Inter-Individual Differences Explain More Variance in Conditioned Pain Modulation Than Age, Sex and Conditioning Stimulus Intensity Combined
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pooled Data
2.2. Participants
2.3. Conditioned Pain Modulation
2.4. Statistical Analysis
3. Results
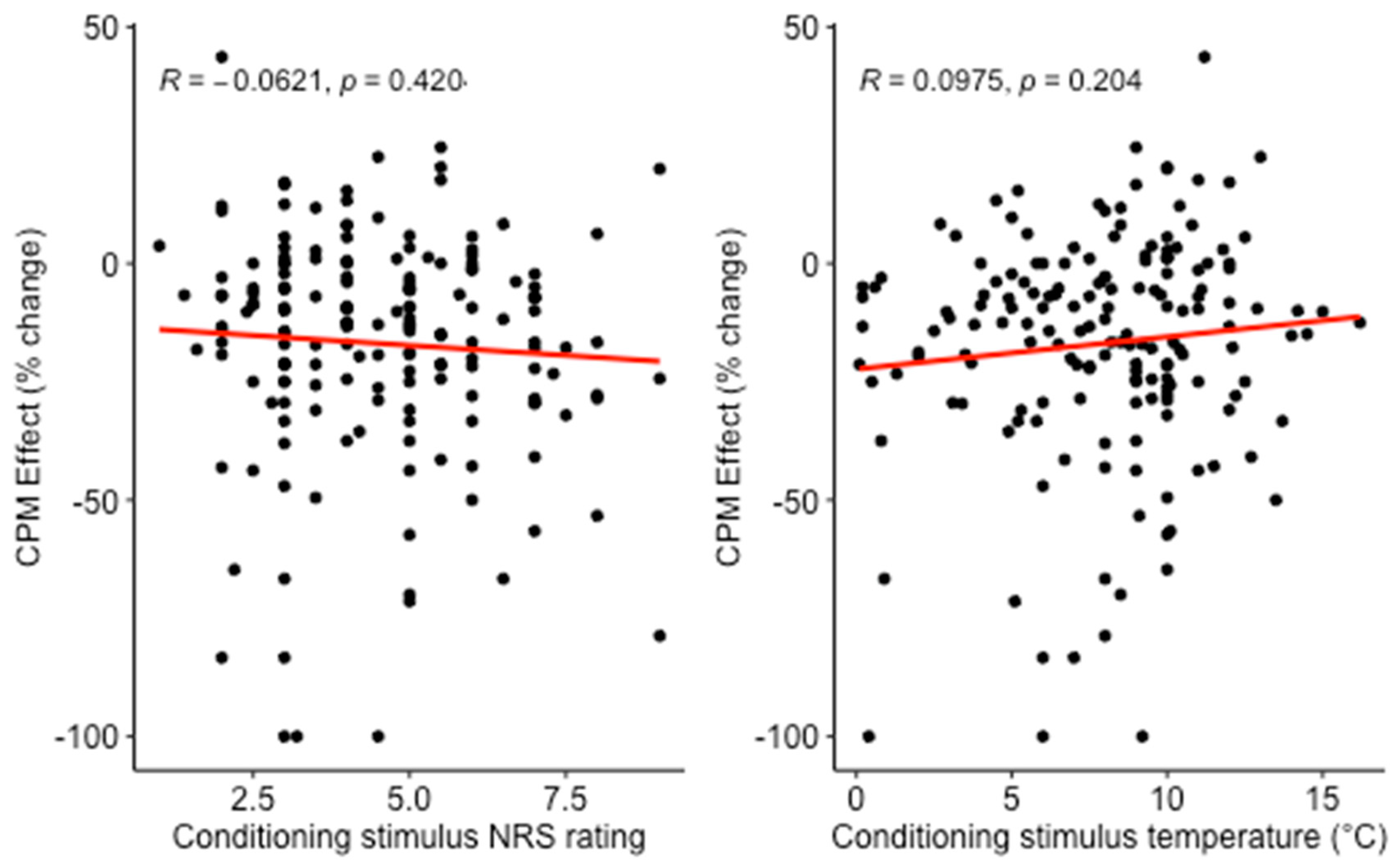
3.1. Cross-Sectional Analysis
3.2. Repeated Measures Analysis of Linear Mixed Models
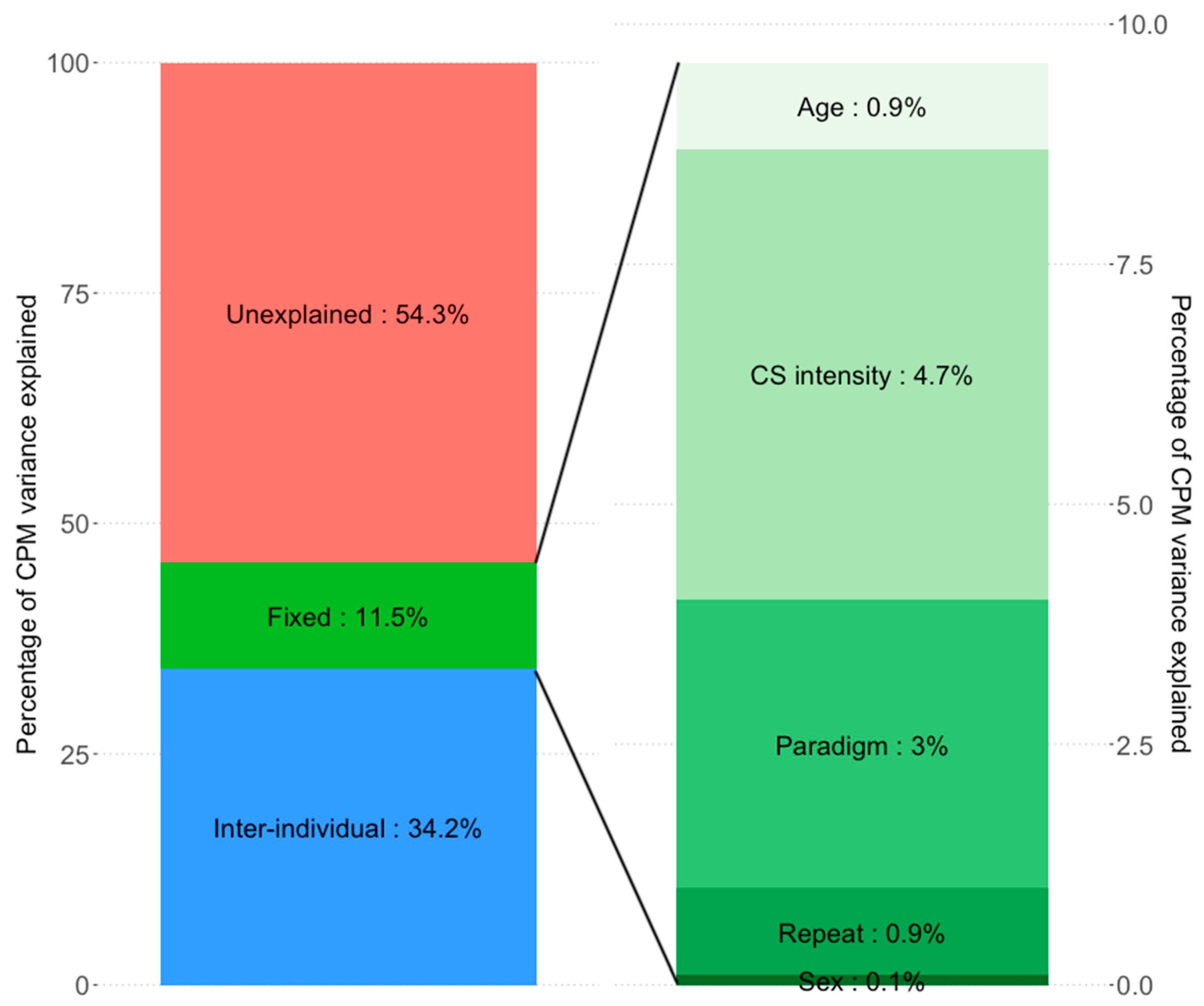
3.3. Repeated Measures Analysis: Decomposition of Explained Variance
4. Discussion
- (i)
- In a large cross-sectional analysis, neither CS physical intensity nor CS pain intensity predicted the CPM effect. In contrast, in a repeated measures analysis, CS physical intensity, but not CS pain intensity predicted the CPM effect.
- (ii)
- Inter-individual differences explained a large proportion of CPM variance (24.0% to 34.2%) while all fixed effects together (CS pain or physical intensity, age, sex, CPM paradigm, measurement repeat) predicted only 3.4% to 11.5% of CPM variance.
4.1. Conditioning Stimulus Physical Intensity and Pain Intensity
4.2. Age, Sex, Measurement Eepeat and CPM Paradigm
4.3. Variance Explained by Inter-Individual Differences vs. Fixed Variables
4.4. Unexplained Variance
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yarnitsky, D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr. Opin. Anaesthesiol. 2010, 23, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.N.; Rice, D.A.; McNair, P.J. Conditioned pain modulation in populations with chronic pain: A systematic review and meta-analysis. J. Pain 2012, 13, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Nir, R.-R.; Yarnitsky, D. Conditioned pain modulation. Curr. Opin. Support. Palliat. Care 2015, 9, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Yarnitsky, D.; Crispel, Y.; Eisenberg, E.; Granovsky, Y.; Ben-Nun, A.; Sprecher, E.; Best, L.-A.; Granot, M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008, 138, 22–28. [Google Scholar] [CrossRef]
- Staud, R.; Robinson, M.E.; Vierck, C.J.; Price, D.D. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain 2003, 101, 167–174. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Sluka, K.A.; Nie, H.L. Experimental muscle pain impairs descending inhibition. Pain 2008, 140, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Riley, J.L.; King, C.D.; Wong, F.; Fillingim, R.B.; Mauderli, A.P. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain 2010, 150, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Larivière, M.; Goffaux, P.; Marchand, S.; Julien, N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin. J. Pain 2007, 23, 506–510. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Nir, R.R.; Sprecher, E.; Yarnitsky, D. Psychological factors and conditioned pain modulation: A meta-analysis. Clin. J. Pain 2016, 32, 541–554. [Google Scholar] [CrossRef]
- Pud, D.; Granovsky, Y.; Yarnitsky, D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 2009, 144, 16–19. [Google Scholar] [CrossRef]
- Nir, R.R.; Granovsky, Y.; Yarnitsky, D.; Sprecher, E.; Granot, M. A psychophysical study of endogenous analgesia: The role of the conditioning pain in the induction and magnitude of conditioned pain modulation. Eur. J. Pain 2011, 15, 491–497. [Google Scholar]
- Granot, M.; Weissman-Fogel, I.; Crispel, Y.; Pud, D.; Granovsky, Y.; Sprecher, E.; Yarnitsky, D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain 2008, 136, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Nahman-Averbuch, H.; Yarnitsky, D.; Granovsky, Y.; Gerber, E.; Dagul, P.; Granot, M. The role of stimulation parameters on the conditioned pain modulation response. Scand. J. Pain 2013, 4, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Graven-Nielsen, T.; Izumi, M.; Petersen, K.K.; Arendt-Nielsen, L. User-independent assessment of conditioning pain modulation by cuff pressure algometry. Eur. J. Pain 2017, 21, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Oono, Y.; Wang, K.; Svensson, P.; Arendt-Nielsen, L. Conditioned pain modulation evoked by different intensities of mechanical stimuli applied to the craniofacial region in healthy men and women. J. Orofac. Pain 2011, 25, 364–375. [Google Scholar]
- Smith, A.; Pedler, A. Conditioned pain modulation is affected by occlusion cuff conditioning stimulus intensity, but not duration. Eur. J. Pain 2018, 22, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Willer, J.C.; Roby, A.; le Bars, D. Psychophysical and electrophysiological approaches to the pain-relieving effects of heterotopic nociceptive stimuli. Brain 1984, 107, 1095–1112. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef] [Green Version]
- Chevan, A.; Sutherland, M. Hierarchical partitioning. Am. Stat. 1991, 45, 90–96. [Google Scholar]
- Lindeman, R.H.; Merenda, P.F.; Gold, R.Z. Introduction to Bivariate and Multivaraite Analysis; Foresman and Company: Glenview, IL, USA, 1980. [Google Scholar]
- Alt, L.K.; Wach, K.; Liebler, E.J.; Straube, A.; Ruscheweyh, R. A randomized sham-controlled cross-over study on the short-term effect of non-invasive cervical vagus nerve stimulation on spinal and supraspinal nociception in healthy subjects. Headache 2020, 60, 1616–1631. [Google Scholar] [CrossRef]
- Krafft, S.; Göhmann, H.D.; Sommer, J.; Straube, A.; Ruscheweyh, R. Learned control over spinal nociception in patients with chronic back pain. Eur. J. Pain 2017, 21, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Ruscheweyh, R.; Kreusch, A.; Albers, C.; Sommer, J.; Marziniak, M. The effect of distraction strategies on pain perception and the nociceptive flexor reflex (RIII reflex). Pain 2011, 152, 2662–2671. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Bartón, K. MuMIn: Multi-Model Inference. 2020. Available online: https://cran.r-project.org/package=MuMIn (accessed on 6 September 2021).
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Nir, R.R.; Yarnitsky, D.; Honigman, L.; Granot, M. Cognitive manipulation targeted at decreasing the conditioning pain perception reduces the efficacy of conditioned pain modulation. Pain 2012, 153, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, C.; Bingel, U.; Büchel, C. Treating pain with pain: Supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain 2011, 152, 428–439. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Roscher, S.; Strian, F. Inhibitory effects do not depend on the subjective experience of pain during heterotopic noxious conditioning stimulation (HNCS): A contribution to the psychophysics of pain inhibition. Eur. J. Pain 2002, 6, 365–374. [Google Scholar] [CrossRef]
- le Bars, D.; Dickenson, A.H.; Besson, J.M. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 1979, 6, 283–304. [Google Scholar] [CrossRef]
- Youssef, A.M.; Macefield, V.G.; Henderson, L.A. Pain inhibits pain; human brainstem mechanisms. Neuroimage 2016, 124, 54–62. [Google Scholar] [CrossRef]
- Tousignant-Laflamme, Y.; Pagé, S.; Goffaux, P.; Marchand, S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Res. 2008, 1230, 73–79. [Google Scholar] [CrossRef]
- Edwards, R.R.; Ness, T.J.; Weigent, D.A.; Fillingim, R.B. Individual differences in diffuse noxious inhibitory controls (DNIC): Association with clinical variables. Pain 2003, 106, 427–437. [Google Scholar] [CrossRef]
- Rosén, A.; Feldreich, A.; Dabirian, N.; Ernberg, M. Effect of heterotopic noxious conditioning stimulation on electrical and pressure pain thresholds in two different anatomical regions. Acta Odontol. Scand. 2008, 66, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treister, R.; Eisenberg, E.; Gershon, E.; Haddad, M.; Pud, D. Factors affecting—And relationships between—Different modes of endogenous pain modulation in healthy volunteers. Eur. J. Pain 2010, 14, 608–614. [Google Scholar] [CrossRef]
- Ge, H.Y.; Madeleine, P.; Arendt-Nielsen, L. Sex differences in temporal characteristics of descending inhibitory control: An evaluation using repeated bilateral experimental induction of muscle pain. Pain 2004, 110, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gruener, H.; Zeilig, G.; Gaidukov, E.; Rachamim-Katz, O.; Ringler, E.; Blumen, N.; Engel-Haber, E.; Defrin, R. Biomarkers for predicting central neuropathic pain occurrence and severity after spinal cord injury: Results of a long-term longitudinal study. Pain 2020, 161, 545–556. [Google Scholar] [CrossRef]
- Müller, M.; Bütikofer, L.; Andersen, O.K.; Heini, P.; Arendt-Nielsen, L.; Jüni, P.; Curatolo, M. Cold pain hypersensitivity predicts trajectories of pain and disability after low back surgery: A prospective cohort study. Pain 2021, 162, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Yarnitsky, D.; Granot, M.; Granovsky, Y. Pain modulation profile and pain therapy: Between pro- and antinociception. Pain 2014, 155, 663–665. [Google Scholar] [CrossRef]
- Lindstedt, F.; Berrebi, J.; Greayer, E.; Lonsdorf, T.B.; Schalling, M.; Ingvar, M.; Kosek, E. Conditioned pain modulation is associated with common Polymorphisms in the serotonin transporter gene. PLoS ONE 2011, 6, e18252. [Google Scholar] [CrossRef]
- Chalaye, P.; Devoize, L.; Lafrenaye, S.; Dallel, R.; Marchand, S. Cardiovascular influences on conditioned pain modulation. Pain 2013, 154, 1377–1382. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; Albers, C.; Kreusch, A.; Sommer, J.; Marziniak, M. The effect of catastrophizing self-statements on pain perception and the nociceptive flexor reflex (RIII reflex). Clin. J. Pain 2013, 29, 725–732. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; Weinges, F.; Schiffer, M.; Bäumler, M.; Feller, M.; Krafft, S.; Straube, A.; Sommer, J.; Marziniak, M. Control over spinal nociception as quantified by the nociceptive flexor reflex (RIII reflex) can be achieved under feedback of the RIII reflex. Eur. J. Pain 2015, 19, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Granovsky, Y.; Miller-Barmak, A.; Goldstein, O.; Sprecher, E.; Yarnitsky, D. CPM test-retest reliability: “Standard” vs “single test-stimulus” protocols. Pain Med. 2016, 17, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurth, C.; Rehberg, B.; von Dincklage, F. Reliability of subjective pain ratings and nociceptive flexion reflex responses as measures of conditioned pain modulation. Pain Res. Manag. 2014, 19, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.L.; Kemp, H.I.; Ridout, D.; Yarnitsky, D.; Rice, A.S.C. Reliability of conditioned pain modulation: A systematic review. Pain 2016, 157, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Rhudy, J.L.; Williams, A.E.; McCabe, K.M.; Nguyên, M.A.T.V.; Rambo, P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology 2005, 42, 579–587. [Google Scholar] [CrossRef] [PubMed]
| Study | Age | M/F | Conditioning Stimulus | Test Stimulus | Repeated Measures | Citation |
|---|---|---|---|---|---|---|
| 1 | 25 ± 6 | 18/12 | Cold water (120 s) | Electrical | Yes | Unpublished |
| 2 | 23 ± 4 | 15/5 | Cold water (60 s) | Contact heat (30 s) | Yes | Unpublished |
| 3 | 27 ± 6 | 14/9 | Cold water (60 s) | Contact heat (30 s) | Yes | [22] |
| 4 | 47 ± 10 | 27/0 | Cold water (90 s) | Contact heat (60 s) | No | [23] |
| 5 | 23 ± 5 | 17/9 | Cold water (60 s) | Contact heat (30 s) | Yes | Unpublished |
| 6 | 25 ± 5 | 9/19 | Cold water (60 s) | Contact heat (30 s) | No | Unpublished |
| 7 | 25 ± 3 | 7/10 | Cold water (60 s) | Contact heat (30 s) | No | Unpublished |
| Model | Predictor | Estimate | Std. Error | p-Value | Multiple R2 |
|---|---|---|---|---|---|
| Model 1 | Intercept | −17.385 | 9.933 | 0.082 | 0.0109 |
| NRScond | −0.916 | 1.101 | 0.407 | ||
| Age | −0.053 | 0.288 | 0.854 | ||
| Sex | 4.181 | 4.080 | 0.307 | ||
| 30 s heat/60 s cold | 3.643 | 8.853 | 0.681 | ||
| Electrical/120 s cold | −1.190 | 4.988 | 0.812 | ||
| Model 2 | Intercept | −27.890 | 10.237 | 0.007 | 0.0194 |
| Temperaturecond | 0.866 | 0.594 | 0.147 | ||
| Age | −0.102 | 0.288 | 0.723 | ||
| Sex | 4.818 | 4.096 | 0.242 | ||
| Heat30 s/Cold60 s | 1.825 | 8.715 | 0.834 | ||
| Electrical/120s cold | 0.470 | 5.141 | 0.927 |
| Model | Predictor | Estimate | Std. Error | p-Value | REML Criterium at Convergence |
|---|---|---|---|---|---|
| Model 3 | Intercept | −11.685 | 13.461 | - | 1649.6 |
| NRScond | −1.485 | 1.056 | 0.159 | ||
| Age | 0.257 | 0.388 | 0.506 | ||
| Sex | 2.452 | 4.402 | 0.578 | ||
| Paradigm | −7.815 | 4.808 | 0.104 | ||
| Repeat | −2.617 | 2.637 | 0.321 | ||
| Model 4 | Intercept | −21.712 | 13.935 | - | 1015.8 |
| Temperaturecond | 1.532 | 0.482 | 0.002 | ||
| Age | 0.258 | 0.428 | 0.546 | ||
| Sex | −1.860 | 4.831 | 0.700 | ||
| Paradigm | −8.468 | 4.910 | 0.085 | ||
| Repeat | −3.073 | 2.343 | 0.190 |
| Model 5 | Model 6 | ||
|---|---|---|---|
| Predictor | R2 | Predictor | R2 |
| NRScond | 0.00681 | Temperaturecond | 0.04650 |
| Age | 0.00438 | Age | 0.00886 |
| Sex | 0.00559 | Sex | 0.00091 |
| Paradigm | 0.01491 | Paradigm | 0.03010 |
| Repeat | 0.00325 | Repeat | 0.00871 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graeff, P.; Itter, A.; Wach, K.; Ruscheweyh, R. Inter-Individual Differences Explain More Variance in Conditioned Pain Modulation Than Age, Sex and Conditioning Stimulus Intensity Combined. Brain Sci. 2021, 11, 1186. https://doi.org/10.3390/brainsci11091186
Graeff P, Itter A, Wach K, Ruscheweyh R. Inter-Individual Differences Explain More Variance in Conditioned Pain Modulation Than Age, Sex and Conditioning Stimulus Intensity Combined. Brain Sciences. 2021; 11(9):1186. https://doi.org/10.3390/brainsci11091186
Chicago/Turabian StyleGraeff, Philipp, Alina Itter, Katharina Wach, and Ruth Ruscheweyh. 2021. "Inter-Individual Differences Explain More Variance in Conditioned Pain Modulation Than Age, Sex and Conditioning Stimulus Intensity Combined" Brain Sciences 11, no. 9: 1186. https://doi.org/10.3390/brainsci11091186
APA StyleGraeff, P., Itter, A., Wach, K., & Ruscheweyh, R. (2021). Inter-Individual Differences Explain More Variance in Conditioned Pain Modulation Than Age, Sex and Conditioning Stimulus Intensity Combined. Brain Sciences, 11(9), 1186. https://doi.org/10.3390/brainsci11091186







