Neurophysiological Characterization of Posteromedial Hypothalamus in Anaesthetized Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Procedures
2.3. Analysis of Extracellular Action Potentials
- Non-overlapping 0.2 s windowing was performed and power spectra (PS) were computed by means of fast Fourier transform (FFT) for every window. Mean () and SD () were calculated and high frequency artifact was identified by deviation exceeding > . These periods were subsequently removed.
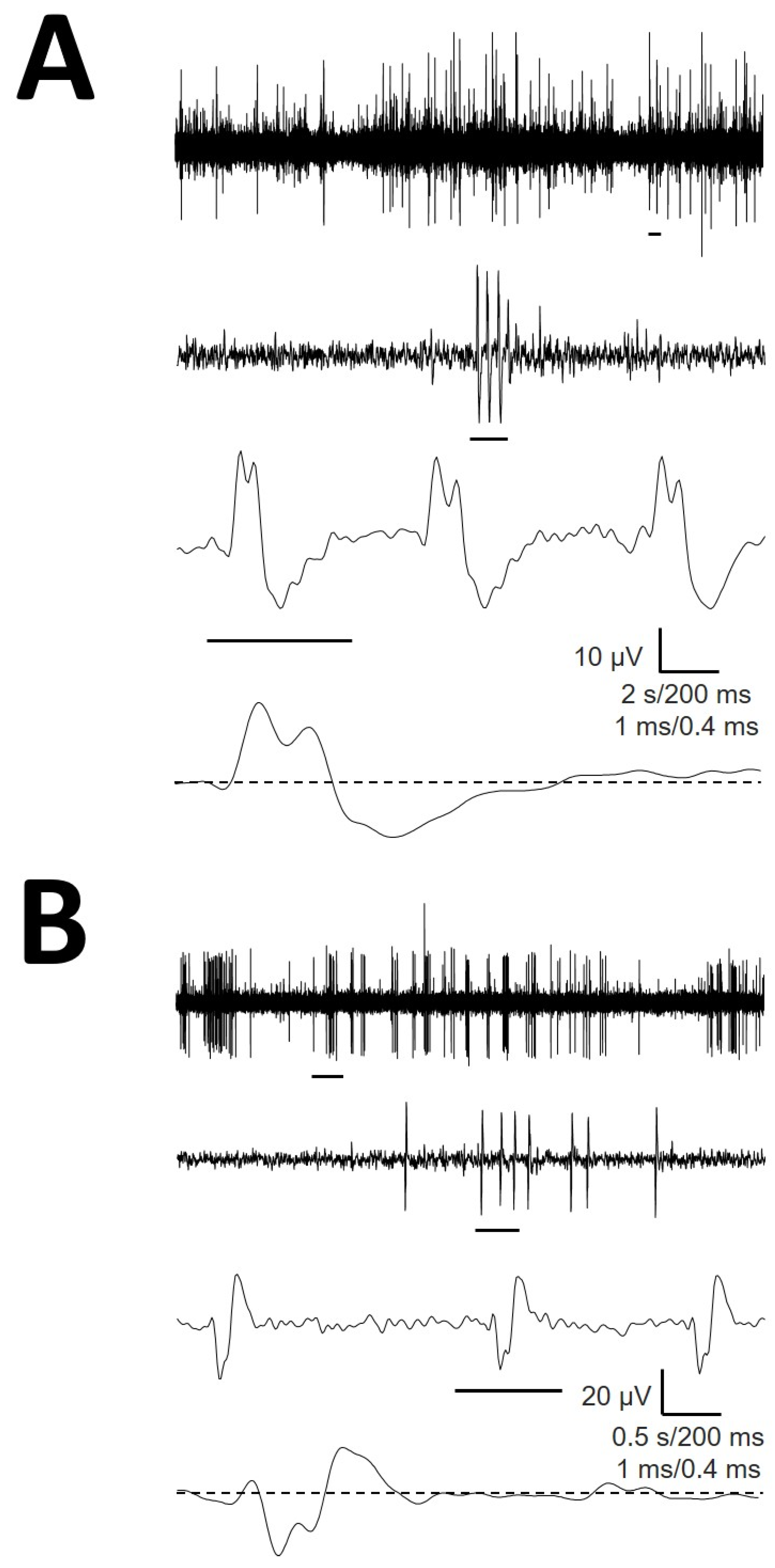
- Identification of EAPs. For every trace (Figure 1A), we computed a maximum (V+) and minimum (V−) voltage threshold (in µV), defined as , where is the mean and is the standard deviation. EAPs must have two phases (depolarization and repolarization); therefore, we identified a tentative EAP when a positive/negative (P/N) phase was followed by a negative/positive (N/P) phase in a period of 0.20–0.65 ms. EAPs were defined as positive or negative according to the highest component identified. Modifications of spike definition and thresholds at this step were the most relevant.

- 3.
- Clustering was performed by an agglomerative hierarchical method, with distance between groups computed by farthest procedure. EAPs sharing similar morphologies were ascribed to the same neuron. For every EAP, we measured the maximum (Vmax) and minimum voltages (Vmin, in µV), durations of negative (dtN) and positive phases at half-amplitude (dtP in ms), and maximum (dVmax) and minimum values of the first derivative (dVmin, in mV/s). These measures can be considered as a six-dimension vector for every k-EAP, (Figure 1B).
- 4.
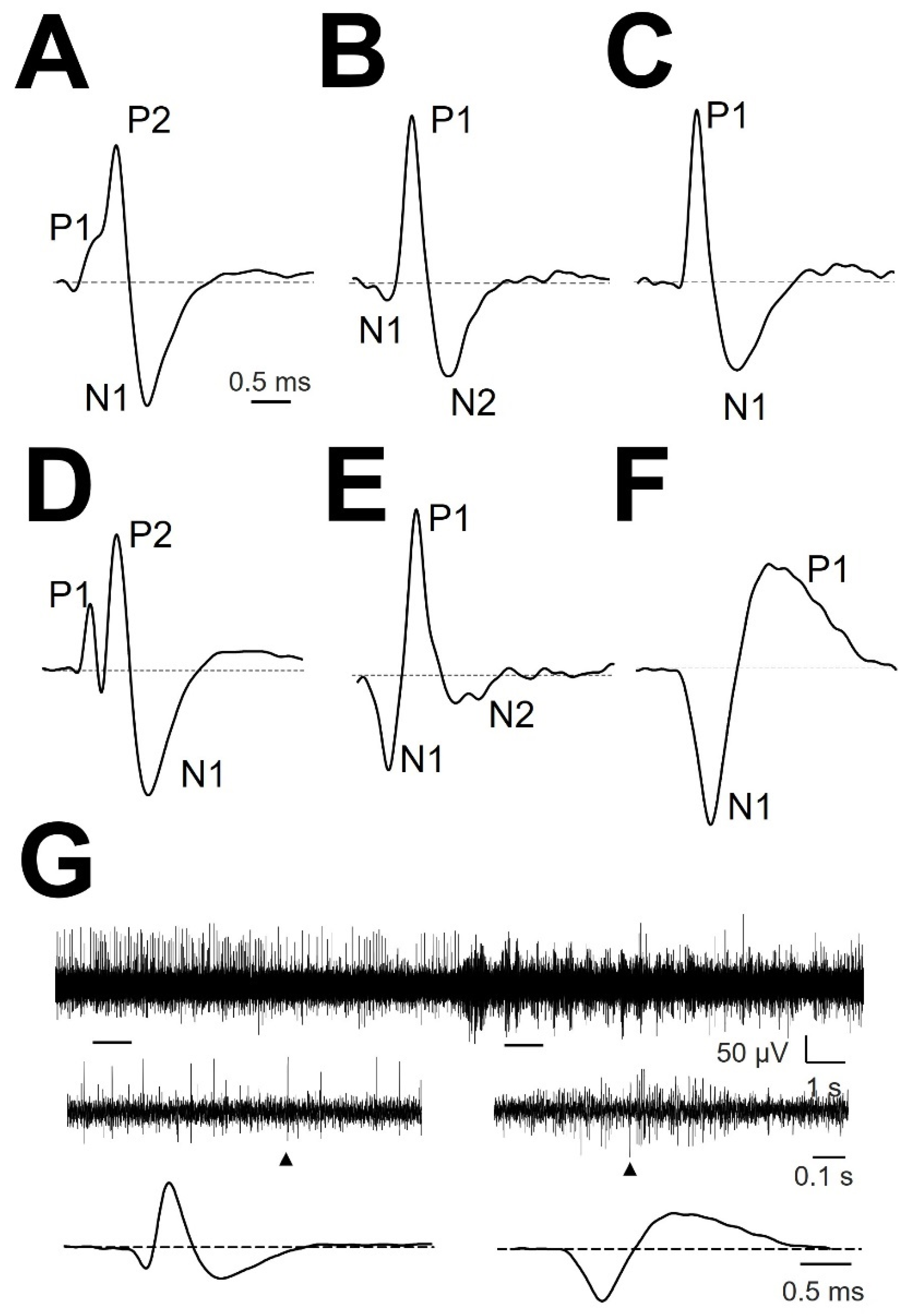
- Construction of the mean action potential (mAP). All the EAPs from the same cluster (Figure 1C) were averaged to obtain a canonical waveform (Figure 1D, upper row), as were the derivatives to obtain the mean derivative (1D, lower row). A minimum of 10 EAPs was averaged. The first 300 µs (72 points) of baseline were used to compute the maximum (VAP+) and minimum (VAP−) voltage thresholds (in µV), defined as , where is the mean and the standard deviation. We used these thresholds to identify hallmark points in mAPs (Figure 1E). Every phase can be characterized by its polarity (P/N), duration (dti) and amplitude (Vi, i = 1, 2, 3). Automatic identification of polarity of phases was done at this point (see Figure A1, (see Appendix A).
2.4. Analysis of the Discharge Pattern
- Mean frequency of the raw (, in Hz) trace and for every neuron (, in Hz). Both values were obtained from the inverse of the instant period.
- Burst Index (BI). Defined as the total number of interspikes intervals (ISI) < 20 ms, divided by the number of ISI > 20 ms. It informs about the number of bursts respecting individual discharges.
- Pause Index (PI). Defined as the total number of interspikes intervals (ISI) > 100 ms, divided by the number of ISI < 100 ms. In some sense, is the opposite to BI.
- Pause Ratio (PR). Defined as the total duration of pauses (ISI > 100 ms), divided by the duration of non-pauses (ISI < 100 ms). The information given by this index is completely different from PI, because it represents the total amount of time the cell remains quiescent, compared with the duration of the burst. This ratio will be high when the refractory period of the cell after the burst would be long.
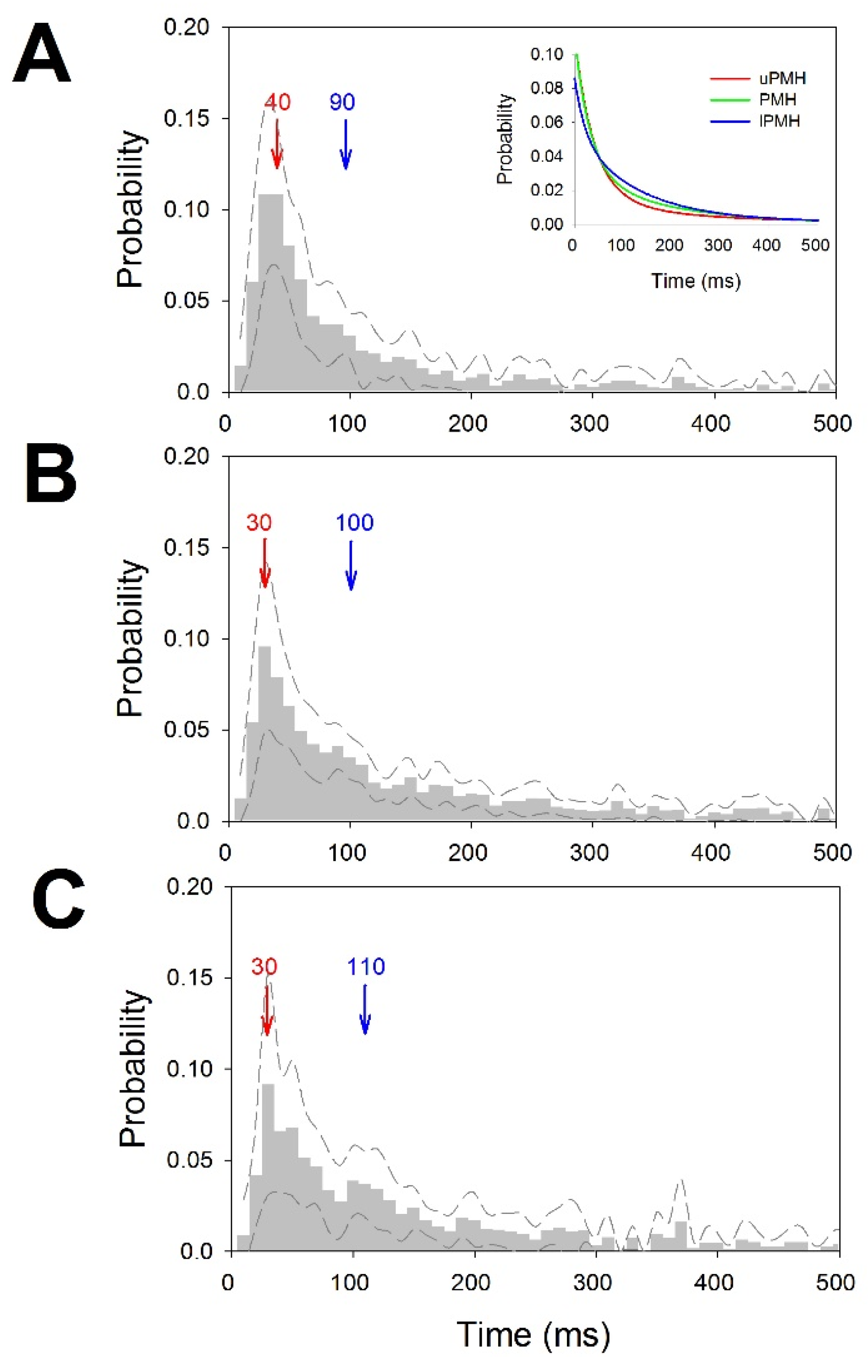
- Probability mass functions (pmf) of ISI for every trace. Relative frequency was computed for 10 ms bins, and the probability/bin (pi) was calculated with the following expression.
2.5. Statistics
2.6. Ethics Statement
3. Results
3.1. Types of mAP According to Structure
3.2. Cellullar Structure of the Different Regions
3.3. Tonic Properties
3.4. Atypical mAP
3.5. Comparison with Thalamic Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Script Availability Statement
Appendix A
| Acronym | Definition |
|---|---|
| amAP | Atypical Mean Action Potential |
| AP | Action Potential |
| BI | Burst Index |
| DBS | Deep Brain Stimulation |
| dmAP | Duration of the mean Action Potential |
| dtN/dtP | Duration of Negative/Positive phases at half-amplitude of EAP |
| dDep | Duration of the Depolarization |
| dFP | Duration of the First Phase |
| dRep | Duration of the Repolarization |
| dVmax/dVmin | Maximum/Minimum values of the first derivative |
| EAP | Extracellular Action Potential |
| Freqcell | Frequency of the individual neuron |
| Freqraw | Frequency of the raw trace |
| ISI | Inter-Stimulus Interval |
| lPMH | Lower Posteromedial Hypothalamus |
| MER | Microelectrode Recording |
| mAP | Mean Action Potential |
| MRI | Magnetic Resonance Imaging |
| N1P1 | Negative (Dep) and Positive (Rep) phases cell |
| N1P1N1 | Negative (FP), Positive (Dep) and Negative (Rep) phases cell |
| N1N2P1 | Negative (FP), Negative (Dep) and Positive (Rep) phases cell |
| PI | Pause Index |
| PMH | Posteromedial hypothalamus |
| pmf | Probability Mass Function |
| PR | Pause Ratio |
| PS | Power Spectra |
| P/N | Positive/Negative phase |
| P1N1 | Positive (Dep) and Negative (Rep) phases cell |
| P1P2N1 | Positive (FP), Positive (Dep) and Negative (Rep) phases cell |
| P1N1P2 | Positive (FP), Negative (Dep) and Positive (Rep) phases cell |
| SW | Schaltenbrand-Wahren |
| uPMH | Upper Posteromedial Hypothalamus |
| VAP± | Positive/negative voltage thresholds of mAP |
| VDep | Amplitude (voltage) of the Depolarization phase |
| VFP | Amplitude (voltage) of the Firs Phase |
| VmAP | Amplitude (voltage) of the mean Action Potential |
| Vmax/Vmin | Maximum/Minimum voltages of EAP |
| VRep | Amplitude (voltage) of the Repolarization phase |
| V± | Maximum/minimum voltage threshold |
| Patient | Age (yrs) | Sex | Intellectual Capacity | Medical History | Medication | MRI |
|---|---|---|---|---|---|---|
| 1 | 22 | F | severe mental retardation | cluster headache, epilepsy | TPM, CLZ, RIS, LVM, OLZ | moderate diffuse cortico-subcortical atrophy; pineal cyst |
| 2 | 22 | M | moderate mental retardation | perinatal hypoxia | GBP, VPA, CYP, Li, OLZ | normal |
| 3 | 48 | M | moderate mental retardation | OCD, AVM, complex partial seizure | CBM, GBP, ZPX, CTP, CLZ | extensive encephalomalacia in right temporal lobe |
| 4 | 37 | F | severe mental retardation | epilepsy | LVM, OLZ, TPM, RIS, ARP | normal |
| Region | Freqcell (Hz) | Freqraw (Hz) | BI | PI | PR |
|---|---|---|---|---|---|
| uPMH | 3.4 ± 0.6 | 6.4 ± 1.2 | 0.220 ± 0.038 | 1.612 ± 0.322 | 1.556 ± 0.724 |
| PMH | 6.1 ± 2.3 | 7.9 ± 1.9 | 0.176 ± 0.044 | 2.438 ± 1.108 | 0.606 ± 1.004 |
| lPMH | 7.1 ± 3.2 | 6.6 ± 1.5 | 0.225 ± 0.123 | 2.759 ± 0.708 | 2.360 ± 1.443 |

References
- Nieuwenhuys, R.; Voogd, J.; Van Huijzen, C. The Human Central Nervous System, 4th ed.; Springer: New York, NY, USA, 2008; pp. 289–323. ISBN 978-3540346845. [Google Scholar]
- Franzini, A.; Messina, G.; Cordella, R.; Marras, C.; Broggi, G. Deep brain stimulation of the posteromedial hypothalamus: Indications, long-term results, and neurophysiological considerations. Neurosurg. Focus 2010, 29, e13. [Google Scholar] [CrossRef]
- Hernando, V.; Pastor, J.; Pedrosa, M.; Peña, E.; Sola, R.G. Low frequency bilateral hypothalamic stimulation for treatment of drug-resistant aggressiveness in a young man with mental retardation. Stereotact. Funct. Neurosurg. 2008, 86, 219–223. [Google Scholar] [CrossRef]
- Micieli, R.; Lopez, A.L.; Plata, R.; Botero, L.F.; Hutchison, W.D. Single-unit analysis of the human posterior hypothalamus and red nucleus during deep brain stimulation for aggressivity. J. Neurosurg. 2017, 126, 1158–1164. [Google Scholar] [CrossRef] [Green Version]
- Torres, C.V.; Blasco, G.; Navas, M.; Ezquiaga, E.; Pastor, J.; Vega-Zelaya, L.; Pulido, P.; Pérez, S.; Manzanares, R. Deep brain stimulation for aggressiveness: Long term follow-up and tractography study of the stimulated brain areas. J. Neurosurg. 2020, 134, 366–375. [Google Scholar] [CrossRef]
- Torres, C.V.; Sola, R.G.; Pastor, J.; Pedrosa, M.; Navas, M.; García-Navarrete, E.; Ezquiaga, E.; Garcia-Camba, E. Long-term results of posteromedial hypothalamic deep brain stimulation for patients with resistant aggressiveness. J. Neurosurg. 2013, 119, 277–287. [Google Scholar] [CrossRef]
- Heinricher, M.M. Microelectrode Recording in Movement Disorder Surgery, 1st ed.; Thieme: New York, NY, USA, 2004; pp. 8–13. ISBN 9781588901743. [Google Scholar]
- Pastor, J.; Vega-Zelaya, L. Features of action potentials from identified thalamic nuclei in anesthetized patients. Brain Sci. 2020, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Vega-Zelaya, L.; Torres, C.V.; Navas, M.; Pastor, J. Neurophysiological characterization of thalamic nuclei in anaesthetized patients. Brain Sci. 2019, 9, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obwegeser, A.A.; Uitti, R.J.; Turk, M.F.; Strongosky, A.J.; Wharen, R.E. Thalamic stimulation for the treatment of midline tremors in essential tremor patients. Neurology 2000, 54, 2342–2344. [Google Scholar] [CrossRef]
- Wu, D.; Wang, S.; Stein, J.F.; Aziz, T.Z.; Green, A.L. Reciprocal interactions between the human thalamus and periaqueductal gray may be important for pain perception. Exp. Brain Res. 2014, 232, 527–534. [Google Scholar] [CrossRef]
- Franzini, A.; Marras, C.; Ferroli, P.; Bugiani, O.; Broggi, G. Stimulation of the posterior hypothalamus for medically intractable impulsive and violent behavior. Stereotact. Funct. Neurosurg. 2005, 83, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Blasco, G.; Navas, M.; González, O.; Bocos, A.; Ezquiaga, E.; Ayuso-Mateos, J.L.; Pastor, J.; Vega-Zelaya, L.; Torres, C.V. Posteromedial hypothalamic Deep Brain Stimulation for refractory aggressiveness in a patient with Weaver syndrome: Clinical, technical report and operative video. Oper. Neurosurg. 2021, 21, 454–456. [Google Scholar] [CrossRef]
- Shimamoto, S.A.; Larson, P.S.; Ostrem, J.L.; Glass, G.A.; Turner, R.S.; Starr, P.A. Physiological identification of the human pedunculopontine nucleus. J. Neurol. Neurosurg. Psychiatry 2010, 81, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Sanghera, M.K.; Schwabe, K.; Lütjens, G.; Jin, X.; Song, J.; Von Wrangel, C.; Stewart, R.M.; Jankovic, J.; Grossman, R.G.; et al. Globus pallidus internus neuronal activity: A comparative study of linear and non-linear features in patients with dystonia or Parkinson’s disease. J. Neural Transm. 2016, 123, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhuang, P.; Hallett, M.; Zhang, Y.; Li, J.; Li, Y. Subthalamic oscillatory activity in parkinsonian patients with off-period dystonia. Acta Neurol. Scand. 2016, 134, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Mayanagi, Y.; Sekino, H.; Ogashiwa, M.; Ishijima, B. Results of stimulation and destruction of the posterior hypothalamus in man. J. Neurosurg. 1970, 33, 689–707. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Mayanagi, Y. Posteromedial hypothalamotomy in the treatment of violent, aggressive behaviour. Acta Neurochir. Suppl. 1988, 44, 145–151. [Google Scholar] [PubMed]
- Hassler, R. Introduction to Stereotaxis with an Atlas of the Human Brain, 1st ed.; Thieme: New York, NY, USA, 1959; pp. 230–290. [Google Scholar]
- Pastor, J.; Vega-Zelaya, L. A new potential specifically marks the sensory thalamus in anaesthetized patients. Clin. Neurophysiol. 2019, 130, 1926–1936. [Google Scholar] [CrossRef]
- Favre, J.; Taha, J.M.; Baumann, T.; Burchiel, K.J. Computer analysis of the tonic, phasic, and kinesthetic activity of pallidal discharges in Parkinson patients. Surg. Neurol. 1999, 51, 665–672. [Google Scholar] [CrossRef]
- Peña, D.; Prieto, F.J. The kurtosis coefficient and the linear discriminant function. Stat. Probab. Lett. 2000, 49, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Favre, J.; Baumann, T. Signal processing and pattern recognition. In Microelectrode Recording in Movement Disorder Surgery; Zvi, I., Kim, J.B., Eds.; Thieme: New York, NY, USA, 2004; ISBN 9781588901743. [Google Scholar]
- Marceglia, S.; Servello, D.; Foffani, G.; Porta, M.; Sassi, M.; Mrakic-Sposta, S.; Rosa, M.; Barbieri, S.; Priori, A. Thalamic single-unit and local field potential activity in Tourette syndrome. Mov. Disord. 2010, 25, 300–308. [Google Scholar] [CrossRef]
- Gold, C.; Henze, D.A.; Koch, C.; Buzsáki, G. On the origin of the extracellular action potential waveform: A modeling study. J. Neurophysiol. 2006, 95, 3113–3128. [Google Scholar] [CrossRef]
- Henze, D.A.; Borhegyi, Z.; Csicsvari, J.; Mamiya, A.; Harris, K.D.; Buzsáki, G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J. Neurophysiol. 2000, 84, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.; Hamani, C.; Hutchison, W.D.; Moro, E.; Lozano, A.M.; Dostrovsky, J.O. Pedunculopontine nucleus microelectrode recordings in movement disorder patients. Exp. Brain Res. 2008, 188, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Galazky, I.; Kaufmann, J.; Voges, J.; Hinrichs, H.; Heinze, H.J.; Sweeney-Reed, C.M. Neuronal spiking in the pedunculopontine nucleus in progressive supranuclear palsy and in idiopathic Parkinson’s disease. J. Neurol. 2019, 266, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- De La Prida, L.M.; Benavides-Piccione, R.; Sola, R.; Pozo, M.A. Electrophysiological properties of interneurons from intraoperative spiking areas of epileptic human temporal neocortex. Neuroreport 2002, 13, 1421–1425. [Google Scholar] [CrossRef] [Green Version]
- Anastassiou, C.A.; Perin, R.; Buzsáki, G.; Markram, H.; Koch, C. Cell type- and activity-dependent extracellular correlates of intracellular spiking. J. Neurophysiol. 2015, 114, 608–623. [Google Scholar] [CrossRef]
- Zhuang, P.; Li, Y.; Hallett, M. Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clin. Neurophysiol. 2004, 115, 2542–2557. [Google Scholar] [CrossRef]
- Alam, M.; Schwabe, K.; Lütjens, G.; Capelle, H.H.; Manu, M.; von Wrangel, C.; Müller-Vahl, K.; Schrader, C.; Scheinichen, D.; Blahak, C.; et al. Comparative characterization of single cell activity in the globus pallidus internus of patients with dystonia or Tourette syndrome. J. Neural Transm. 2015, 122, 687–699. [Google Scholar] [CrossRef]
- Malekmohammadi, M.; Sparks, H.; AuYong, N.; Hudson, A.; Pouratian, N. Propofol Anesthesia Precludes LFP-Based Functional Mapping of Pallidum during DBS Implantation. Stereotact. Funct. Neurosurg. 2018, 96, 249–258. [Google Scholar] [CrossRef]
- Harris, K.D.; Henze, D.A.; Csicsvari, J.; Hirase, H.; Buzsáki, G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 2000, 84, 401–414. [Google Scholar] [CrossRef]
- Holt, G.R.; Koch, C. Electrical Interactions via the Extracellular Potential near Cell Bodies. J. Comput. Neurosci. 1999, 6, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Bakkum, D.J.; Obien, M.E.J.; Radivojevic, M.; Jäckel, D.; Frey, U.; Takahashi, H.; Hierlemannb, A. The Axon Initial Segment is the Dominant Contributor to the Neuron’s Extracellular Electrical Potential Landscape. Adv. Biosyst. 2019, 3, e1800308. [Google Scholar] [CrossRef] [PubMed]
- Koester, J.; Siegelbaum, S.A. Membrane Potential. In Principles of Neural Science, 5th ed.; Kandel, E.R., Schwartz, J.H., Jessell, T., Siegelbaum, S.A., Hudspeth, A.J., Eds.; Elsevier: New York, NY, USA, 2013; ISBN 978-0-07-139011-8. [Google Scholar]
- Gold, C.; Girardin, C.C.; Martin, K.A.C.; Koch, K. High-amplitude positive spikes recorded extracellularly in cat visual cortex. J. Neurophysiol. 2009, 102, 3340–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor, J.; Vega-Zelaya, L. Can we put aside microelectrode recordings in Deep Brain Stimulation surgery? Brain Sci. 2020, 10, 571. [Google Scholar] [CrossRef] [PubMed]
| Region | Type of mAP | ||||||
|---|---|---|---|---|---|---|---|
| P1N1 | P1P2N | N1P1N2 | P1N1 | P1N1P2 | amAP | Total | |
| uPMH | 25 (36) | 9 (13) | 23 (33) | 2 (3) | 4 (6) | 7 (10) | 70 (100) |
| PMH | 12 (21) | 12 (21) | 12 (21) | 5 (9) | 4 (7) | 11 (20) | 56 (100) |
| lPMH | 18 (35) | 8 (15) | 18 (35) | 4 (8) | 1 (2) | 3 (6) | 52 (100) |
| mAP | Region | VFP (µV) | VDep (µV) | VRep (µV) | VmAP (µV) | dFP (ms) | dDep (ms) | dRep (ms) | dmAP (ms) | dVmax (mV/s) | dVmin (mV/s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| uPMH | - | 45.5 ± 3.1 | −25.5 ± 1.6 | 71.4 ± 3.7 | - | 0.43 ± 0.02 ** | 1.16 ± 0.08 | 1.59 ± 0.08 | 3.4 ± 0.3 | −3.3 ± 0.3 | |
| P1N1 | PMH | - | 50.6 ± 9.0 | −33.7 ± 5.1 | 84.3 ± 13.8 | - | 0.47 ± 0.03 * | 1.20 ± 0.08 * | 1.66 ± 0.08 | 3.5 ± 0.5 | −3.8 ± 0.8 |
| lPMH | - | 43.5 ± 2.2 | −28.4 ± 2.1 | 71.9 ± 3.8 | - | 0.47 ± 0.02 * | 0.94 ± 0.03 * | 1.41 ± 0.02 | 2.7 ± 0.2 | −3.4 ± 0.3 | |
| uPMH | 11.3 ± 3.0 | 41.8 ± 4.1 | −27.4 ± 2.9 | 69.3 ± 6.8 | 0.25 ± 0.02 | 0.38 ± 0.02 | 0.91 ± 0.11 ** | 1.54 ± 0.12 ** | 2.9 ± 0.4 | −3.7 ± 0.5 | |
| P1P2N1 | PMH | 18.8 ± 3.4 | 50.3 ± 7.1 | −31.7 ± 3.8 | 82.0 ± 10.5 | 0.28 ± 0.02 | 0.37 ± 0.01 | 1.39 ± 0.09 * | 2.04 ± 0.10 * | 2.4 ± 0.5 | −3.5 ± 0.5 |
| lPMH | 8.8 ± 1.4 | 38.9 ± 7.1 | −32.4 ± 5.8 | 71.2 ± 12.8 | 0.25 ± 0.03 | 0.38 ± 0.02 | 1.04 ± 0.09 * | 1.64 ± 0.11 * | 2.2 ± 0.4 | −3.0 ± 0.7 | |
| uPMH | −9.0 ± 1.1 | 54.2 ± 7.0 | −33.2 ± 2.6 | 87.4 ± 9.1 | 0.28 ± 0.03 | 0.39 ± 0.02 | 1.11 ± 0.10 | 1.79 ± 0.10 | 4.6 ± 0.6 | −4.1 ± 0.4 | |
| N1P1N2 | PMH | −9.6 ± 1.8 | 45.8 ± 7.4 | −25.2 ± 2.1 | 71.0 ± 8.9 | 0.23 ± 0.02 | 0.36 ± 0.02 | 1.33 ± 0.14 | 1.92 ± 0.14 * | 4.4 ± 0.6 | −3.3 ± 0.5 |
| lPMH | −7.7 ± 0.9 | 50.5 ± 5.5 | −31.7 ± 3.2 | 82.2 ± 8.2 | 0.27 ± 0.06 | 0.33 ± 0.02 | 0.98 ± 0.05 | 1.57 ± 0.11 * | 4.9 ± 0.6 | −5.0 ± 0.8 | |
| uPMH | −9.0 | −43.1 | 22.4 | 65.6 | 0.05 | 0.57 | 1.19 | 1.81 | 3.0 | −3.0 | |
| N1P1 | PMH | −0.7 ± 1.0 | −47.9 ± 6.9 | 35.1 ± 5.7 | 83.0 ± 12.5 | 0.04 ± 0.04 | 0.69 ± 0.06 | 1.45 ± 0.17 | 2.14 ± 0.24 | 2.5 ± 0.3 | −2.2 ± 0.4 |
| lPMH | −1.4 ± 0.8 | −36.5 ± 3.9 | 27.4 ± 2.2 | 63.9 ± 5.8 | 0.05 ± 0.03 | 0.62 ± 0.09 | 1.34 ± 0.05 | 1.99 ± 0.10 | 2.4 ± 0.5 | −1.8 ± 0.1 | |
| uPMH | 5.6 ± 1.5 | −29.2 ± 5.8 | 25.1 ± 2.5 | 54.4 ± 6.8 | 0.42 ± 0.09 | 0.51 ± 0.09 | 1.35 ± 0.14 | 2.29 ± 0.17 | 1.8 ± 0.7 | −2.4 ± 0.7 | |
| P1N1P2 | PMH | 0.5 | −58.5 | 38.1 | 96.6 | 0.09 | 0.70 | 1.53 | 2.23 | 2.61 | −2.2 |
| lPMH | 5.5 | −33.1 | 33.8 | 66.9 | 1.15 | 0.53 | 1.20 | 1.73 | 3.0 | −2.1 |
| mAP | Region | N | dVmax (mV/s) | dVmin (mV/s) | VFP (µV) | VDep (µV) | VRep (µV) | VmAP (µV) | dFP (ms) | dDep (ms) | dRep (ms) | dmAP (ms) | Freqcell (Hz) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thalam. | 70 | 4.6 ± 0.4 | −4.0 ± 0.4 | - | 55.8 ± 4.6 | −21.9 ± 2.9 | 78.8 ± 7.2 | - | 0.43 ± 0.02 | 1.53 ± 0.9 | 1.99 ± 0.8 | 0.6 ± 0.1 | |
| P1N1 | Hypoth. | 55 | 3.2 ± 0.2 | −3.5 ± 0.2 | - | 45.9 ± 2.5 | −28.4 ± 1.5 | 74.3 ± 3.6 | - | 0.45 ± 0.01 | 1.10 ± 0.04 | 1.55 ± 0.04 | 1.3 ± 0.3 |
| Signif. | n.s. | n.s. | n.s. | 0.001 | n.s. | 0.05 | 0.001 | 0.001 | 0.001 | ||||
| Thalam. | 811 | 6.6 ± 0.2 | −6.3 ± 0.2 | 18.2 ± 0.6 | 95.3 ± 2.2 | −53.4 ± 1.1 | 148.8 ± 3.3 | 0.11 ± 0.00 | 0.38 ± 0.00 | 1.78 ± 0.01 | 2.83 ± 1.1 | 1.1 ± 0.1 | |
| P1P2N1 | Hypoth. | 29 | 2.5 ± 0.2 | −3.4 ± 0.3 | 13.7 ± 1.9 | 44.5 ± 3.8 | −30.6 ± 2.3 | 75.1 ± 5.9 | 0.27 ± 0.01 | 0.37 ± 0.01 | 1.15 ± 0.07 | 1.77 ± 0.07 | 1.6 ± 0.7 |
| Signif. | 0.001 | 0.001 | n.s. | 0.001 | 0.001 | 0.001 | 0.001 | n.s. | 0.001 | 0.001 | n.s. | ||
| Thalam. | 216 | 8.2 ± 0.4 | −6.1 ± 0.3 | −15.6 ± 0.7 | 85.2 ± 4.0 | −36.9 ± 1.9 | 122.1 ± 5.8 | 0.16 ± 0.00 | 0.36 ± 0.01 | 1.14 ± 0.01 | 1.66 ± 0.03 | 1.7 ± 0.3 | |
| N1P1N2 | Hypoth. | 53 | 4.7 ± 0.3 | −4.2 ± 0.3 | −8.7 ± 0.7 | 51.0 ± 3.9 | −30.9 ± 1.7 | 81.9 ± 5.2 | 0.27 ± 0.02 | 0.34 ± 0.01 | 1.12 ± 0.06 | 1.74 ± 0.07 | 1.3 ± 0.3 |
| Signif. | 0.001 | 0.001 | 0.001 | 0.001 | n.s. | 0.01 | 0.001 | 0.001 | 0.001 | n.s. | 0.001 | ||
| Thalam. | 17 | 8.5 ± 0.9 | −8.6 ± 0.8 | 6.9 ± 1.0 | −102.6 ± 10.9 | 46.4 ± 6.1 | 149.0 ± 16.2 | 0.08 ± 0.03 | 0.46 ± 0.06 | 1.98 ± 0.07 | 2.65 ± 0.12 | 2.5 ± 0.1 | |
| P1N1P2 | Hypoth. | 9 | 2.3 ± 0.3 | −2.3 ± 0.3 | 4.5 ± 1.1 | −36.5 ± 3.7 | 26.1 ± 1.3 | 62.6 ± 4.4 | 0.28 ± 0.07 | 0.56 ± 0.05 | 1.26 ± 0.08 | 2.06 ± 0.15 | 1.0 ± 0.6 |
| Signif. | 0.001 | 0.001 | n.s. | 0.001 | 0.01 | 0.001 | 0.001 | 0.001 | 0.001 | n.s. | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor, J.; Vega-Zelaya, L.; Martín-Abad, E. Neurophysiological Characterization of Posteromedial Hypothalamus in Anaesthetized Patients. Brain Sci. 2022, 12, 43. https://doi.org/10.3390/brainsci12010043
Pastor J, Vega-Zelaya L, Martín-Abad E. Neurophysiological Characterization of Posteromedial Hypothalamus in Anaesthetized Patients. Brain Sciences. 2022; 12(1):43. https://doi.org/10.3390/brainsci12010043
Chicago/Turabian StylePastor, Jesús, Lorena Vega-Zelaya, and Elena Martín-Abad. 2022. "Neurophysiological Characterization of Posteromedial Hypothalamus in Anaesthetized Patients" Brain Sciences 12, no. 1: 43. https://doi.org/10.3390/brainsci12010043
APA StylePastor, J., Vega-Zelaya, L., & Martín-Abad, E. (2022). Neurophysiological Characterization of Posteromedial Hypothalamus in Anaesthetized Patients. Brain Sciences, 12(1), 43. https://doi.org/10.3390/brainsci12010043







