Association between Self-Reported Pain, Cognition, and Neuropathology in Older Adults Admitted to an Outpatient Memory Clinic—A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection and Outcome Measures
2.3. Data Analysis
3. Results
3.1. Demographics and General Health
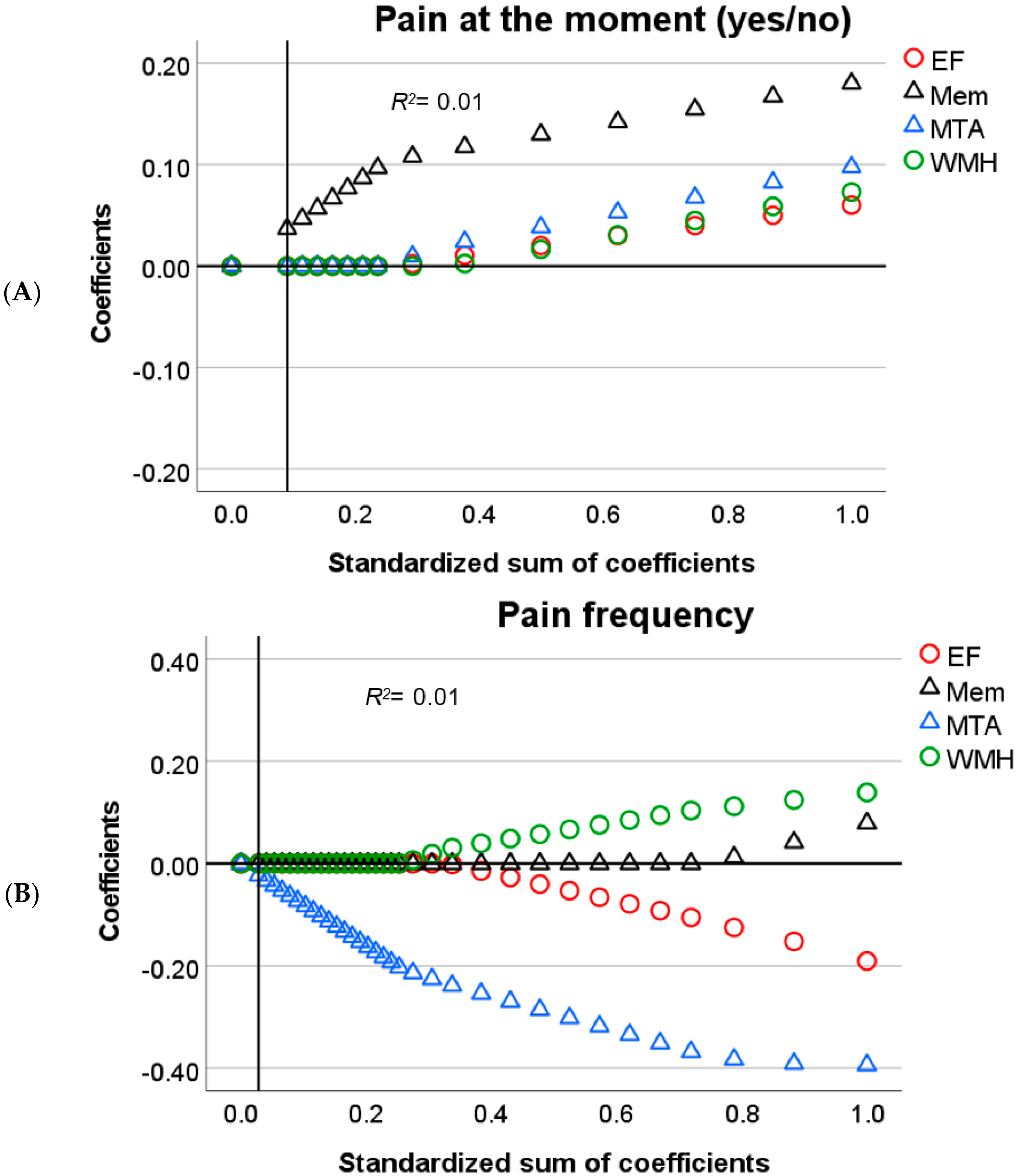
3.2. Predictors of Pain in Cognitive Decline
3.3. Predictors of Depression-Related Complaints
3.4. Pain Profile of Patients at Memory Clinic
3.5. Relation between Cognitive and Neuropathological Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binnekade, T.T.; Scherder, E.J.A.; Maier, A.B.; Lobbezoo, F.; Overdorp, E.J.; Rhebergen, D.; Perez, R.S.G.M.; Oosterman, J.M. Pain in patients with different dementia subtypes, mild cognitive impairment, and subjective cognitive impairment. Pain Med. 2018, 19, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Arduino, C.; Vighetti, S.; Asteggiano, G.; Tarenzi, L.; Rainero, I. Pain reactivity in Alzheimer patients with different degrees of cognitive impairment and brain electrical activity deterioration. Pain 2004, 111, 22–29. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, R.M.; Verma, S.; Mossey, J. Chronic pain: Sources of late-life pain and risk factors for disability. Geriatrics 2000, 55, 40–47. [Google Scholar]
- Scherder, E.; Oosterman, J.; Swaab, D.; Herr, K.; Ooms, M.; Ribbe, M.; Sergeant, J.; Pickering, G.; Benedetti, F. Recent developments in pain in dementia. Br. Med. J. 2005, 330, 461–464. [Google Scholar] [CrossRef] [Green Version]
- Van Kooten, J.; Binnekade, T.T.; Van Der Wouden, J.C.; Stek, M.L.; Scherder, E.J.A.; Husebø, B.S.; Smalbrugge, M.; Hertogh, C.M.P.M. A review of pain prevalence in Alzheimer’s, vascular, frontotemporal and lewy body dementias. Dement. Geriatr. Cogn. Disord. 2016, 41, 220–232. [Google Scholar] [CrossRef]
- Defrin, R.; Amanzio, M.; De Tommaso, M.; Dimova, V.; Filipovic, S.; Finn, D.P.; Gimenez-Llort, L.; Invitto, S.; Jensen-Dahm, C.; Lautenbacher, S.; et al. Experimental pain processing in individuals with cognitive impairment: Current state of the science. Pain 2015, 156, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Scherder, E.J.A.; Plooij, B.; Achterberg, W.P.; Pieper, M.; Wiegersma, M.; Lobbezoo, F.; Oosterman, J.M. Chronic pain in “probable” vascular dementia: Preliminary findings. Pain Med. 2015, 16, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Oosterman, J.M.; De Vries, K.; Dijkerman, H.C.; De Haan, E.H.F.; Scherder, E.J.A. Exploring the relationship between cognition and self-reported pain in residents of homes for the elderly. Int. Psychogeriatr. 2009, 21, 157–163. [Google Scholar] [CrossRef]
- Oosterman, J.M.; Gibson, S.J.; Pulles, W.L.J.A.; Veldhuijzen, D.S. On the moderating role of age in the relationship between pain and cognition. Eur. J. Pain 2013, 17, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherder, E.J.A.; Eggermont, L.; Plooij, B.; Oudshoorn, J.; Vuijk, P.J.; Pickering, G.; Lautenbacher, S.; Achterberg, W.; Oosterman, J. Relationship between chronic pain and cognition in cognitively intact older persons and in patients with Alzheimer’s disease: The need to control for mood. Gerontology 2008, 54, 50–58. [Google Scholar] [CrossRef]
- Reed, B.R.; Mungas, D.M.; Kramer, J.H.; Ellis, W.; Vinters, H.V.; Zarow, C.; Jagust, W.J.; Chui, H.C. Profiles of neuropsychological impairment in autopsy-defined Alzheimer’s disease and cerebrovascular disease. Brain 2007, 130, 731–739. [Google Scholar] [CrossRef]
- Kunz, M.; Mylius, V.; Schepelmann, K.; Lautenbacher, S. Loss in executive functioning best explains changes in pain responsiveness in patients with dementia-related cognitive decline. Behav. Neurol. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Oosterman, J.M.; Traxler, J.; Kunz, M. The Influence of Executive Functioning on Facial and Subjective Pain Responses in Older Adults. Behav. Neurol. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Overdorp, E.J.; Kessels, R.P.C.; Claassen, J.A.; Oosterman, J.M. Cognitive impairments associated with medial temporal atrophy and white matter hyperintensities: An MRI study in memory clinic patients. Front. Aging Neurosci. 2014, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.M.; van Harten, B.; Weinstein, H.C.; Scheltens, P.; Scherder, E.J.A. Pain intensity and pain affect in relation to white matter changes. Pain 2006, 125, 74–81. [Google Scholar] [CrossRef]
- Binnekade, T.T.; Perez, R.S.G.M.; Maier, A.B.; Rhodius-Meester, H.F.M.; Legdeur, N.; Trappenburg, M.C.; Rhebergen, D.; Lobbezoo, F.; Scherder, E.J.A. White matter hyperintensities are related to pain intensity in an outpatient memory clinic population: Preliminary findings. J. Pain Res. 2019, 12, 1621–1629. [Google Scholar] [CrossRef] [Green Version]
- Oosterman, J.M.; Dijkerman, H.C.; Kessels, R.P.C.; Scherder, E.J.A. A unique association between cognitive inhibition and pain sensitivity in healthy participants. Eur. J. Pain 2010, 14, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Bunk, S.; Emch, M.; Koch, K.; Lautenbacher, S.; Zuidema, S.; Kunz, M. Pain processing in older adults and its association with prefrontal characteristics. Brain Sci. 2020, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.M.; Hendriks, H.; Scott, S.; Lord, K.; White, N.; Sampson, E.L. When pain memories are lost: A pilot study of semantic knowledge of pain in dementia. Pain Med. 2014, 15, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Parks, C.M.; Iosif, A.M.; Farias, S.; Reed, B.; Mungas, D.; DeCarli, C. Executive function mediates effects of white matter hyperintensities on episodic memory. Neuropsychologia 2011, 49, 2817–2824. [Google Scholar] [CrossRef] [Green Version]
- van Toutert, M.; Diesfeldt, H.; Hoek, D. De Amsterdamse Dementie-Screeningstest (ADS) bij ouderen zonder neurocognitieve stoornis. Implicaties voor de klinische praktijk. Tijdschr. Gerontol. Geriatr. 2016, 47, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A frontal assessment battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Diesfeldt, H.F.A. De Cognitieve Screening Test (CST) getoetst aan het schaalmodel van Mokken. Tijdschr. Gerontol. Geriatr. 2009, 40, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Lindeboom, J.; Schmand, B.; Tulner, L.; Walstra, G.; Jonker, C. Visual association test to detect early dementia of the Alzheimer type. J. Neurol. Neurosurg. Psychiatry 2002, 73, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Park, H.; Sohn, S.W.; Kim, S.; Park, K.W. Memory performance on the story recall test and prediction of cognitive dysfunction progression in mild cognitive impairment and Alzheimer’s dementia. Geriatr. Gerontol. Int. 2017, 17, 1603–1609. [Google Scholar] [CrossRef]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15, 17–24. [Google Scholar] [CrossRef]
- Chibnall, J.T.; Tait, R.C. Pain assessment in cognitively impaired and unimpaired older adults: A comparison of four scales. Pain 2001, 92, 173–186. [Google Scholar] [CrossRef]
- Kang, Y.; Demiris, G. Self-report pain assessment tools for cognitively intact older adults: Integrative review. Int. J. Older People Nurs. 2018, 13, e12170. [Google Scholar] [CrossRef] [PubMed]
- Conradsson, M.; Rosendahla, E.; Littbranda, H.; Gustafson, Y.; Olofsson, B.; Lövheim, H. Usefulness of the Geriatric Depression Scale 15-item version among very old people with and without cognitive impairment. Aging Ment. Health 2013, 17, 638–645. [Google Scholar] [CrossRef]
- Scheltens, P.; Kuiper, M.; Ch Wolters, E.; Barkhof, F.; Valk, J.; Weinsten, H.C.; Leys, D.; Vermersch, P.; Huglo, D.; Steinling, M. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry 1992, 55, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosterman, J.M.; Oosterveld, S.; Rikkert, M.G.O.; Claassen, J.A.; Kessels, R.P.C. Medial temporal lobe atrophy relates to executive dysfunction in Alzheimer’s disease. Int. Psychogeriatr. 2012, 24, 1474–1482. [Google Scholar] [CrossRef]
- IBM Knowledge Center Modified z Score. Available online: https://www.ibm.com/docs/en/cognos-analytics/11.1.0?topic=terms-modified-z-score (accessed on 28 September 2020).
- Van Der Kooij, A.J. Prediction Accuracy and Stability of Regression with Optimal Scaling Transformations; Leiden University: Leiden, The Netherlands, 2007. [Google Scholar]
- Welmer, A.K.; Rizzuto, D.; Calderón-Larrañaga, A.; Johnell, K. Sex Differences in the Association between Pain and Injurious Falls in Older Adults: A Population-Based Longitudinal Study. Am. J. Epidemiol. 2017, 186, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Geerlings, S.W.; Twisk, J.W.R.; Beekman, A.T.F.; Deeg, D.J.H.; van Tilburg, W. Longitudinal relationship between pain and depression in older adults: Sex, age and physical disability. Soc. Psychiatry Psychiatr. Epidemiol. 2002, 37, 23–30. [Google Scholar] [CrossRef]
- Brazil, I.A.; Hunt, L.T.; Bulten, B.H.; Kessels, R.P.C.; de Bruijn, E.R.A.; Mars, R.B. Psychopathy-related traits and the use of reward and social information: A computational approach. Front. Psychol. 2013, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Smeijers, D.; Brazil, I.A.; Bulten, E.B.H.; Verkes, R.J. Retrospective parental rejection is associated with aggressive behavior as well as cognitive distortions in forensic psychiatric outpatients. Psychol. Violence 2018, 8, 495–504. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Jantsch, H.H.F.; Gawlitza, M.; Geber, C.; Baumgärtner, U.; Krämer, H.H.; Magerl, W.; Treede, R.D.; Birklein, F. Explicit episodic memory for sensory-discriminative components of capsaicin-induced pain: Immediate and delayed ratings. Pain 2009, 143, 97–105. [Google Scholar] [CrossRef]
- Visser, P.J.; Scheltens, P.; Verhey, F.R.J.; Schmand, B.; Launer, L.J.; Jolles, J.; Jonker, C. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J. Neurol. 1999, 246, 477–485. [Google Scholar] [CrossRef]
- Visser, P.J.; Verhey, F.R.J.; Hofman, P.A.M.; Scheltens, P.; Jolles, J. Medial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2002, 72, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Bjekić, J.; Živanović, M.; Purić, D.; Oosterman, J.M.; Filipović, S.R. Pain and executive functions: A unique relationship between Stroop task and experimentally induced pain. Psychol. Res. 2018, 82, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Čeko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.K.; Freedman, D.J.; Wallis, J.D. The prefrontal cortex: Categories, concepts and cognition. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 1123–1136. [Google Scholar] [CrossRef] [Green Version]
- Salzman, C.D.; Fusi, S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu. Rev. Neurosci. 2010, 33, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Carlson, M.D.A.; Morrison, R.S. Study design, precision, and validity in observational studies. J. Palliat. Med. 2009, 12, 77–82. [Google Scholar] [CrossRef]
- Tsuang, M.T. Observational versus experimental studies: Would the results be similar? Psychosom. Med. 1999, 61, 146–147. [Google Scholar] [CrossRef]
- Monroe, T.B.; Herr, K.A.; Mion, L.C.; Cowan, R.L. Ethical and legal issues in pain research in cognitively impaired older adults. Int. J. Nurs. Stud. 2013, 50, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Low, L.A. The impact of pain upon cognition: What have rodent studies told us? Pain 2013, 154, 2603–2605. [Google Scholar] [CrossRef]
- Morogiello, J.M.; Murray, N.G.; Hunt, T.N.; Harris, B.S.; Szekely, B.J.; Shaver, G.W. The effect of acute pain on executive function. J. Clin. Transl. Res. 2018, 4, 113–121. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef] [Green Version]
- Berryman, C.; Stanton, T.R.; Bowering, K.J.; Tabor, A.; McFarlane, A.; Moseley, G.L. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin. Psychol. Rev. 2014, 34, 563–579. [Google Scholar] [CrossRef]
- Lieberman, G.; Shpaner, M.; Watts, R.; Andrews, T.; Filippi, C.G.; Davis, M.; Naylor, M.R. White Matter Involvement in Chronic Musculoskeletal Pain. J. Pain 2014, 15, 1110–1119. [Google Scholar] [CrossRef] [Green Version]
- Apkarian, A.V. The brain in chronic pain: Clinical implications. Pain Manag. 2011, 1, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjistavropoulos, T.; Fitzgerald, T.D.; Marchildon, G.P. Practice guidelines for assessing pain in older persons with dementia residing in long-term care facilities. Physiother. Can. 2010, 62, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Sirsch, E.; Lukas, A.; Drebenstedt, C.; Gnass, I.; Laekeman, M.; Kopke, K.; Fischer, T. Pain Assessment for Older Persons in Nursing Home Care: An Evidence-Based Practice Guideline. J. Am. Med. Dir. Assoc. 2020, 21, 149–163. [Google Scholar] [CrossRef]
- Krulewitch, H.; London, M.R.; Skakel, V.J.; Lundstedt, G.J.; Thomason, H.; Brummel-Smith, K. Assessment of pain in cognitively impaired older adults: A comparison of pain assessment tools and their use by nonprofessional caregivers. J. Am. Geriatr. Soc. 2000, 48, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; de Waal, M.W.M.; Achterberg, W.P.; Gimenez-Llort, L.; Lobbezoo, F.; Sampson, E.L.; van Dalen-Kok, A.H.; Defrin, R.; Invitto, S.; Konstantinovic, L.; et al. The Pain Assessment in Impaired Cognition scale (PAIC15): A multidisciplinary and international approach to develop and test a meta-tool for pain assessment in impaired cognition, especially dementia. Eur. J. Pain 2020, 24, 192–208. [Google Scholar] [CrossRef] [Green Version]
- Schofield, P. The assessment of pain in older people: UK national guidelines. Age Ageing 2018, 47, i1–i22. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Value |
|---|---|
| Demographics | |
| N | 179 (100%) |
| Women | 97 (54.2%) |
| Age | 78 (IQR 12) |
| Low educational level | 104 (58.1%) |
| Comorbidities | |
| Hypertension | 88 (49.2%) |
| Cerebrovascular disease | 37 (20.7%) |
| Diabetes Mellitus | 29 (16.2%) |
| Heart disease | 57 (31.8%) |
| Pain history | |
| Painful condition | 73 (40.8%) |
| Pain medication | 60 (33.5%) |
| GDS-15 (n = 172) | |
| Depressive symptoms | 2.0 (IQR 2.0) |
| Depression (score ≥ 6) | 15 (8.8%) |
| Cognitive diagnoses | |
| Alzheimer’s disease | 60 (33.5%) |
| Amnestic mild cognitive impairment | 45 (25.1%) |
| Subjective cognitive impairment | 22 (12.3%) |
| Other cognitive impairments: | 52 (29.1%) |
| Mixed dementia | 8 (4.5%) |
| Dementia | 4 (2.2%) |
| Parkinsonism | 4 (2.2%) |
| Stroke | 4 (2.2%) |
| Vascular dementia | 4 (2.2%) |
| Delirium | 3 (1.7%) |
| Frontotemporal dementia | 3 (1.7%) |
| Deferred diagnosis | 11 (6.1%) |
| Others (~1% each) | 11 (6.1%) |
| Pain | SCI | aMCI | AD | OCI | Statistical Tests |
|---|---|---|---|---|---|
| Pain occurrence n (%) | 11 (50.0) | 15 (33.0) | 19 (32.0) | 24 (46.0) | X2 = 4.20, p = 0.24 |
| Pain intensity NRS (0–10) Median (IQR) | 6.0 (4.0) | 6.0 (2.6) | 5.5 (4.3) | 6.8 (3.1) | H = 2.53, p = 0.47 |
| Pain severityVDS (0–4) Median (IQR) | 1.0 (1.3) | 1.0 (1.0) | 1.0 (2.0) | 2.0 (2.0) | H = 1.59, p = 0.66 |
| Pain frequency (1–4) Median (IQR) | 3.0 (2.0) | 1.0 (2.0) | 3.0 (2.0) | 3.0 (2.8) | H = 3.93, p = 0.27 |
| Pain locations (1–6) Median (IQR) | 1.0 (2.0) | 1.0 (0.3) | 1.0 (0.5) | 1.0 (1.0) | H = 2.67, p = 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madariaga, V.I.; Overdorp, E.; Claassen, J.A.H.R.; Brazil, I.A.; Oosterman, J.M. Association between Self-Reported Pain, Cognition, and Neuropathology in Older Adults Admitted to an Outpatient Memory Clinic—A Cross-Sectional Study. Brain Sci. 2021, 11, 1156. https://doi.org/10.3390/brainsci11091156
Madariaga VI, Overdorp E, Claassen JAHR, Brazil IA, Oosterman JM. Association between Self-Reported Pain, Cognition, and Neuropathology in Older Adults Admitted to an Outpatient Memory Clinic—A Cross-Sectional Study. Brain Sciences. 2021; 11(9):1156. https://doi.org/10.3390/brainsci11091156
Chicago/Turabian StyleMadariaga, Víctor I., Eduard Overdorp, Jurgen A. H. R. Claassen, Inti A. Brazil, and Joukje M. Oosterman. 2021. "Association between Self-Reported Pain, Cognition, and Neuropathology in Older Adults Admitted to an Outpatient Memory Clinic—A Cross-Sectional Study" Brain Sciences 11, no. 9: 1156. https://doi.org/10.3390/brainsci11091156
APA StyleMadariaga, V. I., Overdorp, E., Claassen, J. A. H. R., Brazil, I. A., & Oosterman, J. M. (2021). Association between Self-Reported Pain, Cognition, and Neuropathology in Older Adults Admitted to an Outpatient Memory Clinic—A Cross-Sectional Study. Brain Sciences, 11(9), 1156. https://doi.org/10.3390/brainsci11091156






