Association between Cerebral Coordination Functions and Clinical Outcomes of Alzheimer’s Dementia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Evaluations
2.3. Apolipoprotein E (APOE) Genotyping
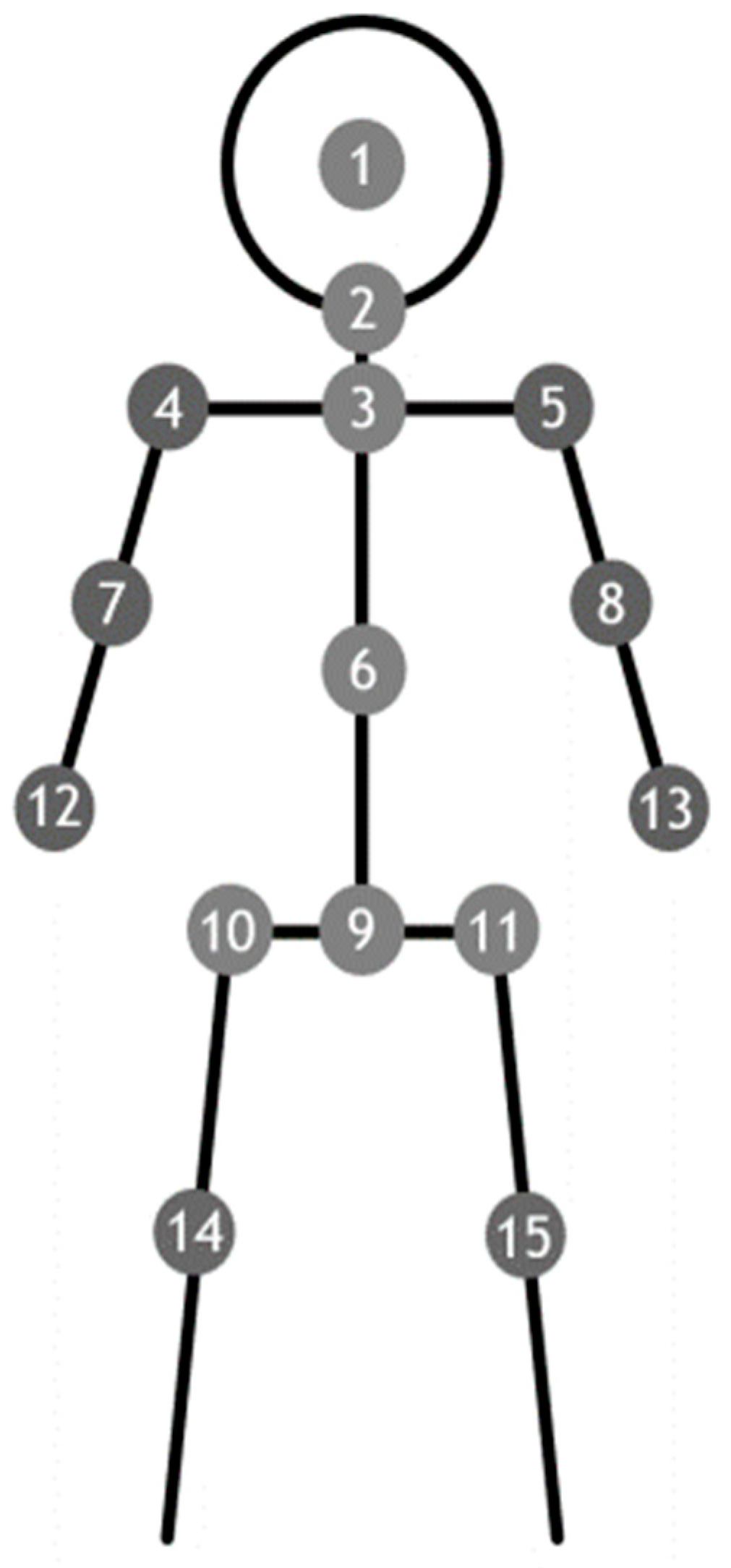
2.4. Posture and Joint Angle Assessments for Coordination Function
2.5. Kinect Depth Sensor System
2.6. Standing Still Position for Data Receiving and Processing
2.7. Angles Formed by the Limbs to the Central Axis
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, E.K.; Tomlinson, B.E.; Blessed, G.; Bergmann, K.; Gibson, P.H.; Perry, R.H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br. Med. J. 1978, 2, 1457–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, S.E. Part III. Neuropathology of Alzheimer’s disease. Disease-a-Month 2000, 46, 688–706. [Google Scholar] [CrossRef]
- Shinotoh, H.; Namba, H.; Fukushi, K.; Nagatsuka, S.I.; Tanaka, N.; Aotsuka, A.; Ota, T.; Tanada, S.; Irie, T. Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer’s disease: A positron emission tomography study. Ann. Neurol. 2000, 48, 194–200. [Google Scholar] [CrossRef]
- Mohs, R.C.; Schmeidler, J.; Aryan, M. Longitudinal studies of cognitive, functional and behavioural change in patients with Alzheimer’s disease. Stat. Med. 2000, 19, 1401–1409. [Google Scholar] [CrossRef]
- Palmqvist, S.; Schöll, M.; Strandberg, O.; Mattsson, N.; Stomrud, E.; Zetterberg, H.; Blennow, K.; Landau, S.; Jagust, W.; Hansson, O. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 2017, 8, 1214. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-H.; Situmeang, R.F.; Ong, P.A. Can blood amyloid levels be used as a biomarker for Alzheimer’s disease? Brain Sci. Adv. 2021, 7, 17–25. [Google Scholar] [CrossRef]
- Herbet, G.; Duffau, H. Revisiting the functional anatomy of the human brain: Toward a meta-networking theory of cerebral functions. Physiol. Rev. 2020, 100, 1181–1228. [Google Scholar] [CrossRef]
- Adesnik, H.; Naka, A. Cracking the function of layers in the sensory cortex. Neuron 2018, 100, 1028–1043. [Google Scholar] [CrossRef] [Green Version]
- Buchman, A.S.; Bennett, D.A. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev. Neurother. 2011, 11, 665–676. [Google Scholar] [CrossRef]
- Schirinzi, T.; Di Lorenzo, F.; Sancesario, G.M.; Di Lazzaro, G.; Ponzo, V.; Pisani, A.; Mercuri, N.B.; Koch, G.; Martorana, A. Amyloid-mediated cholinergic dysfunction in motor impairment related to Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 64, 525–532. [Google Scholar] [CrossRef]
- Scarmeas, N.; Albert, M.; Brandt, J.; Blacker, D.; Hadjigeorgiou, G.; Papadimitriou, A.; Dubois, B.; Sarazin, M.; Wegesin, D.; Marder, K. Motor signs predict poor outcomes in Alzheimer disease. Neurology 2005, 64, 1696–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarmeas, N.; Hadjigeorgiou, G.; Papadimitriou, A.; Dubois, B.; Sarazin, M.; Brandt, J.; Albert, M.; Marder, K.; Bell, K.; Honig, L.S. Motor signs during the course of Alzheimer disease. Neurology 2004, 63, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-P.; Chou, M.-C.; Lai, C.-L.; Chien, I.; Yang, Y.-H. Apolipoprotein E e4 allele is associated with extrapyramidal symptoms in Alzheimer’s disease. Neuropsychiatr. Dis. 2019, 15, 1915–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vöglein, J.; Paumier, K.; Jucker, M.; Preische, O.; McDade, E.; Hassenstab, J.; Benzinger, T.L.; Noble, J.M.; Berman, S.B.; Graff-Radford, N.R. Clinical, pathophysiological and genetic features of motor symptoms in autosomal dominant Alzheimer’s disease. Brain 2019, 142, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Roalf, D.R.; Rupert, P.; Mechanic-Hamilton, D.; Brennan, L.; Duda, J.E.; Weintraub, D.; Trojanowski, J.Q.; Wolk, D.; Moberg, P.J. Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. J. Neurol. 2018, 265, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, S.; Osawa, A.; Maeda, N.; Sano, Y.; Kandori, A.; Mizuguchi, T.; Yin, Y.; Kondo, I. Differences among patients with Alzheimer’s disease, older adults with mild cognitive impairment and healthy older adults in finger dexterity. Geriatr. Gerontol. Int. 2018, 18, 907–914. [Google Scholar] [CrossRef]
- Chrastil, E.R. Heterogeneity in human retrosplenial cortex: A review of function and connectivity. Behav. Neurosci. 2018, 132, 317. [Google Scholar] [CrossRef]
- Liu, C.-H.; Lee, P.; Chen, Y.-L.; Yen, C.-W.; Yu, C.-W. Study of postural stability features by using kinect depth sensors to assess body joint coordination patterns. Sensors 2020, 20, 1291. [Google Scholar] [CrossRef] [Green Version]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-C.; Teng, E.L.; Lin, K.-N.; Chuang, Y.-Y.; Wang, P.-N.; Fuh, J.-L.; Liu, C.-Y. Performance on the cognitive abilities screening instrument at different stages of Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2002, 13, 244–248. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Liscic, R.; Dominguez, J. Framework of treating Alzheimer’s dementia. Brain Sci. Adv. 2019, 5, 82–93. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Wu, M.-N.; Chou, P.-S.; Su, H.-C.; Lin, S.-H.; Sung, P.-S. Longitudinal neuropsychological outcome in Taiwanese Alzheimer’s disease patients treated with medication. Curr. Alzheimer Res. 2018, 15, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1991, 41, 1588–1592. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Huang, L.-C.; Hsieh, S.-W.; Huang, L.-J. Dynamic blood concentrations of Aβ1–40 and Aβ1–42 in Alzheimer’s disease. Front. Cell Dev. Biol. 2020, 8, 768. [Google Scholar] [CrossRef]
- Hebert, L.E.; Bienias, J.L.; McCann, J.J.; Scherr, P.A.; Wilson, R.S.; Evans, D.A. Upper and lower extremity motor performance and functional impairment in Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2010, 25, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Tager, I.B.; Swanson, A.; Satariano, W.A. Reliability of physical performance and self-reported functional measures in an older population. J. Gerontol. Med. Sci. 1998, 53, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Hoeymans, N.; Wouters, E.R.; Feskens, E.J.; van den Bos, G.A.; Kromhout, D. Reproducibility of performance-based and self-reported measures of functional status. J. Gerontol. Med. Sci. 1997, 52, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Reitan, R.M.; Wolfson, D. The Halstead-Reitan Neuropsychological Test Battery. Clin. Gerontol. 1986, 5, 39–61. [Google Scholar] [CrossRef]
- Tiffin, J.; Asher, E.J. The Purdue Pegboard: Norms and studies of reliability and validity. J. Appl. Psychol. 1948, 32, 234. [Google Scholar] [CrossRef]
- Branch, L.G.; Katz, S.; Kniepmann, K.; Papsidero, J.A. A prospective study of functional status among community elders. Am. J. Public Health 1984, 74, 266–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bologna, M.; Guerra, A.; Colella, D.; Cioffi, E.; Paparella, G.; Di Vita, A.; D’Antonio, F.; Trebbastoni, A.; Berardelli, A. Bradykinesia in Alzheimer’s disease and its neurophysiological substrates. Clin. Neurophysiol. 2020, 131, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Nadhif, M.H.; Hadiputra, A.P.; Alief, N.A.; Whulanza, Y.; Supriadi, S. Gait analysis for Alzheimer’s disease therapies using Kinect™: A preliminary report. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; p. 050002. [Google Scholar]
- Lin, Y.-T.; Chou, M.-C.; Wu, S.-J.; Yang, Y.-H. Galantamine plasma concentration and cognitive response in Alzheimer’s disease. PeerJ 2019, 7, e6887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, S.W.; Huang, L.C.; Chang, Y.P.; Hung, C.H.; Yang, Y.H. M2b macrophage subset decrement as an indicator of cognitive function in Alzheimer’s disease. Psychiatry Clin. Neurosci. 2020, 74, 383–391. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N = 113 | ||
|---|---|---|---|
| Age (Mean ± SD), Years | 78.9 ± 6.9 | p-Value | |
| Sex, Female, n (%) | 75 (66.4%) | ||
| Education (mean ± SD) years | 7.6 ± 5.0 | ||
| APOE4 (+)(n/N, %) | 37/111 (32.7%) | ||
| 1st year | 2nd year | ||
| MMSE (mean ± SD) | 17.5 ± 5.0 | 16.4 ± 5.4 | 0.001 |
| CASI (mean ± SD) | 57.7 ± 16.4 | 54.1 ± 18.7 | <0.001 |
| CDR-SB (mean ± SD) | 5.8 ± 2.6 | 6.6 ± 3.0 | <0.001 |
| CDR | <0.001 | ||
| CDR0.5, n (%) | 31(27.4) | 21(18.6) | |
| CDR1.0, n (%) | 75(66.4) | 77(68.1) | |
| CDR2.0, n (%) | 7 (6.2) | 13(11.5) | |
| CDR 3.0, n (%) | 0 | 2(1.8) | |
| Parameter (N = 113) | ||||||||
|---|---|---|---|---|---|---|---|---|
| CDR | CASI | MMSE | CDR-SB | |||||
| Outcome n (%) | Improved 94 (83.2%) | Worse 19 (16.8%) | Improved 44 (38.9%) | Worse 69 (61.1%) | Improved 49 (43.4%) | Worse 64 (56.6%) | Improved 50 (44.2%) | Worse 63 (55.8%) |
| Age (mean ± SD), years | 79.2 ± 6.6 | 77.0 ± 8.0 | 80.0 ± 5.9 | 78.1 ± 7.4 | 80.4 ± 6.3 | 77.7 ± 7.1 | 80.4 ± 6.3 | 77.7 ± 7.2 |
| Sex, female n (%) | 62 (66.0%) | 13 (68.4%) | 31(70.5%) | 44 (63.8%) | 32 (65.3%) | 43 (67.2%) | 32 (64.0%) | 43 (68.3%) |
| Education (mean ± SD), years | 7.5 ± 5.1 | 8.1 ± 4.6 | 6.9 ± 5.0 | 8.1 ± 5.0 | 6.4 ± 5.0 | 8.5 ± 4.9 | 6.8 ± 4.9 | 8.3 ± 5.0 |
| APOE4 (+) (n/N, %) | 32/92 (34.8%) | 5/19 (26.3%) | 19/43 (44.2%) | 18/68 (26.5%) | 17/43 (35.4%) | 20/63 (31.7%) | 15/49 (30.6%) | 22/62 (35.5%) |
| Clinical Outcome Variables | Mean Ratio of Improved Coordination in Improved Clinical Outcome (Mean ± SD) | Mean Ratio of Worse Coordination in Worse Clinical Outcome (Mean ± SD) | Correlation Coefficient for 2 Ratios | p-Value |
|---|---|---|---|---|
| CASI | 0.784 ± 0.124 | 0.495 ± 0.137 | 0.350 | 0.131 |
| MMSE | 0.616 ± 0.133 | 0.458 ± 0.154 | 0.126 | 0.595 |
| CDR-SB | 0.726 ± 0.126 | 0.668 ± 0.121 | 0.241 | 0.306 |
| CDR | 0.516 ± 0.188 | 0.027 ± 0.010 | 1.000 | <0.001 |
| Clinical Outcome Variable | Mean Ratio of Improved Coordination in Improved Clinical Outcome (Mean ± SD) | Mean Ratio of Worse Coordination in Worse Clinical Outcome (Mean ± SD) | Correlation Coefficient for 2 Ratios | p-Value |
|---|---|---|---|---|
| CASI | 0.844 ± 0.182 | 0.533 ± 0.233 | 0.798 | 0.006 |
| MMSE | 0.333 ± 0.174 | 0.037 ± 0.019 | 1.000 | <0.001 |
| CDR-SB | 0.266 ± 0.150 | 0.030 ± 0.017 | 1.000 | <0.001 |
| CDR | 0.511 ± 0.297 | 0.057 ± 0.033 | 1.000 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-H.; Lee, Y.-H.; Yen, C.-W.; Huang, L.-C.; Chang, Y.-P.; Chien, C.-F. Association between Cerebral Coordination Functions and Clinical Outcomes of Alzheimer’s Dementia. Brain Sci. 2022, 12, 1370. https://doi.org/10.3390/brainsci12101370
Yang Y-H, Lee Y-H, Yen C-W, Huang L-C, Chang Y-P, Chien C-F. Association between Cerebral Coordination Functions and Clinical Outcomes of Alzheimer’s Dementia. Brain Sciences. 2022; 12(10):1370. https://doi.org/10.3390/brainsci12101370
Chicago/Turabian StyleYang, Yuan-Han, Ying-Han Lee, Chen-Wen Yen, Ling-Chun Huang, Yang-Pei Chang, and Ching-Fang Chien. 2022. "Association between Cerebral Coordination Functions and Clinical Outcomes of Alzheimer’s Dementia" Brain Sciences 12, no. 10: 1370. https://doi.org/10.3390/brainsci12101370





