A Case Report of Hemifacial Spasm Caused by Vestibular Schwannoma and Literature Review
Abstract
:1. Introduction
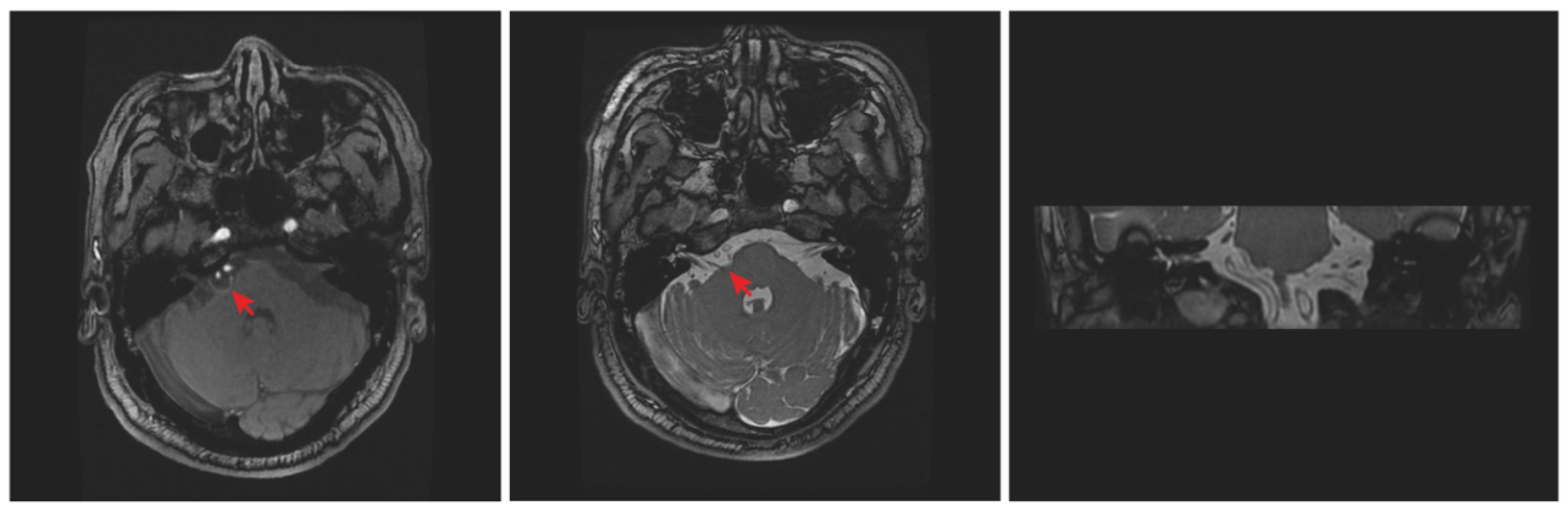
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaltho, T.C.; Jankovic, J. The many faces of hemifacial spasm: Differential diagnosis of unilateral facial spasms. Mov. Disord. 2011, 26, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.G., 2nd; Jannetta, P.J.; Bissonette, D.J.; Shields, P.T.; Larkins, M.V.; Jho, H.D. Microvascular decompression for hemifacial spasm. J. Neurosurg. 1995, 82, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Takayama, K.; Kurokawa, S.; Wada, T.; Nakagawa, H.; Kichikawa, K.; Nakase, H. Hemifacial spasm due to contralateral aneurysmal compression of the facial nerve successfully treated with endovascular coil embolization: Case report. Neurosurgery 2011, 69, E768–E771. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Rhee, B.A.; Choi, S.K.; Koh, J.S.; Lim, Y.J. Cerebellopontine angle tumors causing hemifacial spasm: Types, incidence, and mechanism in nine reported cases and literature review. Acta Neurochir. 2010, 152, 1901–1908. [Google Scholar] [CrossRef]
- Elgamal, E.A.; Coakham, H.B. Hemifacial spasm caused by pontine glioma: Case report and review of the literature. Neurosurg. Rev. 2005, 28, 330–332. [Google Scholar] [CrossRef]
- Matsumoto, K.; Saijo, T.; Kuyama, H.; Asari, S.; Nishimoto, A. Hemifacial spasm caused by a spontaneous dissecting aneurysm of the vertebral artery. Case report. J. Neurosurg. 1991, 74, 650–652. [Google Scholar] [CrossRef]
- Pierry, A.; Cameron, M. Clonic hemifacial spasm from posterior fossa arteriovenous malformation. J. Neurol. Neurosurg. Psychiatry 1979, 42, 670–672. [Google Scholar] [CrossRef]
- Cancelli, I.; Cecotti, L.; Valentinis, L.; Bergonzi, P.; Gigli, G.L. Hemifacial spasm due to a tentorial paramedian meningioma: A case report. Neurol. Sci. 2005, 26, 46–49. [Google Scholar] [CrossRef]
- Ferroli, P.; Broggi, G. Hemifacial spasm due to a subtentorial paramedian meningioma. Neurol. Sci. 2005, 26, 3–4. [Google Scholar] [CrossRef]
- Ruggieri, R.M.; Manfrè, L.; Calbucci, F.; Piccoli, F. Therapeutic considerations in cerebellopontine angle lipomas inducing hemifacial spasm. Neurol. Sci. 2000, 21, 329–331. [Google Scholar] [CrossRef]
- Gálvez-Jiménez, N.; Hanson, M.R.; Desai, M. Unusual causes of hemifacial spasm. Semin. Neurol. 2001, 21, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jain, V.K.; Chhabra, D.K.; Hongo, K.; Kobayashi, S. Hemifacial spasm and cerebellopontine angle epidermoid: Case report and review. Neurol. Res. 1994, 16, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Campos-Benitez, M.; Kaufmann, A.M. Neurovascular compression findings in hemifacial spasm. J. Neurosurg. 2008, 109, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Dannenbaum, M.; Lega, B.C.; Suki, D.; Harper, R.L.; Yoshor, D. Microvascular decompression for hemifacial spasm: Long-term results from 114 operations performed without neurophysiological monitoring. J. Neurosurg. 2008, 109, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Takeuchi, S.; Inenaga, C.; Koide, A.; Kawaguchi, T.; Takahashi, H.; Tanaka, R. Hemifacial spasm associated with an ependymal cyst in the cerebellopontine angle. Case report. J. Neurosurg. 2002, 97, 482–485. [Google Scholar] [CrossRef]
- Nishi, T.; Matsukado, Y.; Nagahiro, S.; Fukushima, M.; Koga, K. Hemifacial spasm due to contralateral acoustic neuroma: Case report. Neurology 1987, 37, 339–342. [Google Scholar] [CrossRef]
- Liu, J.; Liu, P.; Zuo, Y.; Xu, X.; Liu, H.; Du, R.; Yu, Y.; Yuan, Y. Hemifacial Spasm as Rare Clinical Presentation of Vestibular Schwannomas. World Neurosurg. 2018, 116, e889–e894. [Google Scholar] [CrossRef]
- Morita, A.; Fukushima, T.; Miyazaki, S.; Tamagawa, T.; Shimizu, Y.; Atsuji, M. Management of acoustic neurinoma with preserved hearing. No Shinkei Geka. Neurol. Surg. 1987, 15, 821–829. [Google Scholar]
- Sugiura, Y.; Yokoyama, T.; Ryu, H.; Uemura, K.; Ninchoji, T.; Bun, T.; Nishizawa, S. Clinical and electromyographic features of "intermittent tonic facial spasm" due to acoustic neurinoma. Report of two cases. Neurol. Med. Chir. 1988, 28, 1198–1202. [Google Scholar] [CrossRef] [Green Version]
- Nagata, S.; Matsushima, T.; Fujii, K.; Fukui, M.; Kuromatsu, C. Hemifacial spasm due to tumor, aneurysm, or arteriovenous malformation. Surg. Neurol. 1992, 38, 204–209. [Google Scholar] [CrossRef]
- Samii, M.; Matthies, C. Acoustic neurinomas associated with vascular compression syndromes. Acta Neurochir. 1995, 134, 148–154. [Google Scholar] [CrossRef]
- Peker, S.; Ozduman, K.; Kiliç, T.; Pamir, M.N. Relief of hemifacial spasm after radiosurgery for intracanalicular vestibular schwannoma. Minim. Invasive Neurosurg. 2004, 47, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: Treatment recommendations based on a 15 year experience. Neurosurgery 2006, 58, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.G.; Lipson, A.C.; Martin, A.J. Giant vestibular schwannoma in a 12-year-old girl. Pediatr. Neurosurg. 2006, 42, 338–340. [Google Scholar] [CrossRef]
- Han, I.B.; Chang, J.H.; Chang, J.W.; Huh, R.; Chung, S.S. Unusual causes and presentations of hemifacial spasm. Neurosurgery 2009, 65, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chen, G.; Zuo, H. Microsurgical treatment for 55 patients with hemifacial spasm due to cerebellopontine angle tumors. Neurosurg. Rev. 2010, 33, 335–339. [Google Scholar] [CrossRef]
- Chang, C.S.; Chuang, C.C.; Wu, M.F.; Liu, W.S.; Tu, H.T.; Huang, C.F. Gamma Knife surgery for hemifacial spasm related to cerebellopontine angle tumors. J. Neurosurg. 2012, 117, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Bigder, M.; Kaufmann, A.; McDonald, P.J.; Fewer, D.; Butler, J.; Schroeder, G.; West, M. Gamma knife radiosurgery for large vestibular schwannomas: A Canadian experience. Can. J. Neurol. Sci. 2013, 40, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Bouchetemblé, P.; Heathcote, K.; Tollard, E.; Choussy, O.; Dehesdin, D.; Marie, J.P. Intralabyrinthine schwannomas: A case series with discussion of the diagnosis and management. Otol. Neurotol. 2013, 34, 944–951. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Kaufmann, A.M. Two Cases of Secondary Hemifacial Spasm: Pathophysiology and Management. J. Mov. Disord. 2015, 8, 103–105. [Google Scholar] [CrossRef]
- Tuleasca, C.; George, M.; Faouzi, M.; Schiappacasse, L.; Leroy, H.A.; Zeverino, M.; Daniel, R.T.; Maire, R.; Levivier, M. Acute clinical adverse radiation effects after Gamma Knife surgery for vestibular schwannomas. J. Neurosurg. 2016, 125, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Zhong, J.; Dou, N.N.; Xia, L.; Li, B.; Li, S.T. Management of symptomatic hemifacial spasm or trigeminal neuralgia. Neurosurg. Rev. 2016, 39, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Tu, H.T.; Chuang, C.Y.; Chang, C.S.; Chou, H.H.; Lee, M.T.; Huang, C.F. Gamma Knife radiosurgery for large vestibular schwannomas greater than 3 cm in diameter. J. Neurosurg. 2018, 128, 1380–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candanedo, C.; de Jong, M.A.; Michaeli, A.; Moscovici, S.; Cohen, J.E.; Spektor, S. Vestibular schwannoma manifesting with hemifacial spasm in a young woman: Clinical considerations and tumor removal with hearing preservation. 2-Dimensional operative video. Neurosurg. Focus Video 2021, 5, V11. [Google Scholar] [CrossRef]
- Misron, K.; Mfuko, G.; Lee, J.G.; Moon, I.S. Simultaneous Surgical Treatment of Vestibular Schwannoma and Hemifacial Spasm via Minimally Invasive Retrosigmoid Approach. Korean J. Otorhinolaryngol.-Head Neck Surg. 2022, 65, 296–299. [Google Scholar] [CrossRef]
- Roser, F.; Maiti, T.K.; Elhammady, M.S. Various Dissection Techniques for Large Vestibular Schwannomas in Semisitting Position: 2-Dimensional Operative Video. Oper. Neurosurg. 2022, 23, e59. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, K.; Agarwal, S.; Agarwal, D.; Chandra, P.S.; Kale, S.S.; Sharma, B.S.; Mahapatra, A.K. Microvascular decompression for hemifacial spasm: A systematic review of vascular pathology, long term treatment efficacy and safety. Neurol. India 2017, 65, 493–505. [Google Scholar] [CrossRef]
- Sindou, M.; Mercier, P. Microvascular decompression for hemifacial spasm: Outcome on spasm and complications. A review. Neurochirurgie 2018, 64, 106–116. [Google Scholar] [CrossRef]
- Miller, L.E.; Miller, V.M. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: A systematic review. Br. J. Neurosurg. 2012, 26, 438–444. [Google Scholar] [CrossRef]
- Zhong, J.; Li, S.T.; Zhu, J.; Guan, H.X. Is entire nerve root decompression necessary for hemifacial spasm? Int. J. Surg. 2011, 9, 254–257. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hong, W.; Tang, Y.; Ying, T.; Zhang, W.; Li, X.; Zhu, J.; Zhong, J.; Hua, X.; Xu, S.; et al. Re-operation for persistent hemifacial spasm after microvascular decompression with the aid of intraoperative monitoring of abnormal muscle response. Acta Neurochir. 2010, 152, 2113–2118. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Tang, Y.D.; Zhang, Y.; Ying, T.T.; Zhu, J.; Li, S.T. Operative Complications of Microvascular Decompression for Hemifacial Spasm: Experience of 1548 Cases. World Neurosurg. 2017, 107, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, J.; Xu, M.; Zhou, L.F.; Zhang, R.; Lang, L.; Xu, Q.; Zhong, P.; Chen, M.; Wang, Y.; et al. Clinical features of intracranial vestibular schwannomas. Oncol. Lett. 2013, 5, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Stadio, A.; Dipietro, L.; Ralli, M.; Faralli, M.; Della Volpe, A.; Ricci, G.; Messineo, D. Loop characteristics and audio-vestibular symptoms or hemifacial spasm: Is there a correlation? A multiplanar MRI study. Eur. Radiol. 2020, 30, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Yamanaka, K.; Nakajima, H. Hemifacial spasm due to cerebellopontine angle meningiomas--two case reports. Neurol. Med. Chir. 2001, 41, 87–89. [Google Scholar] [CrossRef]


| Year | Author | Sex/Age | Feature of VS | Compression of FN | Management | Outcome |
|---|---|---|---|---|---|---|
| 1987 | T Nishi [16] | / | Contralateral VS | VS | Removal of the VS | Cure after 14 days |
| 1987 | A Morita [18] | / | Ipsilateral VS | VS | Removal of the VS | / |
| 1988 | Y. Sugiura [19] | Female/63 | Ipsilateral VS | VS | Removal of the VS | Immediately cure |
| 1988 | Y. Sugiura | Female/53 | Ipsilateral VS | VS | Removal of the VS + HFA | Immediately cure |
| 1992 | Shinji Nagata [20] | Female/29 | Ipsilateral VS | VS | Removal of the VS | Immediately cure |
| 1995 | M. Samii [21] | Female/54 | Ipsilateral VS | Arterial branches of AICA | Removal of the VS + MVD | Immediately cure |
| 1995 | M. Samii | Female/58 | Ipsilateral VS | Arterial branches of AICA | Removal of the VS + MVD | Immediately cure |
| 1995 | M. Samii | Male/42 | Ipsilateral VS | Arterial branches of AICA | Removal of the VS + MVD | Immediately cure |
| 1995 | M. Samii | Female/47 | Ipsilateral VS | Arterial branches of AICA | Removal of the VS + MVD | Immediately cure |
| 2001 | Néstor Gálvez-Jiménez [11] | Female/67 | Ipsilateral VS | VS | Gamma Knife Radiosurgery + botulinum toxin type A | / |
| 2004 | S. Peker [22] | Male/49 | Ipsilateral VS | VS | Gamma Knife Radiosurgery | Delayed cure |
| 2006 | Bruce E Pollock [23] | Male/66 | Ipsilateral VS | VS | Radiosurgery + Removal of the VS | Delayed cure |
| 2006 | Bruce E Pollock | / | / | VS | Radiosurgery | Recurred |
| 2006 | Jonathan G. Bull [24] | Female/12 | Ipsilateral VS | VS | Removal of the VS | Immediately cure |
| 2009 | In-Bo Han [25] | / | / | VS | Removal of the VS | Immediately cure |
| 2010 | Hongyan Han [26] | / | / | VS + branches of cerebellar arteries | Removal of the VS + MVD | / |
| 2010 | Hongyan Han | / | / | VS + branches of cerebellar arteries | Removal of the VS + MVD | / |
| 2010 | Seung Hwan Lee [4] | Female/60 | Ipsilateral VS | VS + PICA | Removal of the VS + MVD | Immediately cure |
| 2010 | Seung Hwan Lee | Female/60 | Ipsilateral VS | AICA + VA | Removal of the VS + MVD | Recurred |
| 2012 | Cheng-Siu Chang [27] | Female/51 | Ipsilateral VS | VS | Gamma Knife Radiosurgery | Failing |
| 2012 | Cheng-Siu Chang | Male/50 | Ipsilateral VS | VS | Gamma Knife Radiosurgery | Delayed cure |
| 2013 | F. A. Zeiler [28] | Female/52 | Ipsilateral VS | VS | Gamma Knife Radiosurgery | Worsening |
| 2013 | Pierre Bouchetemble’ [29] | / | Ipsilateral VS | VS | Removal of the VS | / |
| 2015 | Frederick A. Zeiler [30] | Female/56 | Ipsilateral VS | VS + PICA | Removal of the VS + MVD | Delayed cure |
| 2016 | Constantin Tuleasca [31] | / | / | VS | Gamma Knife Radiosurgery | Partial relieving |
| 2016 | Ming-Xing Liu [32] | Female/44 | Ipsilateral VS | VS + AICA | Removal of the VS + MVD | Immediately cure |
| 2016 | Ming-Xing Liu | Female/51 | Ipsilateral VS | VS | Removal of the VS | Partial relieving |
| 2016 | Ming-Xing Liu | Male/58 | Ipsilateral VS | VS | Removal of the VS | Partial relieving |
| 2016 | Ming-Xing Liu | Male/59 | Ipsilateral VS | VS | Removal of the VS | Immediately cure |
| 2018 | Jiang Liu [17] | Female/58 | Ipsilateral VS | AICA | Subtotal removal of the VS | Immediately cure |
| 2018 | Jiang Liu | Male/43 | Ipsilateral VS | VS | Gross total removal of the VS | Immediately cure |
| 2018 | Jiang Liu | Female/39 | Ipsilateral VS | AICA | Gross total removal of the VS | Immediately cure |
| 2018 | Jiang Liu | Female/68 | Ipsilateral VS | AICA | Subtotal removal of the VS | Immediately cure |
| 2018 | Jiang Liu | Male/53 | Ipsilateral VS | PICA | Gross total removal of the VS | Immediately cure |
| 2018 | Jiang Liu | Male/71 | Ipsilateral VS | VS | Gross total removal of the VS | Immediately cure |
| 2018 | Jiang Liu | Female/49 | Ipsilateral VS | AICA | Gross total removal of the VS | Immediately cure |
| 2018 | Jiang Liu | Female/60 | Ipsilateral VS | PICA | Subtotal removal of the VS | Immediately cure |
| 2018 | Jiang Liu | Female/57 | Ipsilateral VS | AICA | Gross total removal of the VS | Immediately cure |
| 2018 | Jiang Liu | Male/62 | Contralateral VS | VS | Gross total removal of the VS | Delayed cure |
| 2018 | Cheng-Wei Huang [33] | / | / | VS | Gamma Knife Radiosurgery | Cure after 3 years |
| 2021 | Carlos Candanedo [34] | Female/27 | Ipsilateral VS | VS + AICA | Removal of the VS + MVD | Immediately cure |
| 2022 | Khairunnisak Misron [35] | Male/68 | Ipsilateral VS | VS + AICA | Removal of the VS + MVD | Immediately cure |
| 2022 | Roser, Florian [36] | Female/42 | Ipsilateral VS | VS | Removal of the VS | Immediately cure |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Tang, Y.; Zhao, H.; Chen, Z.; Wang, H.; Zhu, W.; Li, S. A Case Report of Hemifacial Spasm Caused by Vestibular Schwannoma and Literature Review. Brain Sci. 2022, 12, 1347. https://doi.org/10.3390/brainsci12101347
Cai X, Tang Y, Zhao H, Chen Z, Wang H, Zhu W, Li S. A Case Report of Hemifacial Spasm Caused by Vestibular Schwannoma and Literature Review. Brain Sciences. 2022; 12(10):1347. https://doi.org/10.3390/brainsci12101347
Chicago/Turabian StyleCai, Xiaomin, Yinda Tang, Hua Zhao, Zheng Chen, Haopeng Wang, Wanchun Zhu, and Shiting Li. 2022. "A Case Report of Hemifacial Spasm Caused by Vestibular Schwannoma and Literature Review" Brain Sciences 12, no. 10: 1347. https://doi.org/10.3390/brainsci12101347




