The Role of Bypass Surgery for the Management of Complex Intracranial Aneurysms in the Anterior Circulation in the Flow-Diverter Era: A Single-Center Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Bypass Strategy
2.3. Aneurysm Occlusion
2.4. Clinical and Radiological Pre- and Postoperative Management
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Bypass Strategies
- A.
- In two patients, an EC–IC bypass with radial artery graft was performed, connecting one of the branches of the external carotid artery (ECA) with the M1 segment of the MCA (patients no. 4 and 16). Both these patients harbored aneurysms in the ICA, of 18 and 25 mm, respectively. Patient no. 4 presented a right large cavernous ICA aneurysm, with symptoms related to mass effect. After she failed the BTO with the need to replace flow in both ACA and MCA territory, a revascularization strategy with high-flow EC–IC bypass with radial artery graft was performed, with subsequent surgical trapping of the aneurysm (the patient presented a stage IV renal insufficiency, preventing an endovascular approach from being safely performed). Patient no. 16 presented with two giant bilateral aneurysms of the cavernous ICA, with mass effect and symptoms related to the left one. Before coming to our attention, after an episode of significant headache, a spontaneous right ICA thrombosis with aneurysm occlusion was found. Therefore, due to the need to replace the only artery assuring flow in the anterior circulation, a high-flow left EC–IC bypass with radial artery graft was performed, with subsequent endovascular occlusion of the left ICA, including the cavernous aneurysm.
- B.
- In eight patients, a double-barrel STA–MCA bypass was performed. In most of these cases (patients no. 1, 3, 8, 9, 12 and 14), this type of bypass was chosen for a complex MCA aneurysm that involvedtwo large branches of the MCA and whose flow needed to be preserved before addressing the aneurysm sac. In two cases (patients no. 7 and 13), this type of bypass was instead performed in complex ICA aneurysms where the BTO demonstrated a neurological deficit only after hypotensive challenge (see above).
- C.
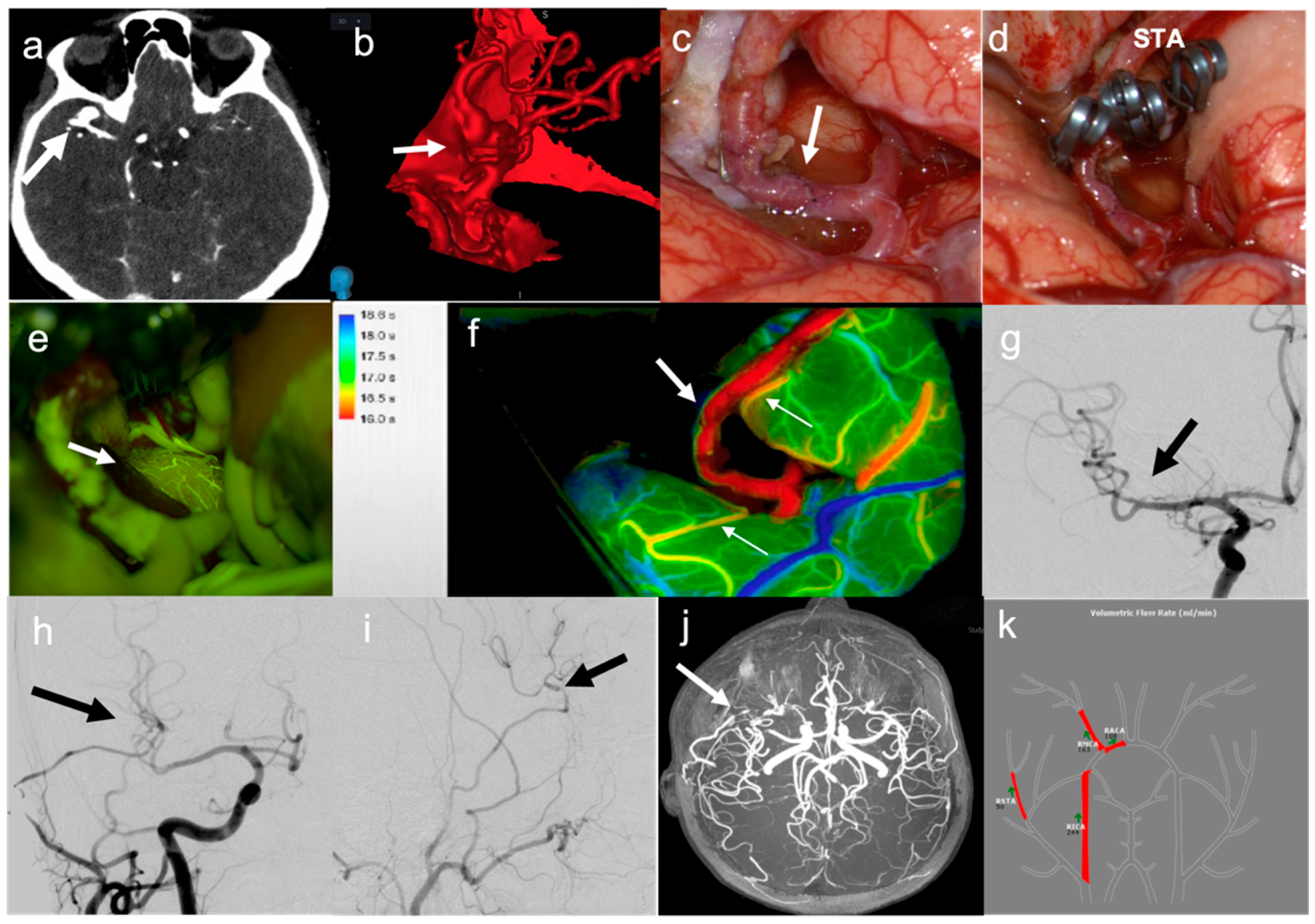
- In six patients, a simple STA–MCA bypass was performed. In two cases (patients no. 2 and 17), this type of bypass was performed to replace a single MCA branch involved in the aneurysm sac as part of the occlusion strategy (Figure 1). In two cases (patients no. 19 and 20), STA–MCA bypasses were performed after having addressed a partially thrombosed aneurysm of the MCA with a temporary clipping, removal of the thrombus and clip reconstruction, with ICG videoangiographic evidence of occlusion of an MCA branch coming out from the aneurysm sac; in these cases, the parietal branch of the STA had been prepared at the beginning of surgery to be ready in case a bypass was needed. In one case (patient no. 6), the patient presented a giant, partially thrombosed and calcified aneurysm of the left ICA, compressing the M1 tract of the left MCA, with a resultant hypoperfusion in the MCA territory and recurrent transient ischemic attacks (TIAs); the STA–MCA bypass was therefore performed to correct the hypoperfusion in the MCA territory before endovascular occlusion of the ICA aneurysm (Figure 2). In one case (patient no. 22), the patient presented a giant aneurysm of the supraclinoid prebifurcation ICA, and the STA–MCA bypass was used to replace flow in the MCA territory before partial trapping of the aneurysm, taking into consideration that flow in the ACA territory was assured by a large AcommA (Figure 3).
- D.
- In one case (patient no. 21), a combined bypass strategy was performed for a complex MCA aneurysm, in which an STA–MCA bypass was used to preserve flow in one of the branches coming out from the MCA aneurysm, while the second larger branch was re-connected (IC–IC bypass) with the proximal afferent artery though an end-to-end microanastomosis after sectioning of the unclippable aneurysm sac from the MCA circulation.
- E.
- In three patients (patients no. 10, 15 and 18), a side-to-side pericallosal artery–pericallosal artery (perA–perA) bypass was performed to preserve distal flow in the ACA territory, before endovascular treatment of complex aneurysm in the proximal ACA (Figure 4).
- F.
- In three patients, a combined procedure involving multiple bypasses to preserve flow in different territories of the distal ACA was performed. In one case (patient no. 5), a side-to-side perA–perA bypass was performed together with a right STA–CmaA artery bypass using a contralateral STA as a graft, to replace both perA and CmaA territories before endovascularly occluding a complex aneurysm of the proximal right ACA that was already submitted to an unsuccessful endovascular treatment in another institution [24]. In one case (patient no. 11), PerA–PerA and CmaA–CmaA artery bypasses were performed in the same patient to preserve the two distal territories in a complex large aneurysm of the proximal ACA, involving both pericallosal and callosomarginal arteries [20]. In one case (patient no. 23), a similar strategy was planned. However, after intraoperative thrombosis of perA–perA bypass occurred two times, a salvage strategy was considered, by grafting the STA segment at its bifurcation into parietal and frontal branches to create a bridge between the right perA proximal to the thrombus and the two perA distal to the thrombus.
- G.
- In one case, an OA–MCA was performed in the same patient (no. 16) that previously received a high-flow EC–IC bypass with radial artery graft, due to the reduced flow in the graft for subsequent vasospasm, with left hemispheric hypoperfusion.
3.3. Strategy of Aneurysm Occlusion
3.3.1. MCA Aneurysms
3.3.2. ACA Aneurysms
3.3.3. ICA Aneurysms
3.4. Periprocedural Complications
3.5. Bypass Patency, Short- and Long-Term Results
4. Discussion
Limitations and Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, R.D.; Broderick, J.P. Unruptured Intracranial Aneurysms: Epidemiology, Natural History, Management Options, and Familial Screening. Lancet. Neurol. 2014, 13, 393–404. [Google Scholar] [CrossRef]
- Li, J.; Shen, B.; Ma, C.; Liu, L.; Ren, L.; Fang, Y.; Dai, D.; Chen, S.; Lu, J. 3D Contrast Enhancement-MR Angiography for Imaging of Unruptured Cerebral Aneurysms: A Hospital-Based Prevalence Study. PLoS ONE 2014, 9, e114157. [Google Scholar] [CrossRef] [Green Version]
- Greving, J.P.; Wermer, M.J.H.; Brown, R.D.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.E.; et al. Development of the PHASES Score for Prediction of Risk of Rupture of Intracranial Aneurysms: A Pooled Analysis of Six Prospective Cohort Studies. Lancet. Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Tominari, S.; Morita, A.; Ishibashi, T.; Yamazaki, T.; Takao, H.; Murayama, Y.; Sonobe, M.; Yonekura, M.; Saito, N.; Shiokawa, Y.; et al. Prediction Model for 3-Year Rupture Risk of Unruptured Cerebral Aneurysms in Japanese Patients. Ann. Neurol. 2015, 77, 1050–1059. [Google Scholar] [CrossRef] [Green Version]
- Juvela, S. PHASES Score and Treatment Scoring with Cigarette Smoking in the Long-Term Prediction of Rupturing of Unruptured Intracranial Aneurysms. J. Neurosurg. 2021, 136, 156–162. [Google Scholar] [CrossRef]
- Etminan, N.; de Sousa, D.A.; Tiseo, C.; Bourcier, R.; Desal, H.; Lindgren, A.; Koivisto, T.; Netuka, D.; Peschillo, S.; Lémeret, S.; et al. European Stroke Organisation (ESO) Guidelines on Management ofunruptured Intracranial Aneurysms. Eur. Stroke J. 2022, 7, V. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.J.S.; Tampieri, D.; Iancu, D.; Cortes, M.; Agid, R.; Krings, T.; Wong, J.; Lavoie, P.; Ghostine, J.; Shettar, B.; et al. SILK Flow Diverter for Complex Intracranial Aneurysms: A Canadian Registry. J. Neurointerv. Surg. 2016, 8, 273–278. [Google Scholar] [CrossRef]
- Dabus, G.; Grossberg, J.A.; Cawley, C.M.; Dion, J.E.; Puri, A.S.; Wakhloo, A.K.; Gonsales, D.; Aguilar-Salinas, P.; Sauvageau, E.; Linfante, I.; et al. Treatment of Complex Anterior Cerebral Artery Aneurysms with Pipeline Flow Diversion: Mid-Term Results. J. Neurointerv. Surg. 2017, 9, 147–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becske, T.; Kallmes, D.F.; Saatci, I.; McDougall, C.G.; Szikora, I.; Lanzino, G.; Moran, C.J.; Woo, H.H.; Lopes, D.K.; Berez, A.L.; et al. Pipeline for Uncoilable or Failed Aneurysms: Results from a Multicenter Clinical Trial. Radiology 2013, 267, 858–868. [Google Scholar] [CrossRef]
- Becske, T.; Brinjikji, W.; Potts, M.B.; Kallmes, D.F.; Shapiro, M.; Moran, C.J.; Levy, E.I.; McDougall, C.G.; Szikora, I.; Lanzino, G.; et al. Long-Term Clinical and Angiographic Outcomes Following Pipeline Embolization Device Treatment of Complex Internal Carotid Artery Aneurysms: Five-Year Results of the Pipeline for Uncoilable or Failed Aneurysms Trial. Neurosurgery 2017, 80, 40–48. [Google Scholar] [CrossRef]
- Kiselev, R.; Orlov, K.; Dubovoy, A.; Berestov, V.; Gorbatykh, A.; Kislitsin, D.; Shayakhmetov, T.; Tasenko, A.; Seleznev, P.; Strelnikov, N.; et al. Flow Diversion versus Parent Artery Occlusion with Bypass in the Treatment of Complex Intracranial Aneurysms: Immediate and Short-Term Outcomes of the Randomized Trial. Clin. Neurol. Neurosurg. 2018, 172, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Nurminen, V.; Raj, R.; Numminen, J.; Kivisaari, R.; Niemelä, M.; Lehecka, M. Flow Diversion for Internal Carotid Artery Aneurysms: Impact of Complex Aneurysm Features and Overview of Outcome. Clin. Neurol. Neurosurg. 2020, 193, 105782. [Google Scholar] [CrossRef] [PubMed]
- Hanel, R.A.; Spetzler, R.F. Surgical Treatment of Complex Intracranial Aneurysms. Neurosurgery 2008, 62, SHC1289–SHC1299. [Google Scholar] [CrossRef]
- Sanai, N.; Zador, Z.; Lawton, M.T. Bypass Surgery for Complex Brain Aneurysms: An Assessment of Intracranial-Intracranial Bypass. Neurosurgery 2009, 65, 670–683. [Google Scholar] [CrossRef]
- Kalani, M.Y.S.; Ramey, W.; Albuquerque, F.C.; McDougall, C.G.; Nakaji, P.; Zabramski, J.M.; Spetzler, R.F. Revascularization and Aneurysm Surgery: Techniques, Indications, and Outcomes in the Endovascular Era. Neurosurgery 2014, 74, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Clarençon, F.; Bonneville, F.; Boch, A.L.; Lejean, L.; Biondi, A. Parent Artery Occlusion Is Not Obsolete in Giant Aneurysms of the ICA. Experience with Very-Long-Term Follow-Up. Neuroradiology 2011, 53, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, L.; Niemelä, M.; Meling, T.; Lehecka, M.; Lehto, H.; Hernesniemi, J. Bypass Surgery for Complex Middle Cerebral Artery Aneurysms: Impact of the Exact Location in the MCA Tree. J. Neurosurg. 2014, 120, 398–408. [Google Scholar] [CrossRef] [Green Version]
- Andaluz, N.; Zuccarello, M. Treatment Strategies for Complex Intracranial Aneurysms: Review of a 12-Year Experience at the University of Cincinnati. Skull Base 2011, 21, 233. [Google Scholar] [CrossRef]
- Lawton, M.T.; Hamilton, M.G.; Morcos, J.J.; Spetzler, R.F. Revascularization and Aneurysm Surgery: Current Techniques, Indications, and Outcome. Neurosurgery 1996, 38, 83–94. [Google Scholar] [CrossRef]
- Acerbi, F.; Vetrano, I.G.; Falco, J.; Gioppo, A.; Ciuffi, A.; Ziliani, V.; Schiariti, M.; Broggi, M.; Faragò, G.; Ferroli, P. In Situ Side-to-Side Pericallosal-Pericallosal Artery and Callosomarginal-Callosomarginal Artery Bypasses for Complex Distal Anterior Cerebral Artery Aneurysms: A Technical Note. Oper. Neurosurg. 2020, 19, E487–E495. [Google Scholar] [CrossRef]
- Mohit, A.A.; Sekhar, L.N.; Natarajan, S.K.; Britz, G.W.; Ghodke, B. High-Flow Bypass Grafts in the Management of Complex Intracranial Aneurysms. Neurosurgery 2007, 60, ONS105-22. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, L.N.; Natarajan, S.K.; Ellenbogen, R.G.; Ghodke, B. Cerebral Revascularization for Ischemia, Aneurysms, and Cranial Base Tumors. Neurosurgery 2008, 62, SHC1373–SHC1410. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Saloner, D.; Rayz, V.L.; Lawton, M.T. Giant Intracranial Aneurysms: Evolution of Management in a Contemporary Surgical Series. Neurosurgery 2011, 69, 1261–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferroli, P.; Lacorte, E.; Bertolini, G.; Serrao, G.; Acerbi, F. Anterior Cerebral Artery Revascularization: Superficial Temporal Artery Callosomarginal Artery Bypass Using a Contralateral Superficial Temporal Artery Interposition Graft. J. Neurosurg. Sci. 2018, 62, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Prada, F.; Vetrano, I.G.; Falco, J.; Faragò, G.; Ferroli, P.; DiMeco, F. Indocyanine Green and Contrast-Enhanced Ultrasound Videoangiography: A Synergistic Approach for Real-Time Verification of Distal Revascularization and Aneurysm Occlusion in a Complex Distal Middle Cerebral Artery Aneurysm. World Neurosurg. 2019, 125, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ferroli, P.; Brock, S.; Leonardi, M.; Schiavolin, S.; Acerbi, F.; Broggi, M. Complications in Neurosurgery: Application of Landriel Ibañez Classification and Preliminary Considerations on 1000 Cases. World Neurosurg. 2014, 82, E576–E577. [Google Scholar] [CrossRef]
- Ferroli, P.; Vetrano, I.G.; Schiavolin, S.; Acerbi, F.; Zattra, C.M.; Schiariti, M.; Leonardi, M.; Broggi, M. Brain Tumor Resection in Elderly Patients: Potential Factors of Postoperative Worsening in a Predictive Outcome Model. Cancers 2021, 13, 2320. [Google Scholar] [CrossRef]
- Ferroli, P.; Broggi, M.; Schiavolin, S.; Acerbi, F.; Bettamio, V.; Caldiroli, D.; Cusin, A.; La Corte, E.; Leonardi, M.; Raggi, A.; et al. Predicting Functional Impairment in Brain Tumor Surgery: The Big Five and the Milan Complexity Scale. Neurosurg. Focus 2015, 39, E14. [Google Scholar] [CrossRef] [Green Version]
- Narducci, A.; Onken, J.; Czabanka, M.; Hecht, N.; Vajkoczy, P. Fluorescein Videoangiography during Extracranial-to-Intracranial Bypass Surgery: Preliminary Results. Acta Neurochir. 2018, 160, 767–774. [Google Scholar] [CrossRef]
- Lawton, M.T.; Lang, M.J. The Future of Open Vascular Neurosurgery: Perspectives on Cavernous Malformations, AVMs, and Bypasses for Complex Aneurysms. J. Neurosurg. 2019, 130, 1409–1425. [Google Scholar] [CrossRef]
- Karnchanapandh, K.; Imizu, M.; Kato, Y.; Sano, H.; Hayakawa, M.; Kanno, T. Successful Obliteration of a Ruptured Partially Thrombosed Giant M1 Fusiform Aneurysm with Coil Embolization at Distal M1 after Extracranial-Intracranial Bypass. Minim. Invasive Neurosurg. 2002, 45, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Rodrĺguez-Hernández, A.; Lawton, M.T. Flash Fluorescence with Indocyanine Green Videoangiography to Identify the Recipient Artery for Bypass with Distal Middle Cerebral Artery Aneurysms: Operative Technique. Neurosurgery 2012, 70, 209–220. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, e2–e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, B.; Bohnstedt, B.N.; Cohen-Gadol, A.A. A Prospective Comparative Study of Microscope-Integrated Intraoperative Fluorescein and Indocyanine Videoangiography for Clip Ligation of Complex Cerebral Aneurysms. J. Neurosurg. 2015, 122, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, M.B.; Yonas, H.; Jungreis, C.; Hung, T.K. Management of a Giant Middle Cerebral Artery Fusiform Serpentine Aneurysm with Distal Clip Application and Retrograde Thrombosis: Case Report and Review of the Literature. Surg. Neurol. 1994, 41, 221–225. [Google Scholar] [CrossRef]
- Horie, N.; Takahashi, N.; Furuichi, S.; Mori, K.; Onizuka, M.; Tsutsumi, K.; Shibata, S. Giant Fusiform Aneurysms in the Middle Cerebral Artery Presenting with Hemorrhages of Different Origins. Report of Three Cases and Review of the Literature. J. Neurosurg. 2003, 99, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Abou-Ismail, M.Y.; Diamond, A.; Kapoor, S.; Arafah, Y.; Nayak, L. The Hypercoagulable State in COVID-19: Incidence, Pathophysiology, and Management. Thromb. Res. 2020, 194, 101. [Google Scholar] [CrossRef]
- Seibert, B.; Tummala, R.P.; Chow, R.; Faridar, A.; Mousavi, S.A.; Divani, A.A. Intracranial Aneurysms: Review of Current Treatment Options and Outcomes. Front. Neurol. 2011, 2, 45. [Google Scholar] [CrossRef] [Green Version]
- Briganti, F.; Leone, G.; Marseglia, M.; Mariniello, G.; Caranci, F.; Brunetti, A.; Maiuri, F. Endovascular Treatment of Cerebral Aneurysms Using Flow-Diverter Devices: A Systematic Review. Neuroradiol. J. 2015, 28, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Kan, P.; Sweid, A.; Srivatsan, A.; Jabbour, P. Expanding Indications for Flow Diverters: Ruptured Aneurysms, Blister Aneurysms, and Dissecting Aneurysms. Neurosurgery 2020, 86, S96–S103. [Google Scholar] [CrossRef]
- Bonney, P.A.; Connor, M.; Fujii, T.; Singh, P.; Koch, M.J.; Stapleton, C.J.; Mack, W.J.; Walcott, B.P. Failure of Flow Diverter Therapy: Predictors and Management Strategies. Neurosurgery 2020, 86, S64–S73. [Google Scholar] [CrossRef] [PubMed]
- Zanaty, M.; Chalouhi, N.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.; Jabbour, P. Flow Diversion for Complex Middle Cerebral Artery Aneurysms. Neuroradiology 2014, 56, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Abla, A.A.; McDougall, C.M.; Breshears, J.D.; Lawton, M.T. Intracranial-to-Intracranial Bypass for Posterior Inferior Cerebellar Artery Aneurysms: Options, Technical Challenges, and Results in 35 Patients. J. Neurosurg. 2016, 124, 1275–1286. [Google Scholar] [CrossRef] [Green Version]
- Amin-Hanjani, S.; Butler, W.E.; Ogilvy, C.S.; Carter, B.S.; Barker, F.G. Extracranial-Intracranial Bypass in the Treatment of Occlusive Cerebrovascular Disease and Intracranial Aneurysms in the United States between 1992 and 2001: A Population-Based Study. J. Neurosurg. 2005, 103, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.G.; Peerless, S.J.; Ferguson, G.G. Hunterian Proximal Arterial Occlusion for Giant Aneurysms of the Carotid Circulation. J. Neurosurg. 1994, 81, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Stieg, P.E.; Fraser, J.F. Surgical Bypass for Intracranial Aneurysms: Navigating Around a Changing Paradigm. World Neurosurg. 2011, 75, 414–417. [Google Scholar] [CrossRef]
- Nussbaum, E.S.; Kallmes, K.M.; Lassig, J.P.; Goddard, J.K.; Madison, M.T.; Nussbaum, L.A. Cerebral Revascularization for the Management of Complex Intracranial Aneurysms: A Single-Center Experience. J. Neurosurg. 2018, 131, 1297–1307. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Shi, X.; Liu, F.; Sun, Y.; Qian, H.; Zhou, Z.; Zhang, Y.; Wang, L. Natural History and Clinical Outcomes in Patients with Complex Intracranial Aneurysms: A Review of 115 Bypass Cases and 22 Nonsurgical Cases. Neurosurg. Rev. 2020, 43, 1605–1613. [Google Scholar] [CrossRef]
- Wiebers, D.O. Unruptured Intracranial Aneurysms: Natural History, Clinical Outcome, and Risks of Surgical and Endovascular Treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured Intracranial Aneurysms—Risk of Rupture and Risks of Surgical Intervention. N. Engl. J. Med. 1998, 339, 1725–1733. [Google Scholar] [CrossRef]
- UCAS Japan Investigators; Morita, A.; Kirino, T.; Hashi, K.; Aoki, N.; Fukuhara, S.; Hashimoto, N.; Nakayama, T.; Sakai, M.; Teramoto, A.; et al. The Natural Course of Unruptured Cerebral Aneurysms in a Japanese Cohort. N. Engl. J. Med. 2012, 366, 2474–2482. [Google Scholar] [CrossRef]
- Hwang, G.; Oh, C.W.; Park, S.Q.; Sheen, S.H.; Bang, J.S.; Kang, H.S. Comparison of Different Microanastomosis Training Models: Model Accuracy and Practicality. J. Korean Neurosurg. Soc. 2010, 47, 287. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Ramachandra, A.B.; Boyd, J.; Marsden, A.L.; Kahn, A.M. Computational Evaluation of Venous Graft Geometries in Coronary Artery Bypass Surgery. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, H.Q.; Li, X.Y.; Tong, X.Z. Double-Barrel Superficial Temporal Artery to Proximal Middle Cerebral Artery Bypass to Treat Complex Intracranial Aneurysms: A Reliable High Blood Flow Bypass. World Neurosurg. 2019, 125, e884–e890. [Google Scholar] [CrossRef] [PubMed]
- Raheja, A.; Suri, A.; Sreenivasan, S.A.; Singla, R. Insurance and Flow-Alteration Superficial Temporal Artery to Middle Cerebral Artery (STA-MCA) Bypass in Management of Complex Anterior Intracranial Circulation Aneurysms in Postendovascular Era. World Neurosurg. 2019, 126, e1387–e1398. [Google Scholar] [CrossRef]
- Stapleton, C.J.; Atwal, G.S.; Hussein, A.E.; Amin-Hanjani, S.; Charbel, F.T. The Cut Flow Index Revisited: Utility of Intraoperative Blood Flow Measurements in Extracranial-Intracranial Bypass Surgery for Ischemic Cerebrovascular Disease. J. Neurosurg. 2019, 133, 1396–1400. [Google Scholar] [CrossRef]
- Grigore, F.N.; Amin-Hanjani, S. A3-A3 Bypass Surgery for Aneurysm: Technical Nuances. Oper. Neurosurg. 2019, 17, 277–285. [Google Scholar] [CrossRef]
- Park, E.S.; Ahn, J.S.; Park, J.C.; Kwon, D.H.; Kwun, B.D.; Kim, C.J. STA-ACA Bypass Using the Contralateral STA as an Interposition Graft for the Treatment of Complex ACA Aneurysms: Report of Two Cases and a Review of the Literature. Acta Neurochir. 2012, 154, 1447–1453. [Google Scholar] [CrossRef]
- Kalani, Y.S.M.; Zabramski, J.M.; Hu, Y.C.; Spetzler, R.F. Extracranial-Intracranial Bypass and Vessel Occlusion for the Treatment of Unclippable Giant Middle Cerebral Artery Aneurysms. Neurosurgery 2013, 72, 428–435. [Google Scholar] [CrossRef]
- Heros, R.C. Distal Arterial Occlusion for Dissecting Aneurysms. J. Neurosurg. 2009, 111, 75–76. [Google Scholar] [CrossRef]
- Ferroli, P.; Ciceri, E.; Parati, E.; Minati, L.; Broggi, G. Obliteration of a Giant Fusiform Carotid Terminus-M1 Aneurysm after Distal Clip Application and Extracranial-Intracranial Bypass: Case Report. J. Neurosurg. Sci. 2007, 51, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Dengler, J.; Kato, N.; Vajkoczy, P. The Y-Shaped Double-Barrel Bypass in the Treatment of Large and Giant Anterior Communicating Artery Aneurysms. J. Neurosurg. 2013, 118, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, G.; Fierstra, J.; Regli, L. Distal Outflow Occlusion with Bypass Revascularization: Last Resort Measure in Managing Complex MCA and PICA Aneurysms. Acta Neurochir. 2016, 158, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.P.; Kim, T.S.; Choi, J.W.; Lee, J.K.; Kim, Y.S.; Moon, K.S.; Kim, J.H.; Kim, S.H. Characteristics and Management of Ruptured Distal Middle Cerebral Artery Aneurysms. Acta Neurochir. 2007, 149, 661–667. [Google Scholar] [CrossRef]
- Lan, L.; Liu, H.; Ip, V.; Soo, Y.; Abrigo, J.; Fan, F.; Ma, S.H.; Ma, K.; Ip, B.; Liu, J.; et al. Regional High Wall Shear Stress Associated with Stenosis Regression in Symptomatic Intracranial Atherosclerotic Disease. Stroke 2020, 51, 3064–3073. [Google Scholar] [CrossRef]
- Abla, A.A.; Lawton, M.T. Anterior Cerebral Artery Bypass for Complex Aneurysms: An Experience with Intracranial-Intracranial Reconstruction and Review of Bypass Options: Clinical Article. J. Neurosurg. 2014, 120, 1364–1377. [Google Scholar] [CrossRef]
- Acerbi, F.; Vetrano, I.G.; Sattin, T.; Falco, J.; de Laurentis, C.; Zattra, C.M.; Bosio, L.; Rossini, Z.; Broggi, M.; Schiariti, M.; et al. Use of ICG Videoangiography and FLOW 800 Analysis to Identify the Patient-Specific Venous Circulation and Predict the Effect of Venous Sacrifice: A Retrospective Study of 172 Patients. Neurosurg. Focus 2018, 45, 1–10. [Google Scholar] [CrossRef]
- Lane, B.C.; Cohen-Gadol, A.A. A Prospective Study of Microscope-Integrated Intraoperative Fluorescein Videoangiography during Arteriovenous Malformation Surgery: Preliminary Results. Neurosurg. Focus 2014, 36, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M.; Broggi, M.; Ferroli, P.; Acerbi, F. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated With a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Acerbi, F.; Restelli, F.; Broggi, M.; Schiariti, M.; Ferroli, P. Feasibility of Simultaneous Sodium Fluorescein and Indocyanine Green Injection in Neurosurgical Procedures. Clin. Neurol. Neurosurg. 2016, 146, 123–129. [Google Scholar] [CrossRef]
- Acerbi, F.; Vetrano, I.G.; Sattin, T.; de Laurentis, C.; Bosio, L.; Rossini, Z.; Broggi, M.; Schiariti, M.; Ferroli, P. The Role of Indocyanine Green Videoangiography with FLOW 800 Analysis for the Surgical Management of Central Nervous System Tumors: An Update. Neurosurg. Focus 2018, 44, E6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajirayat, K.; Gholampour, S.; Sharifi, I.; Bizari, D. Biomechanical Simulation to Compare the Blood Hemodynamics and Cerebral Aneurysm Rupture Risk in Patients with Different Aneurysm Necks. J. Appl. Mech. Tech. Phys. 2018, 58, 968–974. [Google Scholar] [CrossRef]
- Gholampour, S.; Mehrjoo, S. Effect of Bifurcation in the Hemodynamic Changes and Rupture Risk of Small Intracranial Aneurysm. Neurosurg. Rev. 2021, 44, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, S.; Takagi, R.; Araki, Y.; Uda, K.; Sumitomo, M.; Okamoto, S.; Nishihori, M.; Izumi, T.; Nakamura, M.; Saito, R. Blood Flow Stagnation after Treatment of a Giant Internal Carotid Artery Aneurysm: A Computed Fluid Dynamics Analysis. Sci. Rep. 2022, 12, 7283. [Google Scholar] [CrossRef] [PubMed]



| Aneurysm Characteristic | Intraoperative Data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CASE | Location | Size/Shape/ Rupture Status | Circle of Willis Variations | Intramural Thrombosis | Calcification | Vessels Included | Previous Treatment | Bypass Strategy | Aneurysm Exclusion Strategy | Intraoperative Tool |
| 1 | L MCA | 32 mm/ Fusiform | \ | Partially thrombosed | Wall Calcification | M1 | \ | STA–MCA Double Barrel | Trapping | ICG-VA/FLOW 800/CEUS |
| 2 | L MCA (Distal) | 14 mm/ Fusiform | \ | Partially thrombosed | \ | M4 | \ | STA–MCA | Distal Clipping | ICG-VA/FLOW 800/CEUS |
| 3 | R MCA | 35 mm/ Fusiform | Hypoplastic L A1 | Partially thrombosed | Wall Calcification | Lenticulostriate | \ | STA–MCA Double Barrel | Distal Clipping | ICG-VA/FLOW 800 |
| 4 | R ICA (intracavenous) | 18 mm/ Saccular | Duplicated L PComA | \ | \ | \ | \ | EC–IC Radial Graft | Trapping | ICG-VA/FLOW 800 |
| 5 | L ACA (A1-A2) | 40 mm/ Saccular | \ | \ | Wall Calcification | \ | Previous Coiling | PerA–PerA/r STA–r CMA with l STA graft | Endovascular Occlusion | ICG-VA/FLOW 800/CEUS |
| 6 | L ICA | 15 mm/ Saccular | Absent L PcomA | Partially thrombosed | Wall Calcification | \ (Compression M2 Stenosis) | \ | STA–MCA | Endovascular Stenting | ICG-VA/FLOW 800 |
| 7 | R ICA (intracavernous) | 20 mm/ Fusiform | Dysplastic L PcomA, Hypoplastic R A1 | \ | Wall Calcification | ICA | \ | STA–MCADouble Barrel | Endovascular Occlusion | ICG-VA/FLOW 800 |
| 8 | R MCA | 10 mm/ Fusiform | Hypoplastic R A1 | \ | \ | M2 | \ | STA–MCADouble Barrel | Trapping | ICG-VA/FLOW 800 |
| 9 | L MCA | 12 mm/ Saccular/ ruptured | \ | \ | \ | \ | Previous Coiling | STA–MCADouble Barrel | Trapping | ICG-VA/FLOW 800/CEUS |
| 10 | R ACA (A1-A2) | 5 mm/ Saccular | Unilateral L fetal-type variant with ipsilateral dominant PComA and hypoplastic P1 | \ | \ | A1-A2 | Previous Coiling | PerA–PerA | Endovascular Occlusion | ICG-VA/FLOW 800/CEUS |
| 11 | L PerA—CMA | 16 mm/ Fusiform | \ | \ | WallCalcification | L CMA | \ | PerA–PerA/CMA–CMA | Endovascular Occlusion | ICG-VA/FLOW 800/CEUS, fluorescein |
| 12 | L MCA | 9 mm/ Saccular/ ruptured | Bilateral hypoplastic PComA | \ | \ | M2 | \ | STA–MCADouble Barrel | Trapping | ICG-VA/FLOW 800/CEUS/fluorescein |
| 13 | R ICA (intracavernous) | 21 mm/Saccular | Bilateral hypoplastic PComA | \ | Wall Calcification | ICA | Previous Coiling | STA–MCADouble Barrel | Endovascular Occlusion | ICG-VA/FLOW 800 |
| 14 | R MCA | 45 mm/ Saccular/ ruptured | \ | \ | \ | M2 | \ | STA–MCA Double Barrel | Trapping | ICG-VA/FLOW 800 |
| 15 | AcomA | 22 mm/Saccular | \ | \ | \ | \ | \ | PerA–PerA | Endovascular Occlusion | ICG-VA/FLOW 800 |
| 16 | L ICA | 25 mm/Saccular | Absent L PcomA | (R ICA Giant thrombosed Aneurysm) | \ | ICA | \ | EC–IC Radial Graft/OA–MCA | Endovascular Occlusion | ICG-VA/FLOW 800 |
| 17 | R MCA | 7 mm/Fusiform | \ | \ | \ | M2 (trifurcation) | \ | STA–MCA | Trapping | ICG-VA/FLOW 800/CEUS/fluorescein |
| 18 | L ACA (A1) | 33 mm/Saccular | \ | \ | Wall Calcification | Perforating artery | \ | PerA–PerA | Endovascular Occlusion | ICG-VA/FLOW 800/CEUS |
| 19 | L MCA (distal) | 20 mm/Saccular | Hypoplastic R A1 | Partially thrombosed | Wall Calcification | M3 | \ | STA–MCA | Clipping (Vessels Ostium Dissection) | ICG-VA/FLOW 800 |
| 20 | R MCA | 14 mm/Saccular | \ | Partially thrombosed | \ | Temporal M2 | \ | STA–MCA | Clipping Reconstruction | ICG-VA/FLOW 800 |
| 21 | R MCA | 15 mm/ Fusiform | Unilateral R fetal-type variant with ipsilateral dominant PComA and hypoplastic P1 | Partially thrombosed | Wall Calcification | M2 | IC–IC (Major Branch)/STA–MCA (Minor Branch) | Trapping | ICG-VA/FLOW 800/CUES | |
| 22 | R ICA | 37 mm/ Fusiform | \ | \ | \ | M1-A1 | \ | STA–MCA | Partial Trapping and Thrombotic Occlusion | ICG-VA/FLOW 800 |
| 23 | R ACA | 13 mm/Saccular | Bilateral fetal-type variant with dominant PComA and hypoplastic P1 | Partially thrombosed | \ | PerA–CMA origin | \ | PerA–PerA/L A3-Bilateral A4 STA–Graft | Endovascular Flow Diverter | ICG-VA/FLOW 800 |
| CASE | Age/ Gender | BMI | Comorbidity | Onset Symptoms | Preoperative mRS | Preoperative KPS |
|---|---|---|---|---|---|---|
| 1 | 67/F | 32.77 | \ | TIA | 1 | 90 |
| 2 | 20/M | 25.25 | Smoking | Seizure | 0 | 100 |
| 3 | 60/F | 19.84 | \ | Confusion | 1 | 90 |
| 4 | 63/F | 27.5 | Stage IV renal insufficiency | Pain | 0 | 100 |
| 5 | 59/F | 27.34 | Hypertension | Seizure | 0 | 100 |
| 6 | 66/F | 24.3 | \ | Anomic aphasia | 1 | 90 |
| 7 | 51/M | 31.4 | Hypertension | Vertigo | 0 | 100 |
| 8 | 50/F | 22.5 | Smoking | Incidental finding | 1 | 100 |
| 9 | 35/F | 20.6 | \ | FU in SAH | 1 | 100 |
| 10 | 26/M | 29.7 | \ | FU after endovascular treatment | 0 | 100 |
| 11 | 53/M | 26.12 | Smoking | Incidental finding | 1 | 90 |
| 12 | 51/M | 24.34 | \ | Headache | 1 | 90 |
| 13 | 74/F | 28.4 | Hypertension | Diplopia | 2 | 80 |
| 14 | 45/F | 21.22 | \ | SAH | 0 | 90 |
| 15 | 75/F | 20.9 | Hypertension | Reduction in visual acuity (left eye) | 0 | 90 |
| 16 | 60/F | 26.22 | Hypertension and smoking | Diplopia (III c.n. palsy), headache | 0 | 80 |
| 17 | 54/F | 24.77 | \ | Headache | 1 | 100 |
| 18 | 66/F | 27.41 | Hypertension and smoking | Visual field defects | 1 | 80 |
| 19 | 36/F | 20.43 | \ | Incidental finding | 0 | 100 |
| 20 | 62/F | 16.42 | Hypertension | Headache | 0 | 90 |
| 21 | 66/M | 47.75 | Diabetes, cardiovascular disease, obesity, smoking | Confusion | 0 | 90 |
| 22 | 19/M | 20.9 | \ | Reduction in visual acuity (right eye) | 0 | 100 |
| 23 | 32/F | 33.45 | Recent SARS-CoV-2 infection | Incidental finding | 1 | 100 |
| Complication | Clinical Data | Radiological Data | |||||
|---|---|---|---|---|---|---|---|
| CASE | Intraoperative | Postoperative | KPS at Discharge | Last F-U mRS/KPS | Aneurysm Occlusion at Last F-U | Immediate Bypass Patency | Bypass Patency at Last F-U |
| 1 | Aneurysm rupture | Internal capsule stroke (hemiparesis and aphasia) | 30 | 72 months— mRS 2/KPS 80 | 84 months—Yes | Yes | 84 months—Yes |
| 2 | \ | \ | 100 | 84 months— mRS 0/KPS 100 | 84 months—Yes | Yes | 84 months—Yes |
| 3 | \ | Internal capsule stroke (hemiparesis) | 70 | 36 months— mRS 4/KPS 40 | 36 months—Yes | Yes | 36 months—Yes |
| 4 | \ | Internal capsule stroke (monoparesis) | 80 | 3 months— mRS 6/KPS 0 ESRD (Death) | 2 months—Yes | Yes | 2 months—Yes |
| 5 | \ | \ | 90 | 60 months— mRS 0/KPS 100 | 60 months—Yes | Yes ACA–ACA and STA–ACA | 60 months—ACA–ACA Yes/STA–ACA No |
| 6 | \ | \ | 100 | 48 months— mRS 0/KPS 100 | 36 months—Yes | Yes | 48 months—Yes |
| 7 | \ | \ | 100 | 36 months- mRS 0/KPS 100 | 36 months—Yes | Yes | 36 months—Yes |
| 8 | \ | \ | 100 | 48 months— mRS 0/KPS 100 | 48 months—Yes | Yes | 48 months—Yes |
| 9 | Extradural hematoma | \ | 100 | 48 months— mRS 0/KPS 100 | 48 months—Yes | Yes | 48 months—Yes |
| 10 | \ | \ | 100 | 48 months— mRS 0/KPS 100 | 48 months—Yes | Yes | 48 months—Yes |
| 11 | \ | \ | 100 | 24 months— mRS 0/KPS 100 | 24 months—Yes | Yes | 24 months—Yes |
| 12 | \ | \ | 90 | 9 months—mRS 1/KPS 90 | 5 months—Yes | Yes | 5 months—Yes |
| 13 | \ | \ | 80 | 48 months— mRS 2/KPS 70 (Unrelated, CMT) | 48 months—Yes | Yes | 48 months—Yes |
| 14 | Aneurysm rupture | Internal capsule stroke; evidence of hemorrhagic lesion in the brainstem; deep coma | 30 | 12 months— mRS 6/KPS 0—Death | 6 months—Yes | yes | 6 months—Yes |
| 15 | \ | \ | 90 | 3 months— mRS 1/KPS 90 | 3 months—Yes | Yes | 3 months—Yes |
| 16 | \ | Radial artery graft vasospasm with need for angioplasty; left hemispheric hypoperfusion with TIAs | 60 | 24 months— mRS 1/KPS 90 | 24 months—Yes | Yes EC–IC and OA–MCA | 24 months—No EC–IC and OA–MCA |
| 17 | \ | \ | 100 | 24 months— mRS 0/KPS 100 | 24 months—Yes | Yes | 24 months—Yes |
| 18 | \ | Cerebellar hemorrhage— hydrocephalus treated with VP shunt | 40 | 14 months— mRS 1/KPS 90 | 14 monthsYes | Yes | 14 months—Yes |
| 19 | \ | \ | 100 | 12 months— mRS 0/KPS 100 | 12 months—Yes | Yes | 12 months—Yes |
| 20 | \ | \ | 100 | 5 months— mRS 0/KPS 100 | 5 months—Yes | Yes | 5 months—Yes |
| 21 | \ | \ | 100 | 4 months— mRS 0/KPS 100 | 4 months—Yes | Yes | 4 months—Yes |
| 22 | \ | \ | 90 | 6 months— mRS 0/KPS 100 | 6 months—Yes | Yes | 6 months—Yes |
| 23 | Multiple ACA/graft thrombosis and rescue bypass STA to L A3-Bilateral A4 | \ | 40 | 6 months— mRS 1/KPS 90 | 4 months—No (FD positioned) | No | 4 months—no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acerbi, F.; Mazzapicchi, E.; Falco, J.; Vetrano, I.G.; Restelli, F.; Faragò, G.; La Corte, E.; Bonomo, G.; Bersano, A.; Canavero, I.; et al. The Role of Bypass Surgery for the Management of Complex Intracranial Aneurysms in the Anterior Circulation in the Flow-Diverter Era: A Single-Center Series. Brain Sci. 2022, 12, 1339. https://doi.org/10.3390/brainsci12101339
Acerbi F, Mazzapicchi E, Falco J, Vetrano IG, Restelli F, Faragò G, La Corte E, Bonomo G, Bersano A, Canavero I, et al. The Role of Bypass Surgery for the Management of Complex Intracranial Aneurysms in the Anterior Circulation in the Flow-Diverter Era: A Single-Center Series. Brain Sciences. 2022; 12(10):1339. https://doi.org/10.3390/brainsci12101339
Chicago/Turabian StyleAcerbi, Francesco, Elio Mazzapicchi, Jacopo Falco, Ignazio Gaspare Vetrano, Francesco Restelli, Giuseppe Faragò, Emanuele La Corte, Giulio Bonomo, Anna Bersano, Isabella Canavero, and et al. 2022. "The Role of Bypass Surgery for the Management of Complex Intracranial Aneurysms in the Anterior Circulation in the Flow-Diverter Era: A Single-Center Series" Brain Sciences 12, no. 10: 1339. https://doi.org/10.3390/brainsci12101339
APA StyleAcerbi, F., Mazzapicchi, E., Falco, J., Vetrano, I. G., Restelli, F., Faragò, G., La Corte, E., Bonomo, G., Bersano, A., Canavero, I., Gemma, M., Broggi, M., Schiariti, M., Ziliani, V., Raccuia, G., Mangiafico, S., Ganci, G., Ciceri, E., & Ferroli, P. (2022). The Role of Bypass Surgery for the Management of Complex Intracranial Aneurysms in the Anterior Circulation in the Flow-Diverter Era: A Single-Center Series. Brain Sciences, 12(10), 1339. https://doi.org/10.3390/brainsci12101339











