Increased Proximal Wall Shear Stress of Basilar Artery Plaques Associated with Ruptured Fibrous Cap
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Profile
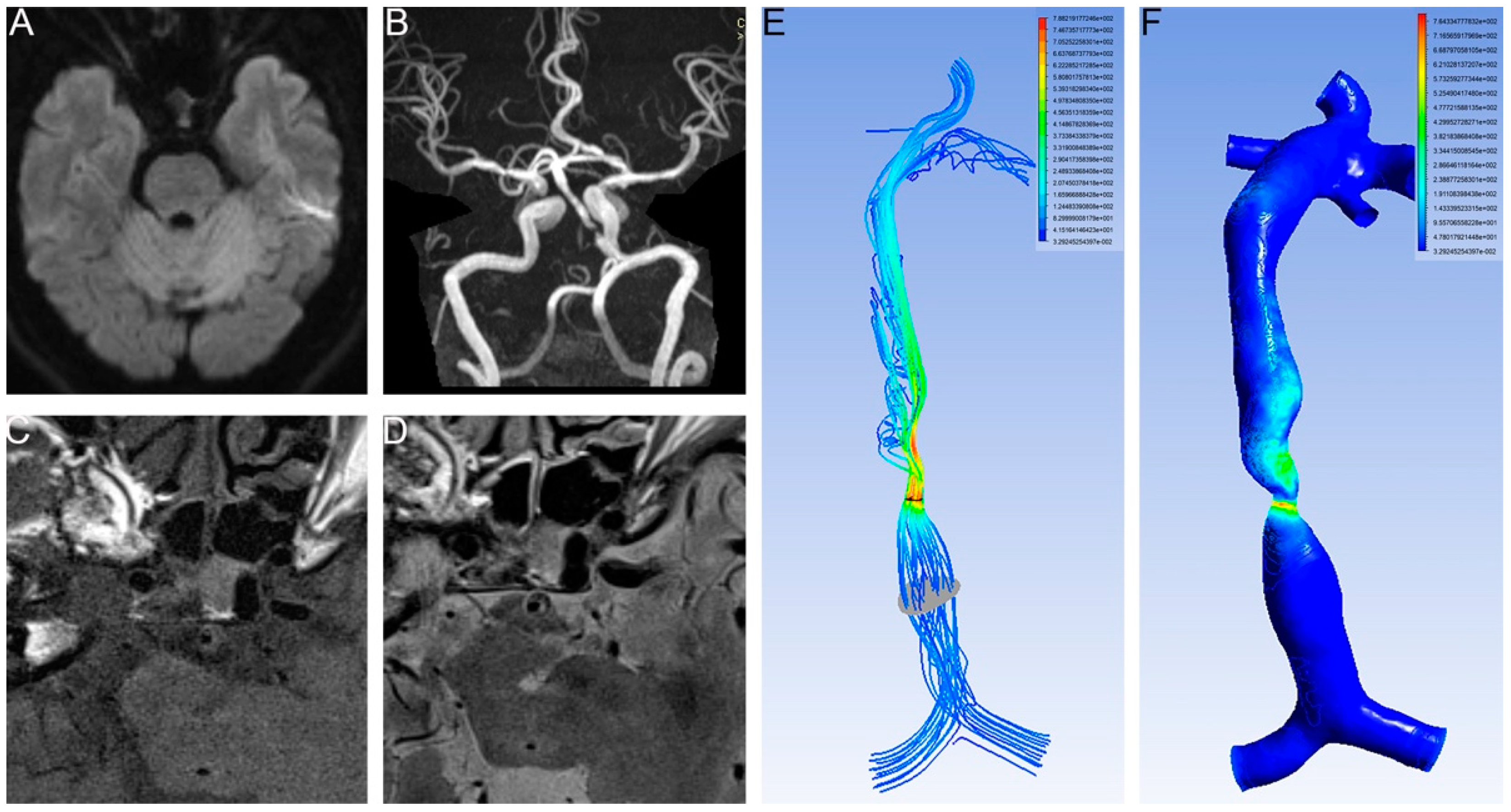
2.2. MRI and High Resolution MRI (HR-MRI) Scanning
2.3. Calculation of the CFD
2.4. Statistics
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmstedt, C.A.; Turan, T.N.; Chimowitz, M.I. Atherosclerotic intracranial arterial stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 2013, 12, 1106–1114. [Google Scholar] [CrossRef] [Green Version]
- Jing, L.N.; Gao, P.Y.; Lin, Y.; Sui, B.B.; Qin, H.Q.; Ma, L.; Xue, J. Distribution of wall shear stress in carotid plaques using magnetic resonance imaging and computational fluid dynamics analysis: A preliminary study. Chin. Med. J. 2011, 124, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Zhang, S.X.; Polissar, N.L.; Echelard, D.; Ortiz, G.; Davis, J.W.; Ellington, E.; Ferguson, M.S.; Hatsukami, T.S. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation 2002, 105, 181–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Yang, C.; Canton, G.; Ferguson, M.; Yuan, C.; Tang, D. Quantifying effect of intraplaque hemorrhage on critical plaque wall stress in human atherosclerotic plaques using three-dimensional fluid-structure interaction models. J. Biomech. Eng. 2012, 134, 121004. [Google Scholar] [CrossRef] [Green Version]
- Katritsis, D.; Kaiktsis, L.; Chaniotis, A.; Pantos, J.; Efstathopoulos, E.P.; Marmarelis, V. Wall shear stress: Theoretical considerations and methods of measurement. Prog. Cardiovasc. Dis. 2007, 49, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Groen, H.C.; Gijsen, F.J.; van der Lugt, A.; Ferguson, M.S.; Hatsukami, T.S.; van der Steen, A.F.; Yuan, C.; Wentzel, J.J. Plaque rupture in the carotid artery is localized at the high shear stress region: A case report. Stroke A J. Cereb. Circ. 2007, 38, 2379–2381. [Google Scholar] [CrossRef] [Green Version]
- Oshida, S.; Mori, F.; Sasaki, M.; Sato, Y.; Kobayshi, M.; Yoshida, K.; Fujiwara, S.; Ogasawara, K. Wall Shear Stress and T1 Contrast Ratio Are Associated With Embolic Signals During Carotid Exposure in Endarterectomy. Stroke A J. Cereb. Circ. 2018, 49, 2061–2066. [Google Scholar] [CrossRef]
- Skodvin, T.O.; Evju, O.; Helland, C.A.; Isaksen, J.G. Rupture prediction of intracranial aneurysms: A nationwide matched case-control study of hemodynamics at the time of diagnosis. J. Neurosurg. 2018, 129, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Leng, X.; Lan, L.; Ip, H.L.; Abrigo, J.; Scalzo, F.; Liu, H.; Feng, X.; Chan, K.L.; Fan, F.S.Y.; Ma, S.H.; et al. Hemodynamics and stroke risk in intracranial atherosclerotic disease. Ann. Neurol. 2019, 85, 752–764. [Google Scholar] [CrossRef]
- Caplan, L.R. Vertebrobasilar Ischemia and Hemorrhage, 2nd ed.; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Suh, D.C.; Park, S.T.; Oh, T.S.; Park, S.O.; Lim, O.K.; Park, S.; Ryu, C.W.; Lee, D.H.; Ko, Y.B.; Lee, S.W.; et al. High shear stress at the surface of enhancing plaque in the systolic phase is related to the symptom presentation of severe M1 stenosis. Korean J. Radiol. Off. J. Korean Radiol. Soc. 2011, 12, 515–518. [Google Scholar] [CrossRef]
- Xu, W. High-resolution MRI of intracranial large artery diseases: How to use it in clinical practice? Stroke Vasc. Neurol. 2019, 4, 102–104. [Google Scholar] [CrossRef]
- Finn, A.V.; Chandrashekhar, Y.; Narula, J. Vulnerable plaques: From PROSPECT to prospects. JACC. Cardiovasc. Imaging 2012, 5, 334–336. [Google Scholar] [CrossRef] [Green Version]
- Sui, B.; Gao, P. High-resolution vessel wall magnetic resonance imaging of carotid and intracranial vessels. Acta Radiol. 2019, 60, 1329–1340. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, S.; Li, M.L.; Ma, Y.; Dong, Y.R.; Lou, M.; Feng, F.; Gao, S.; Wu, S.W.; Xu, W.H. Relationship between the geometry patterns of vertebrobasilar artery and atherosclerosis. BMC Neurol. 2018, 18, 83. [Google Scholar] [CrossRef]
- Yu, J.H.; Kwak, H.S.; Chung, G.H.; Hwang, S.B.; Park, M.S.; Park, S.H. Association of Intraplaque Hemorrhage and Acute Infarction in Patients With Basilar Artery Plaque. Stroke A J. Cereb. Circ. 2015, 46, 2768–2772. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhao, X.Q.; Balu, N.; Neradilek, M.B.; Isquith, D.A.; Yamada, K.; Canton, G.; Crouse, J.R., 3rd; Anderson, T.J.; Huston, J., 3rd; et al. Carotid Plaque Lipid Content and Fibrous Cap Status Predict Systemic CV Outcomes: The MRI Substudy in AIM-HIGH. JACC Cardiovasc. Imaging 2017, 10, 241–249. [Google Scholar] [CrossRef]
- Seyedsaadat, S.M.; Rizvi, A.; Alzuabi, M.; Dugani, S.B.; Murad, M.H.; Huston, J., 3rd; Saba, L.; Brinjikji, W. Correlation of MRI-detected vulnerable carotid plaques with clinical presentation: A systematic review and meta-analysis. J. Neurosurg. Sci. 2019, 64, 263–271. [Google Scholar] [CrossRef]
- Clowes, A.W.; Berceli, S.A. Mechanisms of vascular atrophy and fibrous cap disruption. Ann. N. Y. Acad. Sci. 2000, 902, 153–161. [Google Scholar] [CrossRef]
- Teng, Z.; Canton, G.; Yuan, C.; Ferguson, M.; Yang, C.; Huang, X.; Zheng, J.; Woodard, P.K.; Tang, D. 3D critical plaque wall stress is a better predictor of carotid plaque rupture sites than flow shear stress: An in vivo MRI-based 3D FSI study. J. Biomech. Eng. 2010, 132, 031007. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Chatzizisis, Y.S.; Baker, A.B.; Edelman, E.R.; Stone, P.H.; Feldman, C.L. The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr. Opin. Cardiol. 2009, 24, 580–590. [Google Scholar] [CrossRef]
- Gao, H.; Long, Q.; Graves, M.; Gillard, J.H.; Li, Z.Y. Carotid arterial plaque stress analysis using fluid-structure interactive simulation based on in-vivo magnetic resonance images of four patients. J. Biomech. 2009, 42, 1416–1423. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Q.; Tian, D.; Wang, L.; Liu, B.; Zhang, S. Clinical significance of dynamic monitoring by transcranial doppler ultrasound and intracranial pressure monitor after surgery of hypertensive intracerebral hemorrhage. Int. J. Clin. Exp. Med. 2015, 8, 11456–11462. [Google Scholar]
- Ortega-Gutierrez, S.; Samaniego, E.A.; Huang, A.; Masurkar, A.; Zheng-Lin, B.; Derdeyn, C.P.; Hasan, D.; Marshall, R.; Petersen, N. Effect of Intracranial Stenosis Revascularization on Dynamic and Static Cerebral Autoregulation. J. Vasc. Interv. Neurol. 2018, 10, 1–6. [Google Scholar]
- Guan, J.; Zhou, Q.; Ouyang, H.; Zhang, S.; Lu, Z. The diagnostic accuracy of TCD for intracranial arterial stenosis/occlusion in patients with acute ischemic stroke: The importance of time interval between detection of TCD and CTA. Neurol. Res. 2013, 35, 930–936. [Google Scholar] [CrossRef]
- Xue, Y.J.; Gao, P.Y.; Duan, Q.; Lin, Y.; Dai, C.B. Preliminary study of hemodynamic distribution in patient-specific stenotic carotid bifurcation by image-based computational fluid dynamics. Acta Radiol. 2008, 49, 558–565. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Baker, A.B.; Sukhova, G.K.; Koskinas, K.C.; Papafaklis, M.I.; Beigel, R.; Jonas, M.; Coskun, A.U.; Stone, B.V.; Maynard, C.; et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation 2011, 123, 621–630. [Google Scholar] [CrossRef]


| Characteristics | Fibrous Cap | p | |
|---|---|---|---|
| Ruptured (n= 35) | Nonruptured (n = 141) | ||
| Age (yrs) | 53.3 ± 12.2 | 56.9 ± 12.9 | 0.156 |
| Male | 23 | 103 | 0.389 |
| Medical History | |||
| Hypertension | 15 | 78 | 0.509 |
| Diabetes | 8 | 37 | 0.825 |
| TC (mM) | 3.89 ± 1.19 | 4.31 ± 1.19 | 0.095 |
| LDL (mM) | 2.83 ± 1.52 | 2.92 ± 0.97 | 0.682 |
| TG (mM) | 1.70 ± 0.64 | 1.98 ± 1.00 | 0.174 |
| Smoker | 9 | 54 | 0.387 |
| Drinker | 5 | 32 | 0.336 |
| Medication | |||
| Antihypertensive | 12 | 67 | 0.239 |
| Hypoglycemic | 8 | 37 | 0.825 |
| Statin | 28 | 123 | 0.649 |
| Antiplatelet | 23 | 99 | 0.461 |
| Previous stroke or TIA | 1 | 24 | 0.049 |
| New stroke onset | 27 | 96 | 0.041 |
| CFD | |||
| Proximal WSS | 8.68 ± 17.60 | 8.07 ± 4.55 | 0.014 |
| Distal WSS | 48.62 ± 147.26 | 103.78 ± 522.40 | 0.567 |
| WSS ratio | 9.88 ± 21.12 | 16.04 ± 70.63 | 0.557 |
| Proximal velocity | 0.34 ± 019 | 0.38 ± 0.12 | 0.275 |
| Distal velocity | 0.90 ± 1.31 | 1.05 ± 2.04 | 0.858 |
| Velocity ratio | 2.49 ± 2.85 | 2.91 ± 5.54 | 0.811 |
| Characteristics | B | S.E. | Wald | p | Exp(B) | 95% CI | |
|---|---|---|---|---|---|---|---|
| TC | −0.621 | 0.463 | 1.797 | 0.18 | 0.538 | 0.217 | 1.332 |
| New stroke | −0.177 | 0.754 | 0.055 | 0.815 | 0.838 | 0.191 | 3.672 |
| Stroke history | −2.355 | 1.398 | 2.836 | 0.092 | 0.095 | 0.006 | 1.471 |
| Proximal WSS | 0.447 | 0.179 | 6.234 | 0.013 | 1.564 | 1.101 | 2.222 |
| Distal WSS | −0.015 | 0.05 | 0.09 | 0.765 | 0.985 | 0.893 | 1.086 |
| WSS ratio | 0.252 | 0.153 | 2.721 | 0.099 | 1.287 | 0.954 | 1.737 |
| Proximal velocity | −50.945 | 18 | 8.011 | 0.005 | 0 | 0 | 0 |
| Distal velocity | 12.52 | 5.383 | 5.41 | 0.02 | 273,843.797 | 7.168 | 1.0463 × 1010 |
| Velocity ratio | −4.617 | 2.116 | 4.761 | 0.029 | 0.01 | 0 | 0.625 |
| Proximal pressure | 0 | 0.001 | 0.229 | 0.632 | 1 | 0.998 | 1.001 |
| Distal pressure | −0.001 | 0.004 | 0.087 | 0.768 | 0.999 | 0.99 | 1.007 |
| Pressure ratio | 17.442 | 21.83 | 0.638 | 0.424 | 37,590,804.9 | 0 | 1.4357 × 1026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Chen, H.; Li, C.; Lie, C.; Qiu, Z.; Jiang, Y. Increased Proximal Wall Shear Stress of Basilar Artery Plaques Associated with Ruptured Fibrous Cap. Brain Sci. 2022, 12, 1397. https://doi.org/10.3390/brainsci12101397
Huang R, Chen H, Li C, Lie C, Qiu Z, Jiang Y. Increased Proximal Wall Shear Stress of Basilar Artery Plaques Associated with Ruptured Fibrous Cap. Brain Sciences. 2022; 12(10):1397. https://doi.org/10.3390/brainsci12101397
Chicago/Turabian StyleHuang, Ruiyun, Hongbing Chen, Chenghao Li, Chaowei Lie, Zhihua Qiu, and Yongjun Jiang. 2022. "Increased Proximal Wall Shear Stress of Basilar Artery Plaques Associated with Ruptured Fibrous Cap" Brain Sciences 12, no. 10: 1397. https://doi.org/10.3390/brainsci12101397





