. Psychol. 2018, 54, 2371. [Google Scholar] [CrossRef]
- Zayas, V.; Mischel, W.; Shoda, Y.; Aber, J.L. Roots of adult attachment: Maternal caregiving at 18 months predicts adult peer and partner attachment. Soc. Psychol. Personal. Sci. 2011, 2, 289–297. [Google Scholar] [CrossRef]
- Heshmati, R.; Zemestani, M.; Vujanovic, A. Associations of childhood maltreatment and attachment styles with romantic breakup grief severity: The role of emotional suppression. J. Interpers. Violence 2021, 1–22. [Google Scholar] [CrossRef]
- Crowell, J.; Waters, E.; Grossmann, K. Attachment from Infancy to Adulthood: The Major Longitudinal Studies; Guilford Publications: New York, NY, USA, 2005. [Google Scholar]
- Haydon, K.C.; Collins, W.A.; Salvatore, J.E.; Simpson, J.A.; Roisman, G.I. Shared and distinctive origins and correlates of adult attachment representations: The developmental organization of romantic functioning. Child Dev. 2012, 83, 1689–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sroufe, L.A. Attachment and development: A prospective, longitudinal study from birth to adulthood. Attach. Hum. Dev. 2005, 7, 349–367. [Google Scholar] [CrossRef]
- Feeney, B.C.; Van Vleet, M. Growing through attachment: The interplay of attachment and exploration in adulthood. J. Soc. Pers. Relationsh. 2010, 27, 226–234. [Google Scholar] [CrossRef]
- Belsky, J.; Pluess, M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychol. Bull. 2009, 135, 885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspers, K.M.; Paradiso, S.; Yucuis, R.; Troutman, B.; Arndt, S.; Philibert, R. Association between the serotonin transporter promoter polymorphism (5-HTTLPR) and adult unresolved attachment. Dev. Psychol. 2009, 45, 64. [Google Scholar] [CrossRef] [Green Version]
- Reiner, I.; Spangler, G. Adult attachment and gene polymorphisms of the dopamine D4 receptor and serotonin transporter (5-HTT). Attach. Hum. Dev. 2010, 12, 209–229. [Google Scholar] [CrossRef]
- Bonassi, A.; Cataldo, I.; Tandiono, M.; Foo, J.; Lepri, B.; Esposito, G.P. 112 Effect of early paternal caregiving and genotype rs25531 polymorphisms on the adult relationship with the partner. Eur. Neuropsychopharmacol. 2021, 44 (Suppl. S1), S9–S10. [Google Scholar] [CrossRef]
- Feeney, J.A.; Passmore, N.L.; Peterson, C.C. Adoption, attachment, and relationship concerns: A study of adult adoptees. Pers. Relationsh. 2007, 14, 129–147. [Google Scholar] [CrossRef] [Green Version]
- Fraley, R.C.; Roisman, G.I.; Booth-LaForce, C.; Owen, M.T.; Holland, A.S. Interpersonal and genetic origins of adult attachment styles: A longitudinal study from infancy to early adulthood. J. Personal. Soc. Psychol. 2013, 104, 817. [Google Scholar] [CrossRef] [Green Version]
- Fraley, R.C.; Gillath, O.; Deboeck, P.R. Do life events lead to enduring changes in adult attachment styles? A naturalistic longitudinal investigation. J. Personal. Soc. Psychol. 2020, 120, 1567–1606. [Google Scholar] [CrossRef]
- Emery, J.; Paquette, D.; Bigras, M. Factors predicting attachment patterns in infants of adolescent mothers. J. Fam. Stud. 2008, 14, 65–90. [Google Scholar] [CrossRef]
- Gaffney, M.; Greene, S.M.; Wieczorek-Deering, D.; Nugent, J.K. The concordance between mother-infant attachment at 18 months and maternal attachment 10 years later among married and single mothers. Ir. J. Psychol. 2000, 21, 154–170. [Google Scholar] [CrossRef]
- Berthelot, N.; Ensink, K.; Bernazzani, O.; Normandin, L.; Luyten, P.; Fonagy, P. Intergenerational transmission of attachment in abused and neglected mothers: The role of trauma-specific reflective functioning. Infant Ment. Health J. 2015, 36, 200–212. [Google Scholar] [CrossRef] [Green Version]
- Verhage, M.L.; Schuengel, C.; Madigan, S.; Fearon, R.; Oosterman, M.; Cassibba, R.; Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H. Narrowing the transmission gap: A synthesis of three decades of research on intergenerational transmission of attachment. Psychol. Bull. 2016, 142, 337. [Google Scholar] [CrossRef] [Green Version]
- van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. Bridges across the intergenerational transmission of attachment gap. Curr. Opin. Psychol. 2019, 25, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberstick, B.C.; Smolen, A.; Williams, R.B.; Bishop, G.D.; Foshee, V.A.; Thornberry, T.P.; Conger, R.; Siegler, I.C.; Zhang, X.; Boardman, J.D.; et al. Population frequencies of the triallelic 5HTTLPR in six ethnicially diverse samples from North America, Southeast Asia, and Africa. Behav. Genet. 2015, 45, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Ravitz, P.; Maunder, R.; Hunter, J.; Sthankiya, B.; Lancee, W. Adult attachment measures: A 25-year review. J. Psychosom. Res. 2010, 69, 419–432. [Google Scholar] [CrossRef]
- Fraiberg, S.; Edelson, E.; Shapiro, V. Ghosts in the nursery: A psychoanalytic approach to the problems of impaired infant-mother relationships. J. Am. Acad. Child Psychiatry 1976, 14, 387–421. [Google Scholar] [CrossRef]
- Savelieva, K.; Pulkki-Råback, L.; Jokela, M.; Hintsanen, M.; Merjonen, P.; Hutri-Kähönen, N.; Juonala, M.; Viikari, J.; Raitakari, O.; Keltikangas-Järvinen, L. Intergenerational continuity in qualities of the parent–child relationship: Mediating and moderating mechanisms. J. Child Fam. Stud. 2017, 26, 2191–2201. [Google Scholar] [CrossRef]

| Dimension | Min | Median | Mean | Max | SD | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|
| Maternal Care | 4.00 | 27.00 | 25.97 | 36.00 | 8.33 | −0.95 | 0.11 |
| Maternal Overprotection | 7.00 | 17.00 | 17.45 | 34.00 | 6.00 | 0.66 | 0.17 |
| Paternal Care | 1.00 | 25.00 | 22.91 | 36.00 | 9.44 | −0.51 | −0.63 |
| Paternal Overprotection | 3.00 | 15.00 | 15.51 | 33.00 | 7.36 | 0.66 | −0.19 |
| Anxiety | 21.00 | 53.00 | 56.40 | 116.00 | 20.19 | 0.72 | 0.02 |
| Avoidance | 6.00 | 38.00 | 36.14 | 46.00 | 73.00 | 0.18 | −0.65 |
| Subscale | Maternal Care | Maternal Overp | Paternal Care | Paternal Overp | Anxiety | Avoidance |
|---|---|---|---|---|---|---|
| Maternal Care | −0.26 | 0.59 **** | 0.02 | −0.44 *** | −0.22 | |
| Maternal Overp | −0.23 | 0.36 ** | 0.46 *** | 0.29 * | ||
| Paternal Care | −0.29 * | −0.39 ** | −0.23 | |||
| Paternal Overp | 0.14 | 0.08 | ||||
| Anxiety | 0.43 ** | |||||
| Avoidance |
| ECR-R dimension | L | S/S |
|---|---|---|
| Anxiety | 57.27 (3.01) | 54.57 (4.59) |
| Avoidance | 36.02 (2.49) | 36.38 (3.75) |
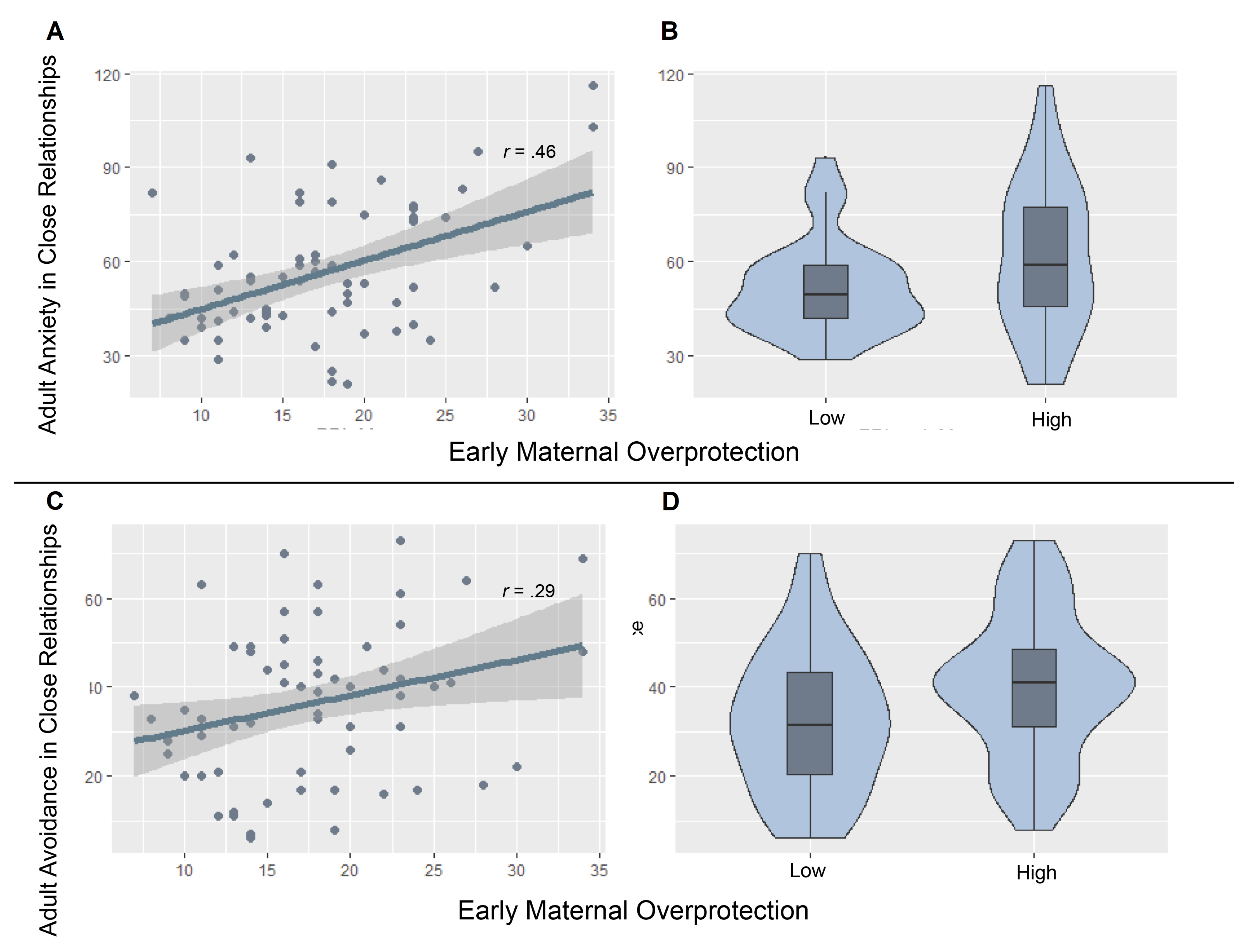
| ECR-R Dimension | Low Maternal Overprotection | High Maternal Overprotection |
| Anxiety | 51.85 (2.56) | 61.39 (4.31) |
| Avoidance | 32.38 (2.76) | 40.26 (2.95) |
| ECR-R Anxiety | ||||
|---|---|---|---|---|
| PBI Dimension | Low/L | Low/SS | High/L | High/SS |
| Maternal Care | 65.00 (4.47) | 62.73 (6.98) | 49.55 (3.37) | 45.60 (4.68) |
| Maternal Overprotection | 53.14 (3.32) | 49.50 (4.06) | 61.41 (4.94) | 61.33 (9.10) |
| Paternal Care | 64.38 (4.16) | 59.23 (6.74) | 48.75 (3.57) | 47.00 (4.32) |
| Paternal Overprotection | 55.00 (3.89) | 53.36 (7.79) | 59.55 (4.63) | 55.90 (4.89) |
| ECR-R Avoidance | ||||
| PBI Dimension | Low/L | Low/SS | High/L | High/SS |
| Maternal Care | 41.14 (3.59) | 42.46 (4.99) | 30.91 (3.17) | 29.07 (5.06) |
| Maternal Overprotection | 31.96 (3.50) | 33.17 (4.67) | 40.09 (3.41) | 40.67 (6.16) |
| Paternal Care | 38.33 (3.76) | 39.77 (4.83) | 33.25 (3.10) | 30.88 (5.76) |
| Paternal Overprotection | 36.14 (3.14) | 38.00 (5.66) | 35.91 (3.94) | 34.60 (5.06) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonassi, A.; Cataldo, I.; Gabrieli, G.; Lepri, B.; Esposito, G. Serotonin Transporter Gene Polymorphisms and Maternal Overprotection Regulate Adult Social Expectations on Close Relationships. Brain Sci. 2021, 11, 1123. https://doi.org/10.3390/brainsci11091123
Bonassi A, Cataldo I, Gabrieli G, Lepri B, Esposito G. Serotonin Transporter Gene Polymorphisms and Maternal Overprotection Regulate Adult Social Expectations on Close Relationships. Brain Sciences. 2021; 11(9):1123. https://doi.org/10.3390/brainsci11091123
Chicago/Turabian StyleBonassi, Andrea, Ilaria Cataldo, Giulio Gabrieli, Bruno Lepri, and Gianluca Esposito. 2021. "Serotonin Transporter Gene Polymorphisms and Maternal Overprotection Regulate Adult Social Expectations on Close Relationships" Brain Sciences 11, no. 9: 1123. https://doi.org/10.3390/brainsci11091123
APA StyleBonassi, A., Cataldo, I., Gabrieli, G., Lepri, B., & Esposito, G. (2021). Serotonin Transporter Gene Polymorphisms and Maternal Overprotection Regulate Adult Social Expectations on Close Relationships. Brain Sciences, 11(9), 1123. https://doi.org/10.3390/brainsci11091123







