Embryonic Deletion of TXNIP in GABAergic Neurons Enhanced Oxidative Stress in PV+ Interneurons in Primary Somatosensory Cortex of Aging Mice: Relevance to Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
3. Results
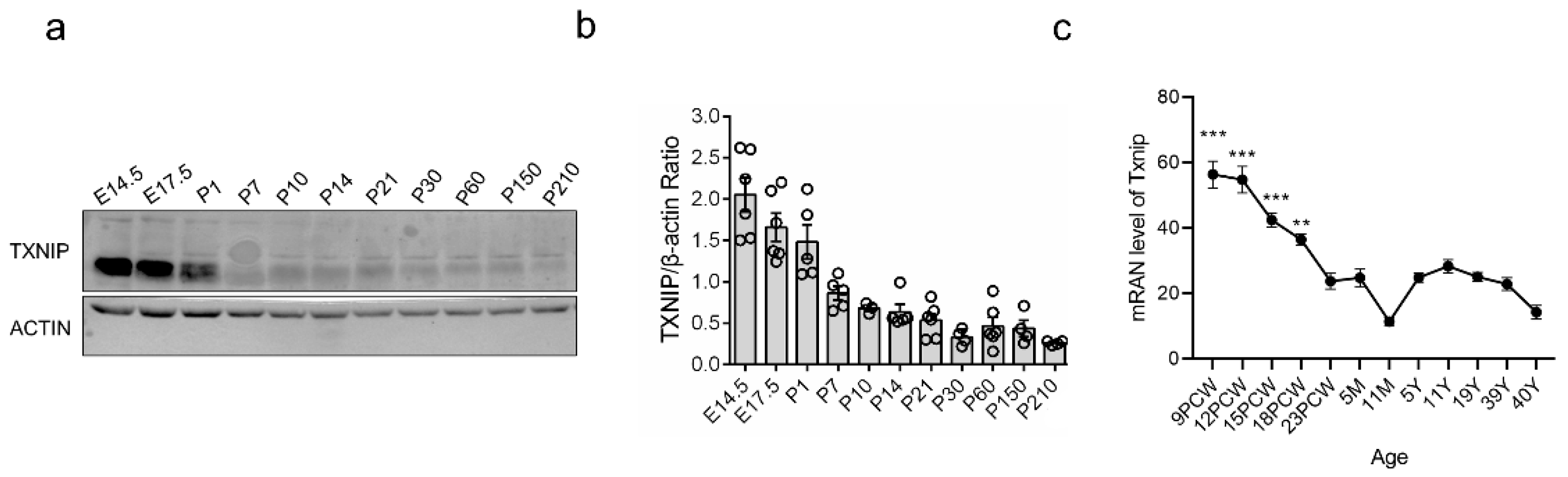
3.1. TXNIP Was Highly Expressed in the Early Prenatal Period, Followed by a Rapid Decrease in PFC of Healthy Mice
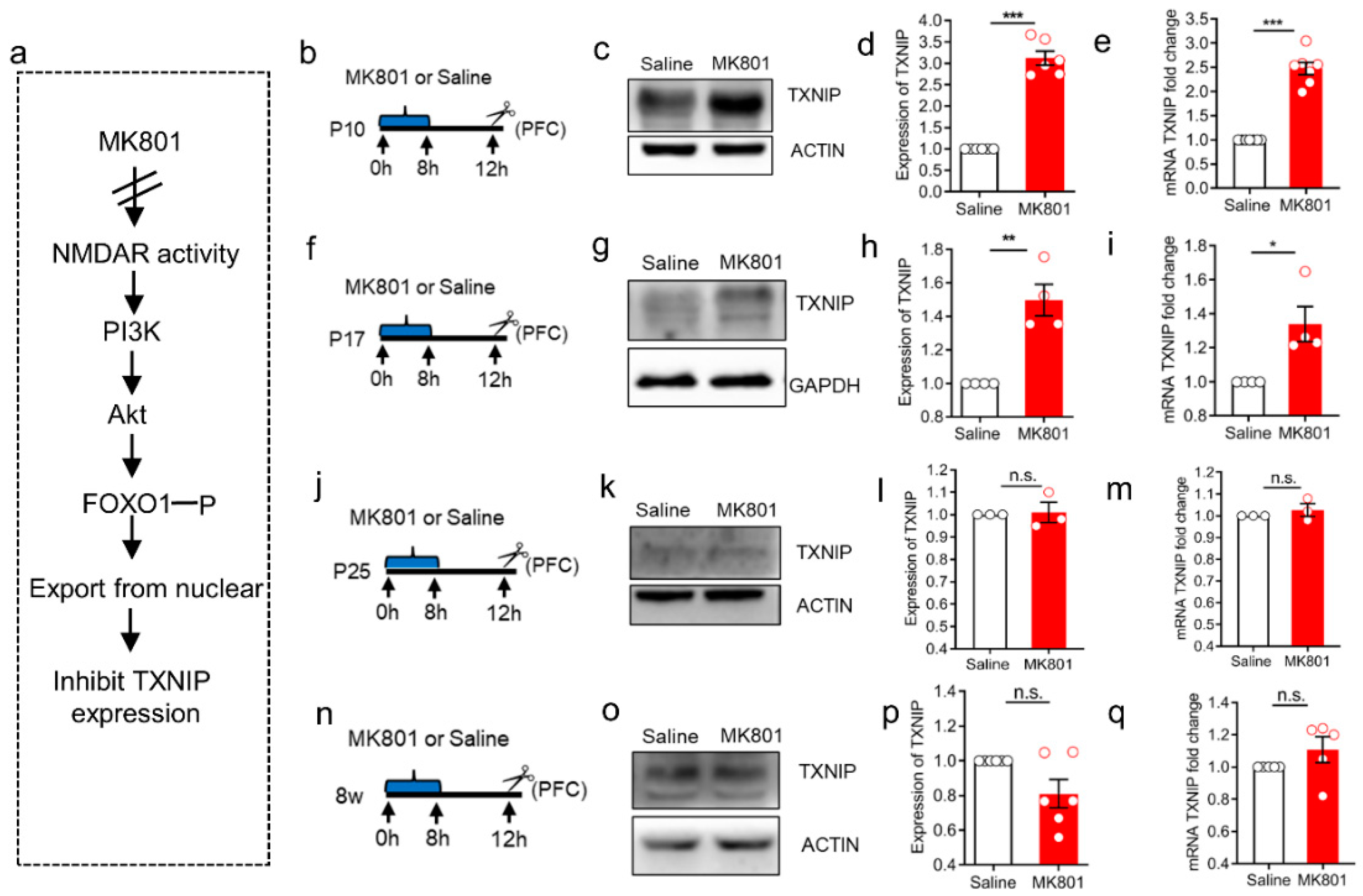
3.2. The Induction of TXNIP Expression via NMDAR Hypofunction Was Only Detected at the Early Stage of Brain Development
3.3. The Cellular Location of TXNIP in PFC during the Early Developmental Period
3.4. Embryonic Conditional Knockout of TXNIP Induced Increased Oxidative Stress in PVIs in S1 Region of Aging Mice
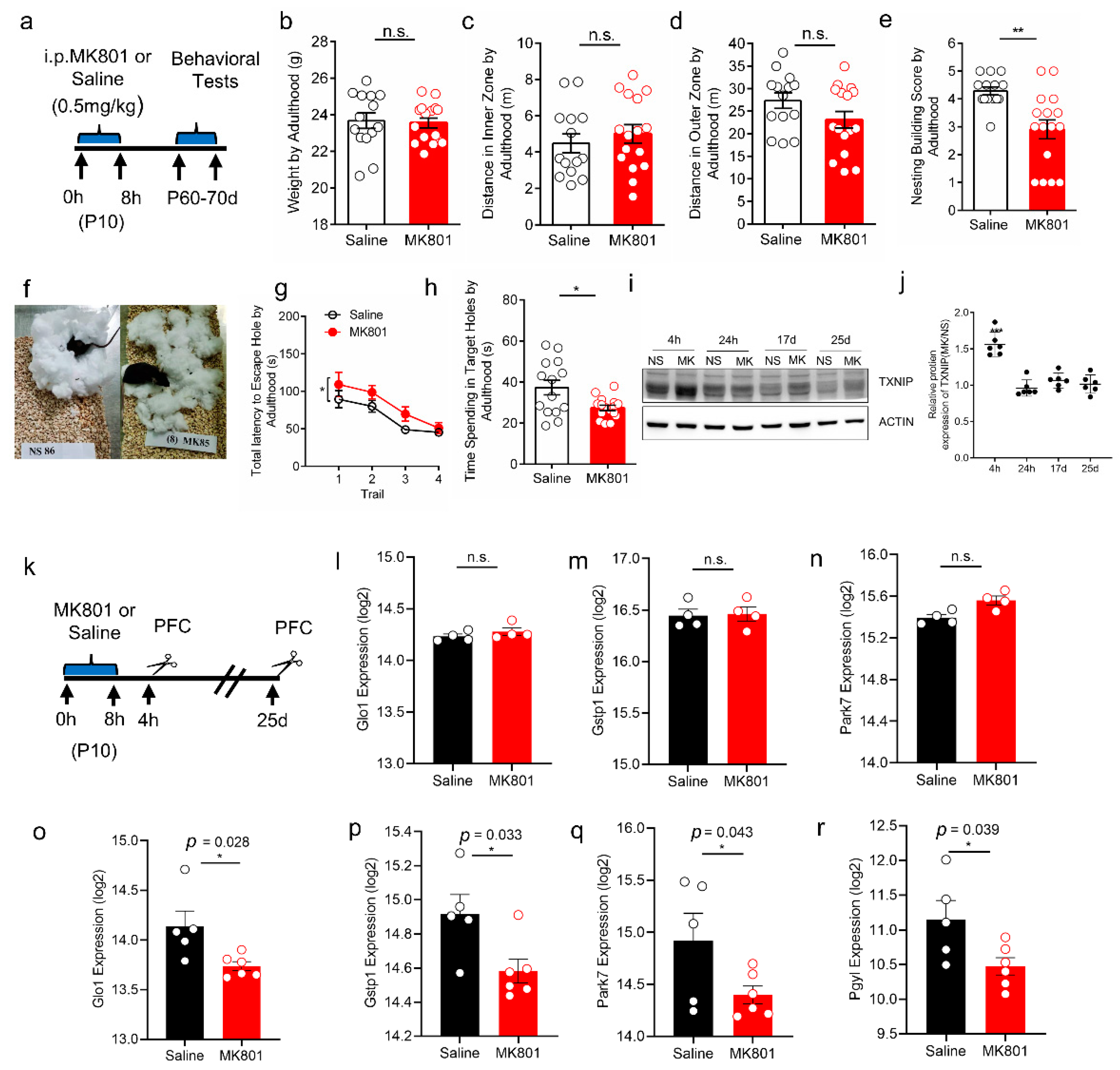
3.5. Increased Expression of TXNIP and Oxidative Stress in PFC of Schizophrenia-like Mice
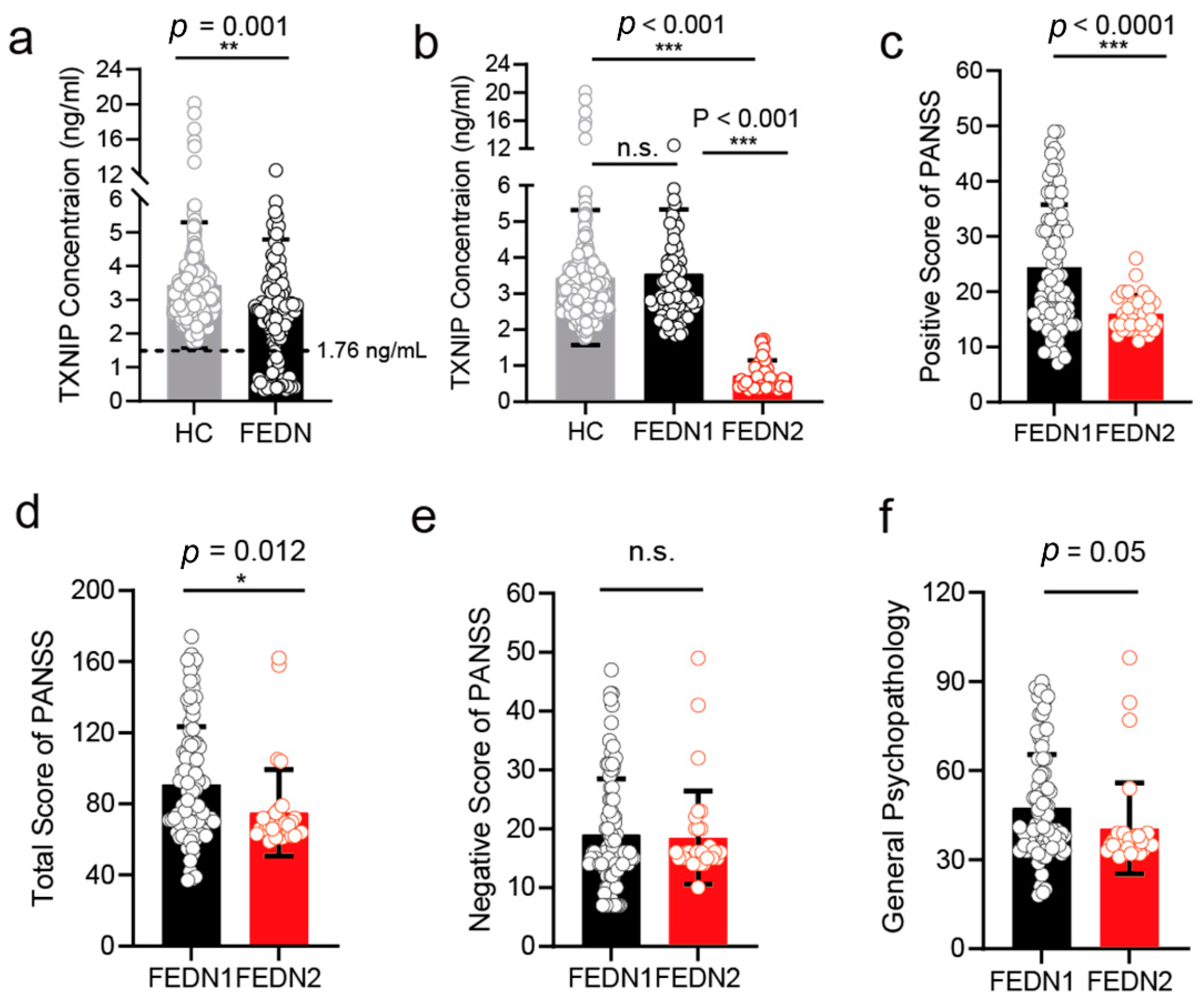
3.6. The Plasma Level of TXNIP in FEDN Schizophrenia Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barron, H.; Hafizi, S.; Andreazza, A.C.; Mizrahi, R. Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int. J. Mol. Sci. 2017, 18, 651. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, D.; Díaz-Caneja, C.M.; Ayora, M.; Hernández-Álvarez, F.; Rodríguez-Quiroga, A.; Recio, S.; Leza, J.C.; Arango, C. Oxidative stress and inflammation in first-episode psychosis: A systematic review and meta-analysis. Schizophr. Bull. 2019, 45, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.Z.; Yahaya, M.F.; Tooyama, I.; Damanhuri, H.A. A mechanistic evaluation of antioxidant nutraceuticals on their potential against age-associated neurodegenerative diseases. Antioxidants 2020, 9, 1019. [Google Scholar] [CrossRef]
- Schulze, P.C.; De Keulenaer, G.W.; Yoshioka, J.; Kassik, K.A.; Lee, R.T. Vitamin D3-upregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ. Res. 2002, 91, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, J.S.; Chatterjee, A.; Castellani, L.W.; Ross, D.A.; Ohmen, J.; Cavalcoli, J.; Wu, C.; Dains, K.M.; Catanese, J.; Chu, M.; et al. Positional cloning of the combined hyperlipidemia gene Hyplip1. Nat. Genet. 2002, 30, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Levendusky, M.C.; Basle, J.; Chang, S.; Mandalaywala, N.V.; Voigt, J.M.; Dearborn, R.E., Jr. Expression and regulation of vitamin D3upregulated protein 1 (VDUP1) is conserved in mammalian and insect brain. J. Comp. Neurol. 2009, 517, 581–600. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidou, C.; Kaindl, A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox Signal. 2011, 14, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Shin, S.M.; Kim, J.K.; Paik, S.G.; Yang, Y.; Choi, I. Heat shock factor regulates VDUP1 gene expression. Biochem. Biophys. Res. Commun. 2004, 315, 369–375. [Google Scholar] [CrossRef]
- Parikh, H.; Carlsson, E.; Chutkow, W.A.; Johansson, L.E.; Storgaard, H.; Poulsen, P.; Saxena, R.; Ladd, C.; Schulze, P.C.; Mazzini, M.J.; et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007, 4, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Papadia, S.; Soriano, F.; Léveillé, F.; Martel, M.-A.; Dakin, K.A.; Hansen, H.H.; Kaindl, A.; Sifringer, M.; Fowler, J.; Stefovska, V.; et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 2008, 11, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Chutkow, W.A.; Patwari, P.; Yoshioka, J.; Lee, R.T. Thioredoxin-interacting Protein (Txnip) Is a Critical Regulator of Hepatic Glucose Production. J. Biol. Chem. 2008, 283, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Saxena, G.; Mungrue, I.N.; Lusis, A.J.; Shalev, A. Thioredoxin-interacting protein—A critical link between glucose toxicity and beta-cell apoptosis. Diabetes 2008, 57, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Li, L.; Ismael, S.; Nasoohi, S.; Sakata, K.; Liao, F.-F.; McDonald, M.P.; Ishrat, T. Thioredoxin-interacting protein (txnip) associated nlrp3 inflammasome activation in human alzheimer’s disease brain. J. Alzheimer’s Dis. 2019, 68, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Fertan, E.; Rodrigues, G.J.; Wheeler, R.V.; Goguen, D.; Wong, A.A.; James, H.; Stadnyk, A.; Brown, R.E.; Weaver, I.C. Cognitive decline, cerebral-spleen tryptophan metabolism, oxidative stress, cytokine production, and regulation of the txnip gene in a triple transgenic mouse model of alzheimer disease. Am. J. Pathol. 2019, 189, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Bharti, V.; Zhou, H.; Hoi, V.; Tan, H.; Wu, Z.; Nagakannan, P.; Eftekharpour, E.; Wang, J.-F. Upregulation of thioredoxin-interacting protein in brain of amyloid-beta protein precursor/presenilin 1 transgenic mice and amyloid-beta treated neuronal cells. J. Alzheimer’s Dis. 2019, 72, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tan, H.; Letourneau, L.; Wang, J.-F. Increased thioredoxin-interacting protein in brain of mice exposed to chronic stress. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 88, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, R.; Ji, Z.; Li, X.; Fu, Q.; Ma, S. Perilla aldehyde attenuates CUMS-induced depressive-like behaviors via regulating TXNIP/TRX/NLRP3 pathway in rats. Life Sci. 2018, 206, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ishrat, T.; Mohamed, I.; Pillai, B.; Soliman, S.; Fouda, A.; Ergul, A.; El-Remessy, A.B.; Fagan, S.C. Thioredoxin-interacting protein: A novel target for neuroprotection in experimental thromboembolic stroke in mice. Mol. Neurobiol. 2015, 51, 779–780. [Google Scholar] [CrossRef]
- Du, S.-Q.; Wang, X.-R.; Zhu, W.; Ye, Y.; Yang, J.-W.; Ma, S.-M.; Ji, C.-S.; Liu, C.-Z. Acupuncture inhibits TXNIP-associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats. CNS Neurosci. Ther. 2018, 24, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-J.; Peng, W.; Gong, H.; Liu, Y.-Z.; Zhang, Y.; Lian, Y.-J.; Cao, Z.-Y.; Wu, R.; Liu, L.-L.; Wang, B.; et al. Antidiabetic drug glyburide modulates depressive-like behavior comorbid with insulin resistance. J. Neuroinflammation 2017, 14, 210. [Google Scholar] [CrossRef]
- Rudy, B.; Fishell, G.; Lee, S.; Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011, 71, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gan, J.; Jonas, P. Fast-spiking, parvalbumin(+) GABAergic interneurons: From cellular design to microcircuit function. Science 2014, 345, 1255263. [Google Scholar] [CrossRef]
- Jensen, O.; Kaiser, J.; Lachaux, J.-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kann, O.; Papageorgiou, I.E.; Draguhn, A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J. Cereb. Blood Flow Metab. 2014, 34, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Steullet, P.; Cabungcal, J.-H.; Coyle, J.; Didriksen, M.; Gill, K.; Grace, A.A.; Hensch, T.K.; LaMantia, A.-S.; Lindemann, L.; Maynard, T.M.; et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol. Psychiatry 2017, 22, 936–943. [Google Scholar] [CrossRef]
- Cabungcal, J.-H.; Steullet, P.; Kraftsik, R.; Cuenod, M.; Do, K.Q. Early-life insults impair parvalbumin interneurons via oxidative stress: Reversal by n-acetylcysteine. Biol. Psychiatry 2013, 73, 574–582. [Google Scholar] [CrossRef]
- Cabungcal, J.-H.; Steullet, P.; Morishita, H.; Kraftsik, R.; Cuenod, M.; Hensch, T.K.; Do, K.Q. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 9130–9135. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.A.; Grieve, S.; Hoth, K.F.; Paul, R.H.; Sweet, L.; Tate, D.; Gunstad, J.; Stroud, L.; McCaffery, J.; Hitsman, B.; et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol. Psychiatry 2006, 59, 975–982. [Google Scholar] [CrossRef]
- Gos, T.; Bock, J.; Poeggel, G.; Braun, K. Stress-induced synaptic changes in the rat anterior cingulate cortex are dependent on endocrine developmental time windows. Synapse 2008, 62, 229–232. [Google Scholar] [CrossRef]
- Fornito, A.; Yücel, M.; Dean, B.; Wood, S.J.; Pantelis, C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: Bridging the gap between neuroimaging and neuropathology. Schizophr. Bull. 2009, 35, 973–993. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, J.; Imahashi, K.; Gabel, S.A.; Chutkow, W.A.; Burds, A.A.; Gannon, J.; Schulze, P.C.; MacGillivray, C.; London, R.E.; Murphy, E.; et al. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ. Res. 2007, 101, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.-T.; Chen, H.; Samaco, R.; Xue, M.; Chahrour, M.; Yoo, J.; Neul, J.; Gong, S.; Lu, H.-C.; Heintz, N.; et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 2010, 468, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Y.; Liu, W.; Ma, Y.; Chen, X.; Xue, T.; Cui, D. Molecular basis of gaba hypofunction in adolescent schizophrenia-like animals. Neural Plast. 2021, 2021, 9983438. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Bell, K.F.S.; Hasel, P.; Kaindl, A.M.; Fricker, M.; Thomson, D.; Cregan, S.P.; Gillingwater, T.H.; Hardingham, G.E. Corrigendum: Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat. Commun. 2017, 8, 16158. [Google Scholar] [CrossRef]
- Nakazawa, K.; Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol. Ther. 2020, 205, 107426. [Google Scholar] [CrossRef] [PubMed]
- Radonjić, N.V.; Knežević, I.D.; Vilimanovich, U.; Kravić-Stevović, T.; Marina, L.V.; Nikolić, T.; Todorović, V.; Bumbaširević, V.; Petronijević, N.D. Decreased glutathione levels and altered antioxidant defense in an animal model of schizophrenia: Long-term effects of perinatal phencyclidine administration. Neuropharmacology 2010, 58, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, B.; Soriano, F.X.; Hardingham, G.E. Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels 2009, 3, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, F.; Hirata, Y.; Akram, H.; Kamitori, K.; Dong, Y.; Sui, L.; Tokuda, M. FOXO/TXNIP pathway is involved in the suppression of hepatocellular carcinoma growth by glutamate antagonist MK-801. BMC Cancer 2013, 13, 468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Che, X.; Zhang, H.; Fan, P.; Tan, G.; Liu, L.; Jiang, D.; Zhao, J.; Xiang, X.; Liang, Y.; et al. Thioredoxin-interacting protein links endoplasmic reticulum stress to inflammatory brain injury and apoptosis after subarachnoid haemorrhage. J. Neuroinflammation 2017, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zuo, D.; Yu, L.; Zhang, L.; Tang, J.; Cui, C.; Bao, L.; Zan, K.; Zhang, Z.; Yang, X.; et al. ROS/TXNIP pathway contributes to thrombin induced NLRP3 inflammasome activation and cell apoptosis in microglia. Biochem. Biophys. Res. Commun. 2017, 485, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.B.; Vreugdenhil, M.; Toescu, E.C. The effect of aging-associated impaired mitochondrial status on kainate-evoked hippocampal gamma oscillations. Neurobiol. Aging 2012, 33, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Sosenko, I.R.S. Failure of premature rabbits to increase antioxidant enzymes during hyperoxic exposure: Increased susceptibility to pulmonary oxygen toxicity compared with term rabbits. Pediatr. Res. 1991, 29, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Lizasoain, I.; Weiner, C.P.; Knowles, R.G.; Moncada, S. The ontogeny of cerebral and cerebellar nitric oxide synthase in the guinea pig and rat. Pediatr. Res. 1996, 39, 779–783. [Google Scholar] [CrossRef][Green Version]
- Lin, G.N.; Guo, S.; Tan, X.; Wang, W.; Qian, W.; Song, W.; Wang, J.; Yu, S.; Wang, Z.; Cui, D.; et al. PsyMuKB: An integrative de novo variant knowledge base for developmental disorders. Genom. Proteom. Bioinform. 2019, 17, 453–464. [Google Scholar] [CrossRef]
- Song, L.; Pan, S.; Zhang, Z.; Jia, L.; Chen, W.-H.; Zhao, X.-M. STAB: A spatio-temporal cell atlas of the human brain. Nucleic Acids Res. 2021, 49, D1029–D1037. [Google Scholar] [CrossRef]
- Ismael, S.; Nasoohi, S.; Li, L.; Aslam, K.S.; Khan, M.M.; El-Remessy, A.B.; McDonald, M.P.; Liao, F.-F.; Ishrat, T. Thioredoxin interacting protein regulates age-associated neuroinflammation. Neurobiol. Dis. 2021, 156, 105399. [Google Scholar] [CrossRef]
- Ding, R.; Ou, W.; Chen, C.; Liu, Y.; Li, H.; Zhang, X.; Chai, H.; Ding, X.; Wang, Q. Endoplasmic reticulum stress and oxidative stress contribute to neuronal pyroptosis caused by cerebral venous sinus thrombosis in rats: Involvement of TXNIP/peroxynitrite-NLRP3 inflammasome activation. Neurochem. Int. 2020, 141, 104856. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, T.; Wang, X.; Hu, Y.; Cheng, Y.; Li, H.; Shi, Y.; Wang, L.; Yin, D.; Cui, D. Embryonic Deletion of TXNIP in GABAergic Neurons Enhanced Oxidative Stress in PV+ Interneurons in Primary Somatosensory Cortex of Aging Mice: Relevance to Schizophrenia. Brain Sci. 2022, 12, 1395. https://doi.org/10.3390/brainsci12101395
Xue T, Wang X, Hu Y, Cheng Y, Li H, Shi Y, Wang L, Yin D, Cui D. Embryonic Deletion of TXNIP in GABAergic Neurons Enhanced Oxidative Stress in PV+ Interneurons in Primary Somatosensory Cortex of Aging Mice: Relevance to Schizophrenia. Brain Sciences. 2022; 12(10):1395. https://doi.org/10.3390/brainsci12101395
Chicago/Turabian StyleXue, Ting, Xiaodan Wang, Ying Hu, Ying Cheng, Han Li, Yuan Shi, Lijun Wang, Dongmin Yin, and Donghong Cui. 2022. "Embryonic Deletion of TXNIP in GABAergic Neurons Enhanced Oxidative Stress in PV+ Interneurons in Primary Somatosensory Cortex of Aging Mice: Relevance to Schizophrenia" Brain Sciences 12, no. 10: 1395. https://doi.org/10.3390/brainsci12101395
APA StyleXue, T., Wang, X., Hu, Y., Cheng, Y., Li, H., Shi, Y., Wang, L., Yin, D., & Cui, D. (2022). Embryonic Deletion of TXNIP in GABAergic Neurons Enhanced Oxidative Stress in PV+ Interneurons in Primary Somatosensory Cortex of Aging Mice: Relevance to Schizophrenia. Brain Sciences, 12(10), 1395. https://doi.org/10.3390/brainsci12101395






