Phase Lag Index of Resting-State EEG for Identification of Mild Cognitive Impairment Patients with Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. EEG Recording and Processing
2.3. Statistical Analysis
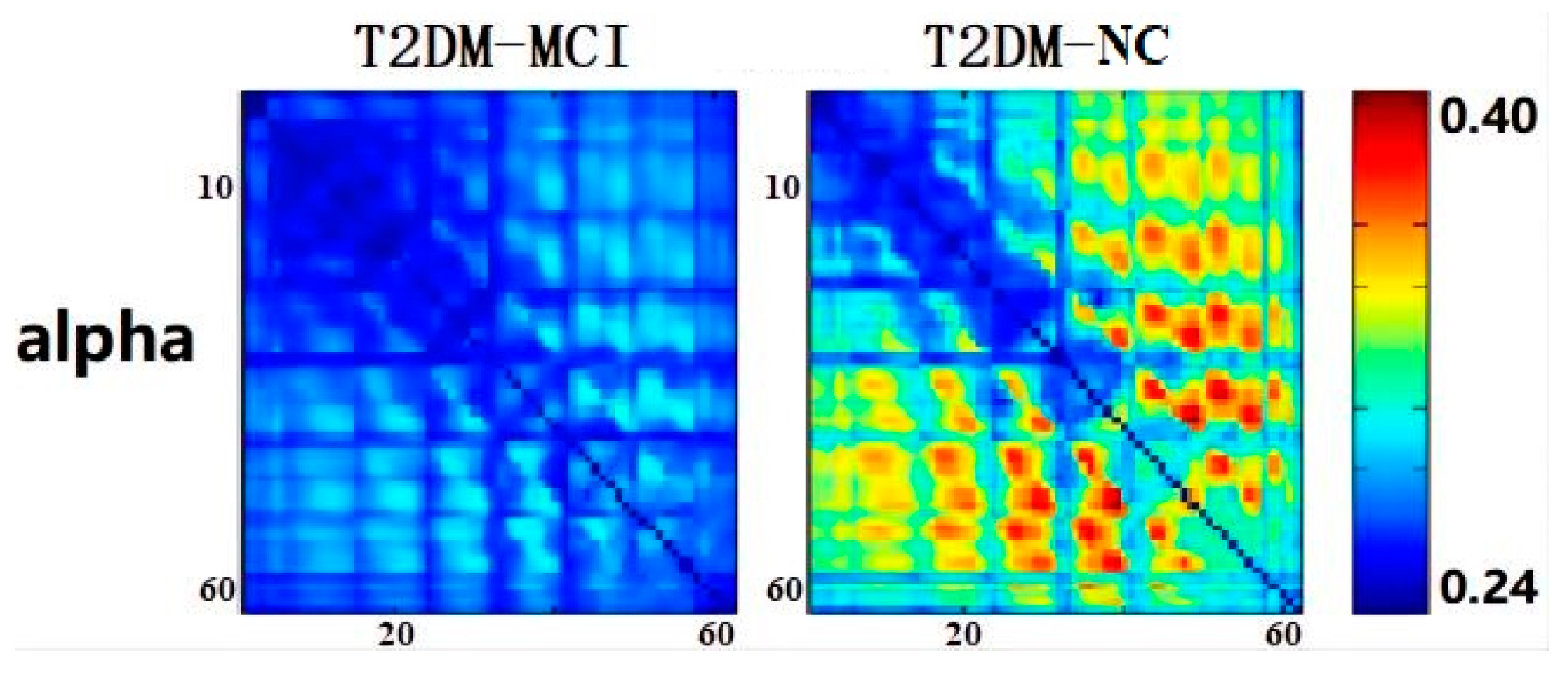
3. Results
3.1. Subject Characteristics
3.2. Electroencephalographic Results
3.3. Machine Learning Applications
3.4. Correlation Analysis
4. Discussion
4.1. This Work
| Study | Study Sample | EEG Features of Neurological Outcome | Main Findings |
|---|---|---|---|
| Zeng et al. [27] | Sixteen T2DM-aMCI patients and twelve T2DM-NC patients | Phase lag index, clustering coefficient, and path length | The complex network-derived biomarkers based on EEG could be employed to track the cognitive function of diabetic patients and provide a new diagnostic tool for T2DM-aMCI patients. |
| Bian et al. [28] | Sixteen T2DM-aMCI patients and twelve T2DM-NC patients | Relative power and coherence | The decreased theta, alpha coherence, and increased delta coherence in corresponding regions may distinguish T2DM-aMCI from T2DM-NC and help the diagnosis of T2DM-aMCI patients. |
| Wen et al. [31] | Nineteen T2DM-aMCI patients and twenty T2DM-NC patients | Permutation conditional mutual information (PCMI) | The coupling strength or directionality of EEG signals calculated by PCMI might be used as a biomarker in distinguishing the T2DM-aMCI from T2DM-NC. |
| Cui et al. [32] | Eight T2DM-aMCI patients and eleven T2DM-NC patients | Synchronization index (SI) and global synchronization index (GSI) | Each of the methods reflected that the cortical source synchronization was significantly different between the aMCI and the control group, and these differences correlated with cognitive functions. |
| Lu et al. [33] | Seventeen T2DM-aMCI patients and ten T2DM-NC patients | Correlation between probabilities of recurrence (CPR) | The synchronization value of the EEG signal was significantly decreased in T2DM-aMCI patients compared with T2DM-NC patients, and the EEG indicator was associated with cognitive impairment in T2DM-aMCI patients. |
| Proposed work | Thirty T2DM-MCI patients and thirty T2DM-NC patients | Phase lag index | The functional connectivity analysis of EEG may offer an effective way to track the cortical dysfunction linked to the cognitive deterioration of T2DM patients, and the α-band phase lag index may have a role in guiding the diagnosis of T2DM-MCI. |
4.2. Contributions
4.3. Limitations
4.4. Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name |
| T2DM | Type 2 Diabetes Mellitus |
| MCI | Mild Cognitive Impairment |
| T2DM-MCI | Mild Cognitive Impairment patients with Type 2 Diabetes |
| EEG | Electroencephalography |
| AD | Alzheimer’s Disease |
| MMSE | Mini-Mental State Examination |
| PLI | Phase Lag Index |
| T2DM-NC | Normal Controls with Type 2 Diabetes |
| EOG | Electrooculography |
| EMG | Electromyography |
| ECG | Electrocardiogram |
| SD | Standard Deviation |
| AUC | Area Under Curve |
| ROC | Receiver Operating Characteristic |
| MoCA | Montreal Cognitive Assessment |
| SVM | Support Vector Machine |
| LDA | Linear Discriminant Analysis |
| PCMI | Permutation Conditional Mutual Information |
| SI | Synchronization Index |
| GSI | Global Synchronization Index |
| CPR | Correlation between Probabilities of Recurrence |
References
- Benwell, C.S.Y.; Davila-Pérez, P.; Fried, P.J.; Jones, R.N.; Travison, T.G.; Santarnecchi, E.; Pascual-Leone, A.; Shafi, M.M. EEG spectral power abnormalities and their relationship with cognitive dysfunction in patients with Alzheimer’s disease and type 2 diabetes. Neurobiol. Aging 2020, 85, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xie, Z.; Wang, C.; Wang, S. Elevated Peripheral Brain-Derived Neurotrophic Factor Level Associated With Decreasing Insulin Secretion May Forecast Memory Dysfunction in Patients With Long-Term Type 2 Diabetes. Front. Physiol. 2022, 12, 686838. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.D. State of the science on mild cognitive impairment (MCI). CNS Spectr. 2019, 24, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Koekkoek, P.S.; Kappelle, L.J.; van den Berg, E.; Rutten, G.E.; Biessels, G.J. Cognitive function in patients with diabetes mellitus: Guidance for daily care. Lancet Neurol. 2015, 14, 329–340. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.J.; Zhang, M.; Xu, Z.Q.; Gao, C.Y.; Fang, C.Q.; Yan, J.C.; Zhou, H.D.; Chongqing Ageing Study Group. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 2011, 76, 1485–1491. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, L.; Pang, J.; Zhu, X.; Ming, D. Characterization of EEG Data Revealing Relationships With Cognitive and Motor Symptoms in Parkinson’s Disease: A Systematic Review. Front. Aging Neurosci. 2020, 12, 587396. [Google Scholar] [CrossRef]
- Hussain, I.; Hossain, M.A.; Jany, R.; Bari, M.A.; Uddin, M.; Kamal, A.R.M.; Ku, Y.; Kim, J.S. Quantitative Evaluation of EEG-Biomarkers for Prediction of Sleep Stages. Sensors 2022, 22, 3079. [Google Scholar] [CrossRef]
- Hussain, I.; Young, S.; Park, S.J. Driving-Induced Neurological Biomarkers in an Advanced Driver-Assistance System. Sensors 2021, 21, 6985. [Google Scholar] [CrossRef]
- Hussain, I.; Young, S.; Kim, C.H.; Benjamin, H.C.M.; Park, S.J. Quantifying Physiological Biomarkers of a Microwave Brain Stimulation Device. Sensors 2021, 21, 1896. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.J. Quantitative Evaluation of Task-Induced Neurological Outcome after Stroke. Brain Sci. 2021, 11, 900. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. HealthSOS: Real-Time Health Monitoring System for Stroke Prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Miraglia, F.; Vecchio, F.; Pappalettera, C.; Nucci, L.; Cotelli, M.; Judica, E.; Ferreri, F.; Rossini, P.M. Brain Connectivity and Graph Theory Analysis in Alzheimer’s and Parkinson’s Disease: The Contribution of Electrophysiological Techniques. Brain Sci. 2022, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hsu, C.Y.; Chiu, H.W.; Hu, C.J.; Lee, T.C. Frequency power and coherence of electroencephalography are correlated with the severity of Alzheimer’s disease: A multicenter analysis in Taiwan. J. Formos. Med. Assoc. 2015, 114, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wang, J.; Yu, H.; Wei, X.; Yang, C.; Deng, B. Decreased coherence and functional connectivity of electroencephalograph in Alzheimer’s disease. Chaos 2014, 24, 033136. [Google Scholar] [CrossRef]
- Musaeus, C.S.; Engedal, K.; Høgh, P.; Jelic, V.; Mørup, M.; Naik, M.; Oeksengaard, A.R.; Snaedal, J.; Wahlund, L.O.; Waldemar, G.; et al. Oscillatory connectivity as a diagnostic marker of dementia due to Alzheimer’s disease. Clin. Neurophysiol. 2019, 130, 1889–1899. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, Y.; Wei, M.; Guo, Y.; Yu, Z.; Wang, H.; Li, Z.; Fan, H. A novel index of functional connectivity: Phase lag based on Wilcoxon signed rank test. Cogn. Neurodyn. 2021, 15, 621–636. [Google Scholar] [CrossRef]
- Yu, M.; Gouw, A.A.; Hillebrand, A.; Tijms, B.M.; Stam, C.J.; van Straaten, E.C.; Pijnenburg, Y.A. Different functional connectivity and network topology in behavioral variant of frontotemporal dementia and Alzheimer’s disease: An EEG study. Neurobiol. Aging. 2016, 42, 150–162. [Google Scholar] [CrossRef]
- Hou, F.; Liu, C.; Yu, Z.; Xu, X.; Zhang, J.; Peng, C.K.; Wu, C.; Yang, A. Age-Related Alterations in Electroencephalography Connectivity and Network Topology During n-Back Working Memory Task. Front. Hum. Neurosci. 2018, 12, 484. [Google Scholar] [CrossRef]
- Cohen, M.X. Effects of time lag and frequency matching on phase-based connectivity. J. Neurosci. Methods. 2015, 250, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Weng, J.; Zhu, D.; Ji, L.; Lu, J.; Zhou, Z.; Zou, D.; Guo, L.; Ji, Q.; Chen, L.; et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 2019, 35, e3158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J.; Nolte, G.; Daffertshofer, A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007, 28, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Hardmeier, M.; Hatz, F.; Bousleiman, H.; Schindler, C.; Stam, C.J.; Fuhr, P. Reproducibility of functional connectivity and graph measures based on the phase lag index (PLI) and weighted phase lag index (wPLI) derived from high resolution EEG. PLoS ONE 2014, 9, e108648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alster, P.; Dunalska, A.; Migda, B.; Madetko, N.; Królicki, L. The Rate of Decrease in Brain Perfusion in Progressive Supranuclear Palsy and Corticobasal Syndrome May Be Impacted by Glycemic Variability-A Pilot Study. Front. Neurol. 2021, 12, 767480. [Google Scholar] [CrossRef]
- Zeng, K.; Wang, Y.; Ouyang, G.; Bian, Z.; Wang, L.; Li, X. Complex network analysis of resting state EEG in amnestic mild cognitive impairment patients with type 2 diabetes. Front. Comput. Neurosci. 2015, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.; Li, Q.; Wang, L.; Lu, C.; Yin, S.; Li, X. Relative power and coherence of EEG series are related to amnestic mild cognitive impairment in diabetes. Front. Aging Neurosci. 2014, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Viccaro, L.J.; Perera, S.; Studenski, S.A. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J. Am. Geriatr. Soc. 2011, 59, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Puthankattil, S.D. Functional Connectivity and Complexity in the Phenomenological Model of Mild Cognitive-Impaired Alzheimer’s Disease. Front. Comput. Neurosci. 2022, 16, 877912. [Google Scholar] [CrossRef]
- Wen, D.; Bian, Z.; Li, Q.; Wang, L.; Lu, C.; Li, X. Resting-state EEG coupling analysis of amnestic mild cognitive impairment with type 2 diabetes mellitus by using permutation conditional mutual information. Clin. Neurophysiol. 2016, 127, 335–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, D.; Liu, J.; Bian, Z.; Li, Q.; Wang, L.; Li, X. Cortical source multivariate EEG synchronization analysis on amnestic mild cognitive impairment in type 2 diabetes. Sci. World J. 2014, 2014, 523216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Liu, J.; Gu, G.; Li, X.; Yin, S.; Cui, D. Nonlinear Phase Synchronization Analysis of EEG Signals in Amnesic Mild Cognitive Impairment with Type 2 Diabetes Mellitus. Neuroscience 2021, 472, 25–34. [Google Scholar] [CrossRef] [PubMed]




| Group | T2DM-MCI (N = 30) | T2DM-NC (N = 30) | χ2/t Value | p-Value |
|---|---|---|---|---|
| Gender (M/F) | 13/17 | 16/14 | 0.601 | 0.438 |
| Age (years) | 67.17 ± 4.12 | 67.73 ± 4.40 | −0.515 | 0.609 |
| Education level (years) | 10.63 ± 3.75 | 10.23 ± 3.23 | 0.443 | 0.660 |
| MoCA(scores) | 22.08 ± 2.24 | 28.08 ± 1.41 | −11.350 | <0.001 * |
| Duration of T2DM (years) | 14.23 ± 7.80 | 15.43 ± 8.34 | −0.576 | 0.567 |
| Duration of MCI (years) | 3.33 ± 1.86 | - | - | - |
| Classifier | Accuracy (%) | Empirical Chance Level Accuracy (%) | Specificity (%) | Sensitivity (%) | AUC |
|---|---|---|---|---|---|
| Logistic Regression | 75.00 | 64.58 | 60.87 | 88.00 | 0.805 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.; Wu, Z.; Xia, R.; Li, X.; Liu, J.; Dai, Y.; Wang, D.; Chen, S. Phase Lag Index of Resting-State EEG for Identification of Mild Cognitive Impairment Patients with Type 2 Diabetes. Brain Sci. 2022, 12, 1399. https://doi.org/10.3390/brainsci12101399
Kuang Y, Wu Z, Xia R, Li X, Liu J, Dai Y, Wang D, Chen S. Phase Lag Index of Resting-State EEG for Identification of Mild Cognitive Impairment Patients with Type 2 Diabetes. Brain Sciences. 2022; 12(10):1399. https://doi.org/10.3390/brainsci12101399
Chicago/Turabian StyleKuang, Yuxing, Ziyi Wu, Rui Xia, Xingjie Li, Jun Liu, Yalan Dai, Dan Wang, and Shangjie Chen. 2022. "Phase Lag Index of Resting-State EEG for Identification of Mild Cognitive Impairment Patients with Type 2 Diabetes" Brain Sciences 12, no. 10: 1399. https://doi.org/10.3390/brainsci12101399





