Relationship between Cortical Thickness and EEG Alterations during Sleep in the Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Polysomnographic Recordings
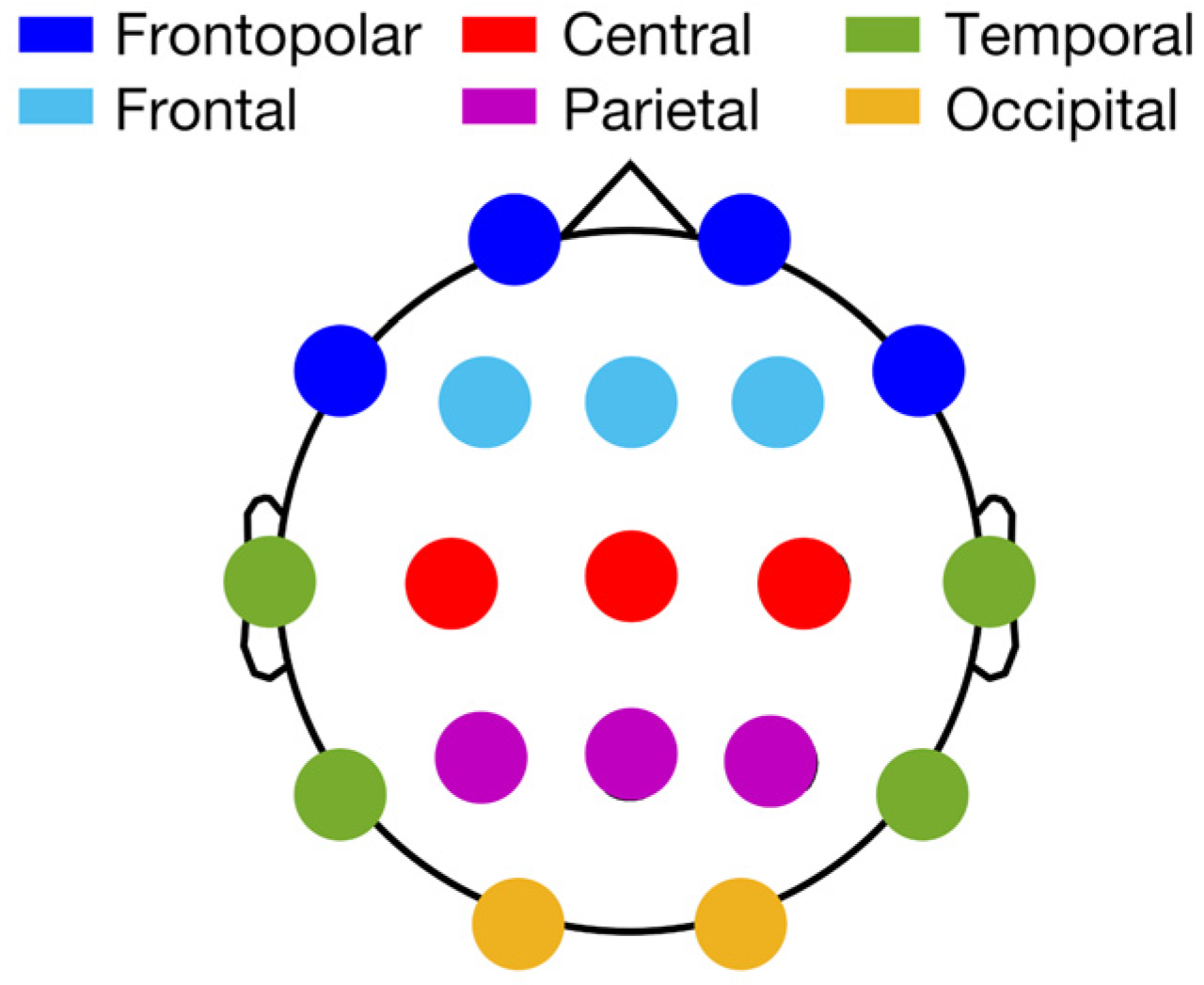
2.3. Sleep EEG Analysis
2.4. MRI Acquisition and Processing
2.5. Statistical Analyses
3. Results
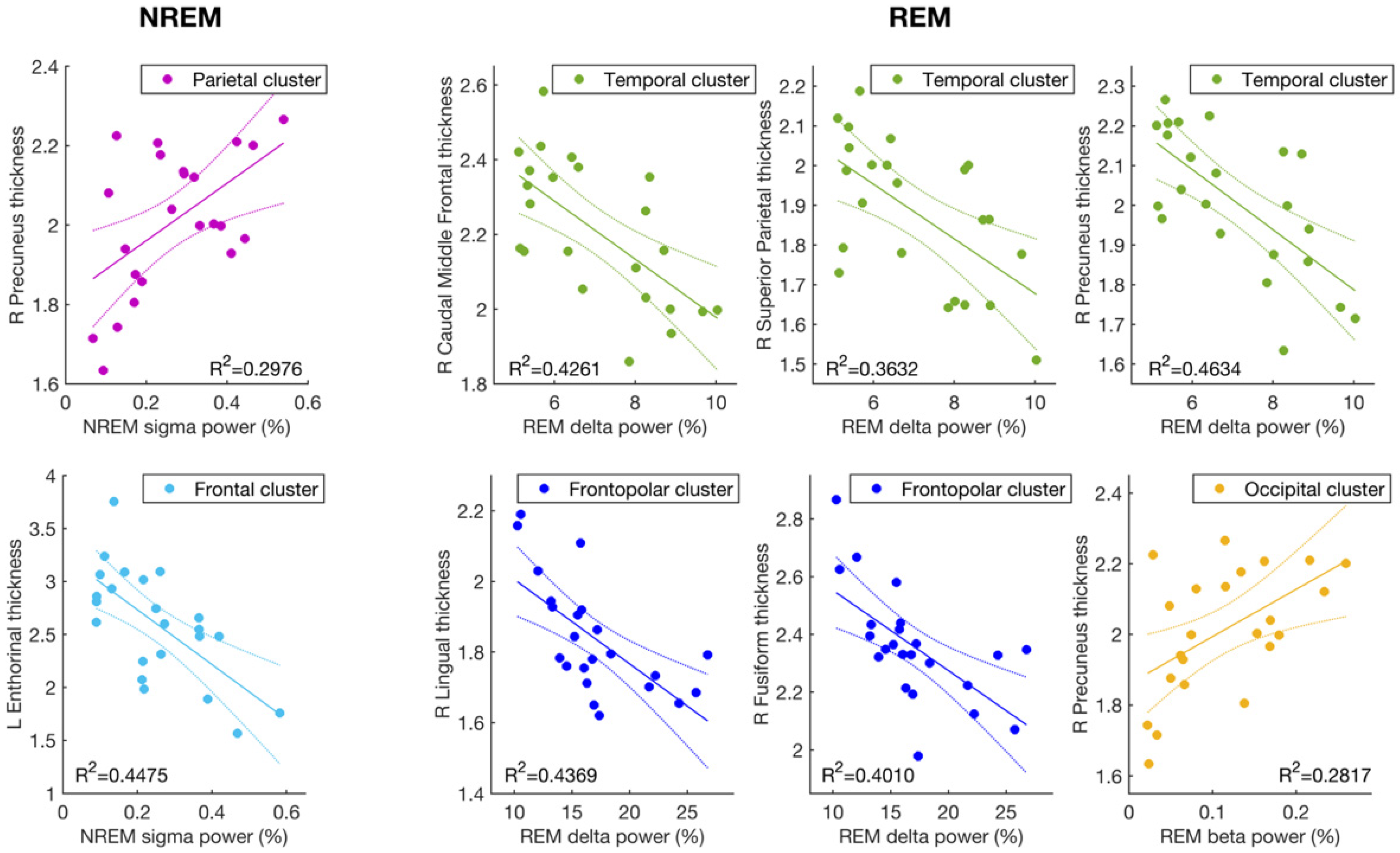
3.1. Cortical Thickness and Sigma Activity of NREM Sleep
3.2. Cortical Thickness and the EEG Slowing (Delta and Beta Activity) during REM Sleep
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, J.-E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid- Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science 2009, 326, 1005–1007. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, Y.-E.S.; Ooms, S.J.; Sutphen, C.; Macauley, S.L.; Zangrilli, M.A.; Jerome, G.; Fagan, A.M.; Mignot, E.; Zempel, J.M.; Claassen, J.A.H.R.; et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 2017, 140, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Lucey, B.P.; Hicks, T.J.; McLeland, J.S.; Toedebusch, C.D.; Boyd, J.; Elbert, D.L.; Patterson, B.W.; Baty, J.; Morris, J.C.; Ovod, V.; et al. Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann. Neurol. 2018, 83, 197–204. [Google Scholar] [CrossRef]
- Ooms, S.; Overeem, S.; Besse, K.; Rikkert, M.O.; Verbeek, M.; Claassen, J.A.H.R. Effect of 1 Night of Total Sleep Deprivation on Cerebrospinal Fluid β-Amyloid 42 in Healthy Middle-Aged Men. JAMA Neurol. 2014, 71, 971. [Google Scholar] [CrossRef]
- Prinz, P.N.; Vitaliano, P.P.; Vitiello, M.V.; Bokan, J.; Raskind, M.; Peskind, E.; Gerber, C. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol. Aging 1982, 3, 361–370. [Google Scholar] [CrossRef]
- Vitiello, M.V.; Prinz, P.N.; Williams, D.E.; Frommlet, M.S.; Ries, R.K. Sleep Disturbances in Patients With Mild-Stage Alzheimer’s Disease. J. Gerontol. 1990, 45, M131–M138. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.E.; Vitiello, M.V.; Larsen, L.H.; Prinz, P.N. Sleep/wake patterns In Alzheimer’s disease: Relationships with cognition and function. J. Sleep Res. 1995, 4, 15–20. [Google Scholar] [CrossRef]
- Cordone, S.; Scarpelli, S.; Alfonsi, V.; De Gennaro, L.; Gorgoni, M. Sleep-Based Interventions in Alzheimer’s Disease: Promising Approaches from Prevention to Treatment along the Disease Trajectory. Pharmaceuticals 2021, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.M.; Gerstner, J.R.; Holtzman, D.M. The sleep-wake cycle and Alzheimer’s disease: What do we know? Neurodegener. Dis. Manag. 2014, 4, 351–362. [Google Scholar] [CrossRef] [Green Version]
- De Gennaro, L.; Ferrara, M. Sleep spindles: An overview. Sleep Med. 2003, 7, 422–440. [Google Scholar] [CrossRef]
- Gorgoni, M.; Lauri, G.; Truglia, I.; Cordone, S.; Sarasso, S.; Scarpelli, S.; Mangiaruga, A.; D’Atri, A.; Tempesta, D.; Ferrara, M.; et al. Parietal Fast Sleep Spindle Density Decrease in Alzheimer’ s Disease and Amnesic Mild Cognitive Impairment. Neural Plast. 2016, 2016, 8376108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauchs, G.; Schabus, M.; Parapatics, S.; Bertran, F.; Clochon, P.; Hot, P.; Denise, P.; Desgranges, B.; Eustache, F.; Gruber, G.; et al. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport 2008, 19, 1159–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerberg, C.E.; Mander, B.A.; Florczak, S.M.; Weintraub, S.; Mesulam, M.-M.; Zee, P.C.; Paller, K.A. Concurrent Impairments in Sleep and Memory in Amnestic Mild Cognitive Impairment. J. Int. Neuropsychol. Soc. 2012, 18, 490–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinzing, J.G.; Niethard, N.; Born, J. Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 2019, 22, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Rao, V.; Lu, B.; Saletin, J.M.; Ancoli-Israel, S.; Jagust, W.J.; Walker, M.P. Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cereb. Cortex 2014, 24, 3301–3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brayet, P.; Petit, D.; Frauscher, B.; Gagnon, J.-F.; Gosselin, N.; Gagnon, K.; Rouleau, I.; Montplaisir, J. Quantitative EEG of Rapid-Eye-Movement Sleep: A Marker of Amnestic Mild Cognitive Impairment. Clin. EEG Neurosci. 2016, 47, 134–141. [Google Scholar] [CrossRef]
- Hassainia, F.; Petit, D.; Nielsen, T.; Gauthier, S.; Montplaisir, J. Quantitative EEG and Statistical Mapping of Wakefulness and REM Sleep in the Evaluation of Mild to Moderate Alzheimer’s Disease. Eur. Neurol. 1997, 37, 219–224. [Google Scholar] [CrossRef]
- D’Atri, A.; Scarpelli, S.; Gorgoni, M.; Truglia, I.; Lauri, G.; Cordone, S.; Ferrara, M.; Marra, C.; Rossini, P.M.; De Gennaro, L. EEG alterations during wake and sleep in mild cognitive impairment and Alzheimer’s disease. iScience 2021, 24, 102386. [Google Scholar] [CrossRef]
- Buchan, R.J.; Nagata, K.; Yokoyama, E.; Langman, P.; Yuya, H.; Hirata, Y.; Hatazawa, J.; Kanno, I. Regional correlations between the EEG and oxygen metabolism in dementia of Alzheimer’s type. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 409–417. [Google Scholar] [CrossRef]
- Nagata, K.; Gross, C.E.; Kindt, G.W.; Geier, J.M.; Adey, G.R. Topographic Electroencephalographic Study with Power Ratio Index Mapping in Patients with Malignant Brain Tumors. Neurosurgery 1985, 17, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.; Lafortune, M.; Bedetti, C.; Bouchard, M.; Gagnon, J.F.; Doyon, J.; Evans, A.C.; Lina, J.-M.; Carrier, J. Cortical thinning explains changes in sleep slow waves during adulthood. J. Neurosci. 2015, 35, 7795–7807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mander, B.A.; Rao, V.; Lu, B.; Saletin, J.M.; Lindquist, J.R.; Ancoli-Israel, S.; Jagust, W.; Walker, M.P. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 2013, 16, 357–364. [Google Scholar] [CrossRef]
- Latreille, V.; Gaubert, M.; Dubé, J.; Lina, J.-M.; Gagnon, J.-F.; Carrier, J. Age-related cortical signatures of human sleep electroencephalography. Neurobiol. Aging 2019, 76, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef] [Green Version]
- Mander, B.A.; Marks, S.M.; Vogel, J.W.; Rao, V.; Lu, B.; Saletin, J.M.; Ancoli-Israel, S.; Jagust, W.J.; Walker, M.P. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat. Neurosci. 2015, 18, 1051–1057. [Google Scholar] [CrossRef] [Green Version]
- Anderer, P.; Klösch, G.; Gruber, G.; Trenker, E.; Pascual-Marqui, R.; Zeitlhofer, J.; Barbanoj, M.; Rappelsberger, P.; Saletu, B. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience 2001, 103, 581–592. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S. The AASM Manual for the Scoring of Sleep and Associates Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical Surface-Based Analysis II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical Surface-Based Analysis I: Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Fischl, B.; Sereno, M.I.; Tootell, R.B.H.; Dale, A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999, 8, 272–284. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yang, H.; Xu, H.; Li, Q.; Jin, Y.; Jiang, W.; Wang, J.; Wu, Y.; Li, W.; Yang, C.; Li, X.; et al. Study of brain morphology change in Alzheimer’s disease and amnestic mild cognitive impairment compared with normal controls. Gen. Psychiatry 2019, 32, e100005. [Google Scholar] [CrossRef]
- Van Hoesen, G.W.; Hyman, B.T.; Damasio, A.R. Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus 1991, 1, 1–8. [Google Scholar] [CrossRef]
- Whitwell, J.L. Progression of Atrophy in Alzheimer’s Disease and Related Disorders. Neurotox. Res. 2010, 18, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Golby, A.; Silverberg, G.; Race, E.; Gabrieli, S.; O’Shea, J.; Knierim, K.; Stebbins, G.; Gabrieli, J. Memory encoding in Alzheimer’s disease: An fMRI study of explicit and implicit memory. Brain 2005, 128, 773–787. [Google Scholar] [CrossRef] [Green Version]
- Bakkour, A.; Morris, J.C.; Dickerson, B.C. The cortical signature of prodromal AD: Regional thinning predicts mild AD dementia. Neurology 2009, 72, 1048–1055. [Google Scholar] [CrossRef] [Green Version]
- Bozzali, M.; Filippi, M.; Magnani, G.; Cercignani, M.; Franceschi, M.; Schiatti, E.; Castiglioni, S.; Mossini, R.; Falautano, M.; Scotti, G.; et al. The contribution of voxel-based morphometry in staging patients with mild cognitive impairment. Neurology 2006, 67, 453–460. [Google Scholar] [CrossRef]
- Chételat, G.; Landeau, B.; Eustache, F.; Mézenge, F.; Viader, F.; de la Sayette, V.; Desgranges, B.; Baron, J.-C. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: A longitudinal MRI study. Neuroimage 2005, 27, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Fischl, B.; Cabral, H.J.; Kemper, T.L.; Guttmann, C.R.G.; Blacker, D.; Hyman, B.T.; Albert, M.S.; Killiany, R.J. MRI measures of temporoparietal regions show differential rates of atrophy during prodromal AD. Neurology 2008, 71, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Cabral, H.J.; Fischl, B.; Guttmann, C.R.G.; Blacker, D.; Hyman, B.T.; Albert, M.S.; Killiany, R.J. Temporoparietal MR Imaging Measures of Atrophy in Subjects with Mild Cognitive Impairment That Predict Subsequent Diagnosis of Alzheimer Disease. Am. J. Neuroradiol. 2009, 30, 532–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hämäläinen, A.; Tervo, S.; Grau-Olivares, M.; Niskanen, E.; Pennanen, C.; Huuskonen, J.; Kivipelto, M.; Hänninen, T.; Tapiola, M.; Vanhanen, M.; et al. Voxel-based morphometry to detect brain atrophy in progressive mild cognitive impairment. Neuroimage 2007, 37, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, V.; Niskanen, E.; Muehlboeck, S.; Pihlajamäki, M.; Könönen, M.; Hallikainen, M.; Kivipelto, M.; Tervo, S.; Vanninen, R.; Evans, A.; et al. Cortical Thickness Analysis to Detect Progressive Mild Cognitive Impairment: A Reference to Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2009, 28, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Karas, G.; Sluimer, J.; Goekoop, R.; van der Flier, W.; Rombouts, S.A.R.B.; Vrenken, H.; Scheltens, P.; Fox, N.; Barkhof, F. Amnestic Mild Cognitive Impairment: Structural MR Imaging Findings Predictive of Conversion to Alzheimer Disease. Am. J. Neuroradiol. 2008, 29, 944–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitwell, J.L.; Shiung, M.M.; Przybelski, S.A.; Weigand, S.D.; Knopman, D.S.; Boeve, B.F.; Petersen, R.C.; Jack, C.R. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology 2008, 70, 512–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckner, R.L. Molecular, Structural, and Functional Characterization of Alzheimer’s Disease: Evidence for a Relationship between Default Activity, Amyloid, and Memory. J. Neurosci. 2005, 25, 7709–7717. [Google Scholar] [CrossRef] [Green Version]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Grambaite, R.; Selnes, P.; Reinvang, I.; Aarsland, D.; Hessen, E.; Gjerstad, L.; Fladby, T. Executive Dysfunction in Mild Cognitive Impairment is Associated with Changes in Frontal and Cingulate White Matter Tracts. J. Alzheimer’s Dis. 2011, 27, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Squire, L.R.; Stark, C.E.L.; Clark, R.E. The medial temporal lobe. Annu. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfonsi, V.; D’Atri, A.; Gorgoni, M.; Scarpelli, S.; Mangiaruga, A.; Ferrara, M.; De Gennaro, L. Spatiotemporal dynamics of sleep spindle sources across NREM sleep cycles. Front. Neurosci. 2019, 13, 727. [Google Scholar] [CrossRef]
- Rosenbaum, R.S.; Furey, M.L.; Horwitz, B.; Grady, C.L. Altered connectivity among emotion-related brain regions during short-term memory in Alzheimer’s disease. Neurobiol. Aging 2010, 31, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Meulenbroek, O.; Rijpkema, M.; Kessels, R.P.C.; Rikkert, M.G.M.O.; Fernández, G. Autobiographical memory retrieval in patients with Alzheimer’s disease. Neuroimage 2010, 53, 331–340. [Google Scholar] [CrossRef]
- Pini, L.; Pievani, M.; Bocchetta, M.; Altomare, D.; Bosco, P.; Cavedo, E.; Galluzzi, S.; Marizzoni, M.; Frisoni, G.B. Brain atrophy in Alzheimer’s Disease and aging. Ageing Res. Rev. 2016, 30, 25–48. [Google Scholar] [CrossRef]
- Bernardi, G.; Betta, M.; Ricciardi, E.; Pietrini, P.; Tononi, G.; Siclari, F. Regional Delta Waves In Human Rapid Eye Movement Sleep. J. Neurosci. 2019, 39, 2686–2697. [Google Scholar] [CrossRef] [Green Version]
- Babiloni, C.; Lizio, R.; Marzano, N.; Capotosto, P.; Soricelli, A.; Triggiani, A.I.; Cordone, S.; Gesualdo, L.; Del Percio, C. Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int. J. Psychophysiol. 2015, 103, 88–102. [Google Scholar] [CrossRef]
- Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Jordan, S.E.; Ahn, S.S. Evaluation of stroke using EEG frequency analysis and topographic mapping. Neurology 1987, 37, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Nita, D.A.; Cisse, Y.; Timofeev, I.; Steriade, M. Waking-Sleep Modulation of Paroxysmal Activities Induced by Partial Cortical Deafferentation. Cereb. Cortex 2007, 17, 272–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasso, S.; D’Ambrosio, S.; Fecchio, M.; Casarotto, S.; Viganò, A.; Landi, C.; Mattavelli, G.; Gosseries, O.; Quarenghi, M.; Laureys, S.; et al. Local sleep-like cortical reactivity in the awake brain after focal injury. Brain 2020, 143, 3672–3684. [Google Scholar] [CrossRef] [PubMed]
| PSG Measure 1 | Mean | s.d. |
|---|---|---|
| SO latency (min) | 26.60 | 19.99 |
| REM latency (min) | 87.03 | 49.04 |
| WASO (min) | 74.71 | 44.46 |
| N1 (%) | 7.05 | 6.37 |
| N2 (%) | 74.33 | 6.95 |
| N3 (%) | 0.56 | 1.28 |
| REM (%) | 18.24 | 6.81 |
| TBT (min) | 373.58 | 70.16 |
| TST (min) | 268.87 | 76.22 |
| SEI (%) | 71.40 | 13.00 |
| ISA (#) | 19.74 | 12.14 |
| MR Cortical Areas | Side | Sleep Index | EEG Cluster | r | p |
|---|---|---|---|---|---|
| Caudal middle frontal | L | REM Delta | Temporal | −0.62 | 0.0016 |
| R | REM Delta | Temporal | −0.65 | 0.00073 | |
| Entorhinal | L | NREM sigma | Frontopolar | −0.62 | 0.0017 |
| L | NREM sigma | Frontal | −0.67 | 0.00048 | |
| L | NREM sigma | Central | −0.62 | 0.0017 | |
| Fusiform | R | REM Delta | Frontopolar | −0.63 | 0.00122 |
| R | REM Delta | Temporal | −0.58 | 0.0035 | |
| Lingual | R | REM Delta | Frontopolar | −0.66 | 0.00060 |
| R | REM Delta | Temporal | −0.60 | 0.0027 | |
| Precuneus | R | NREM sigma | Parietal | 0.55 | 0.0071 |
| R | REM delta | Frontopolar | −0.62 | 0.0017 | |
| R | REM delta | Temporal | −0.68 | 0.00035 | |
| R | REM beta | Temporal | 0.52 | 0.012 | |
| R | REM beta | Occipital | 0.53 | 0.0092 | |
| Superior parietal | R | REM delta | Temporal | −0.60 | 0.0023 |
| Mean thickness | R | REM delta | Frontopolar | −0.60 | 0.0027 |
| R | REM delta | Temporal | −0.59 | 0.0028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Atri, A.; Gorgoni, M.; Scarpelli, S.; Cordone, S.; Alfonsi, V.; Marra, C.; Ferrara, M.; Rossini, P.M.; De Gennaro, L. Relationship between Cortical Thickness and EEG Alterations during Sleep in the Alzheimer’s Disease. Brain Sci. 2021, 11, 1174. https://doi.org/10.3390/brainsci11091174
D’Atri A, Gorgoni M, Scarpelli S, Cordone S, Alfonsi V, Marra C, Ferrara M, Rossini PM, De Gennaro L. Relationship between Cortical Thickness and EEG Alterations during Sleep in the Alzheimer’s Disease. Brain Sciences. 2021; 11(9):1174. https://doi.org/10.3390/brainsci11091174
Chicago/Turabian StyleD’Atri, Aurora, Maurizio Gorgoni, Serena Scarpelli, Susanna Cordone, Valentina Alfonsi, Camillo Marra, Michele Ferrara, Paolo Maria Rossini, and Luigi De Gennaro. 2021. "Relationship between Cortical Thickness and EEG Alterations during Sleep in the Alzheimer’s Disease" Brain Sciences 11, no. 9: 1174. https://doi.org/10.3390/brainsci11091174








