Activation Patterns of Functional Brain Network in Response to Action Observation-Induced and Non-Induced Motor Imagery of Swallowing: A Pilot Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. Methods
2.2.1. Data Acquisition
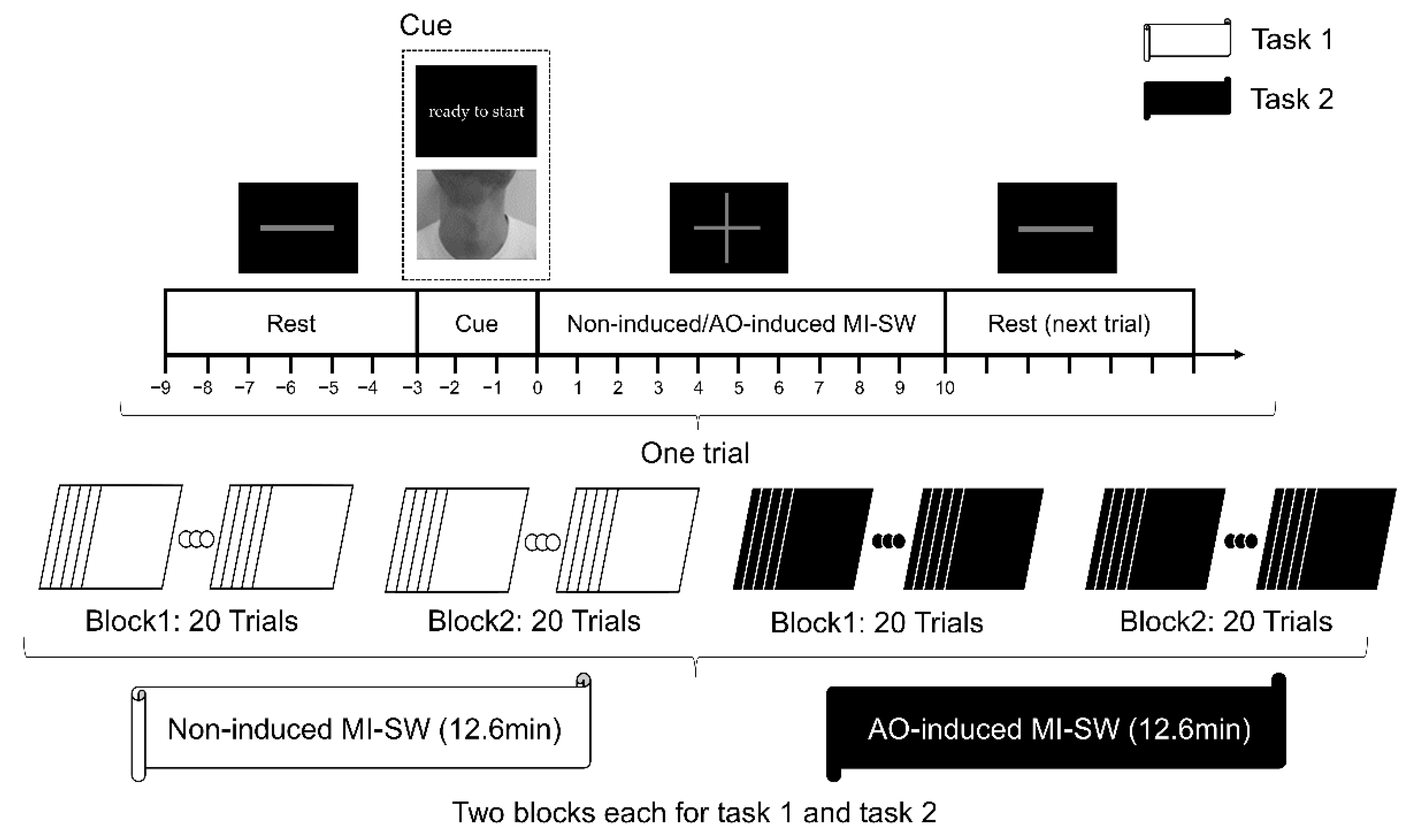
2.2.2. Experimental Design
2.2.3. Data Processing
2.2.4. ROI Activation Indicators
2.2.5. Construction and Analysis of Brain Network
2.2.6. Statistical Analysis
3. Results
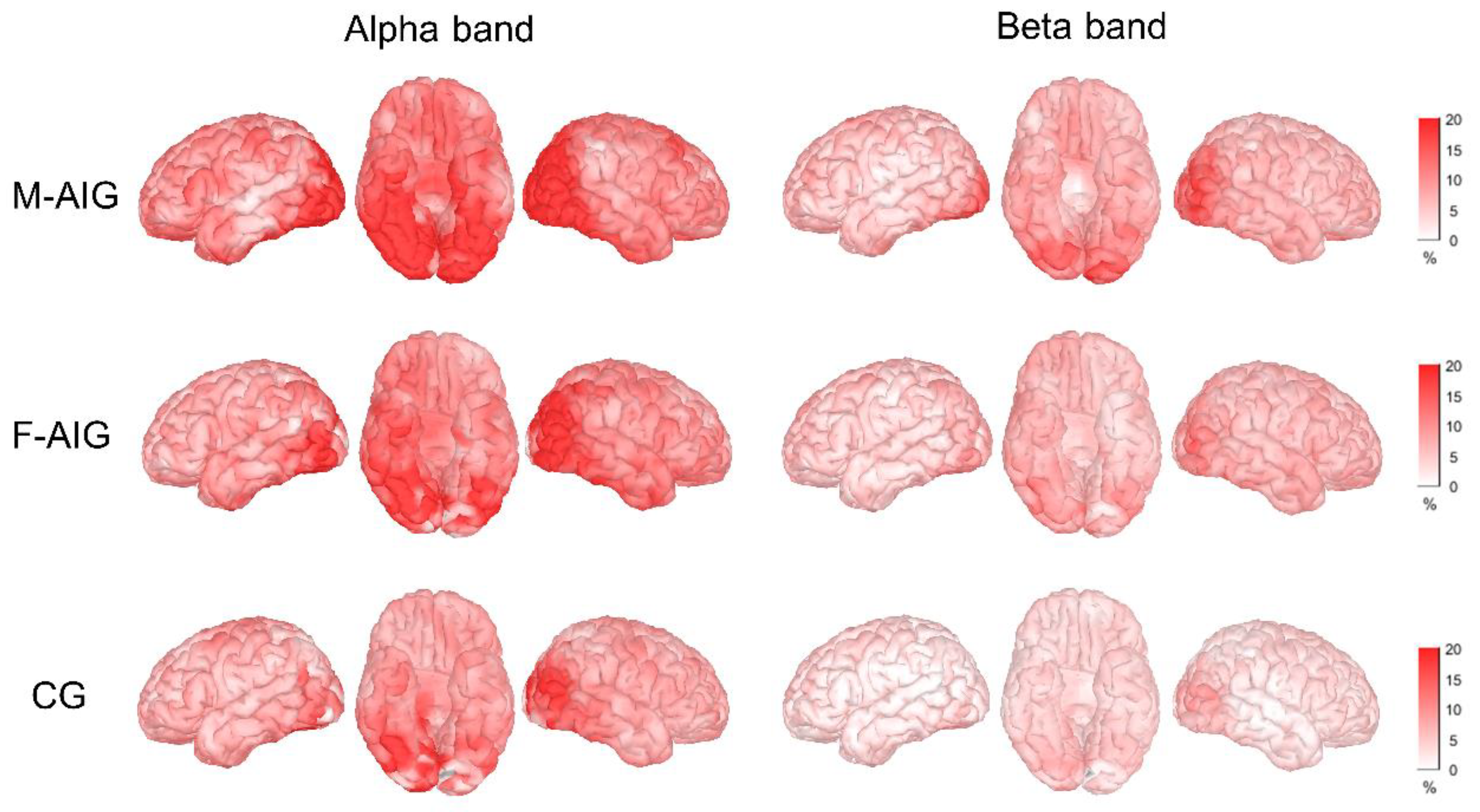
3.1. ERS Activation Mode
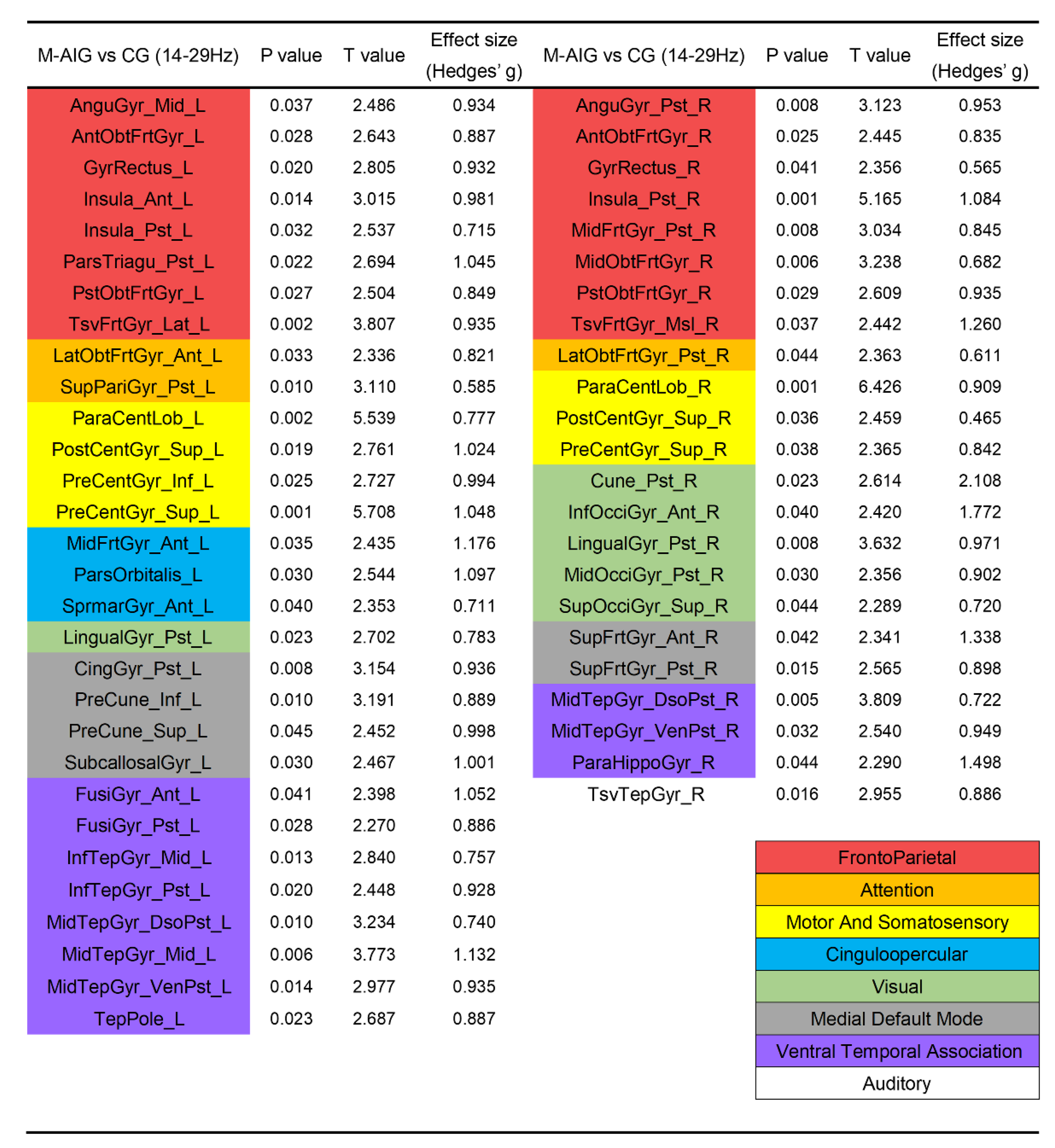
3.2. Comparison of ROIs’ Activation between M-AIG and CG
3.3. Comparison of ROIs’ Activation between F-AIG and CG
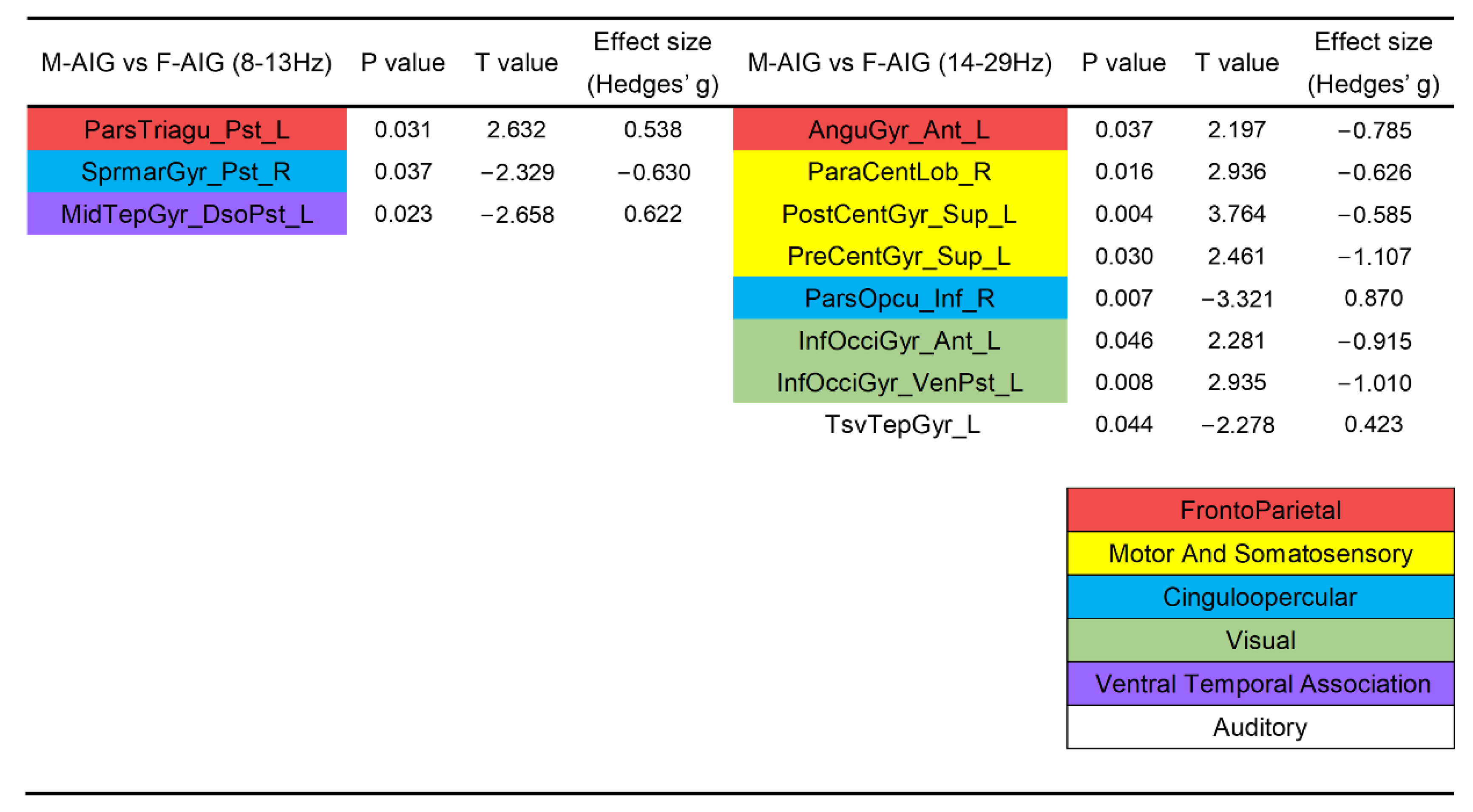
3.4. Comparison of ROIs’ Activation between M-AIG and F-AIG
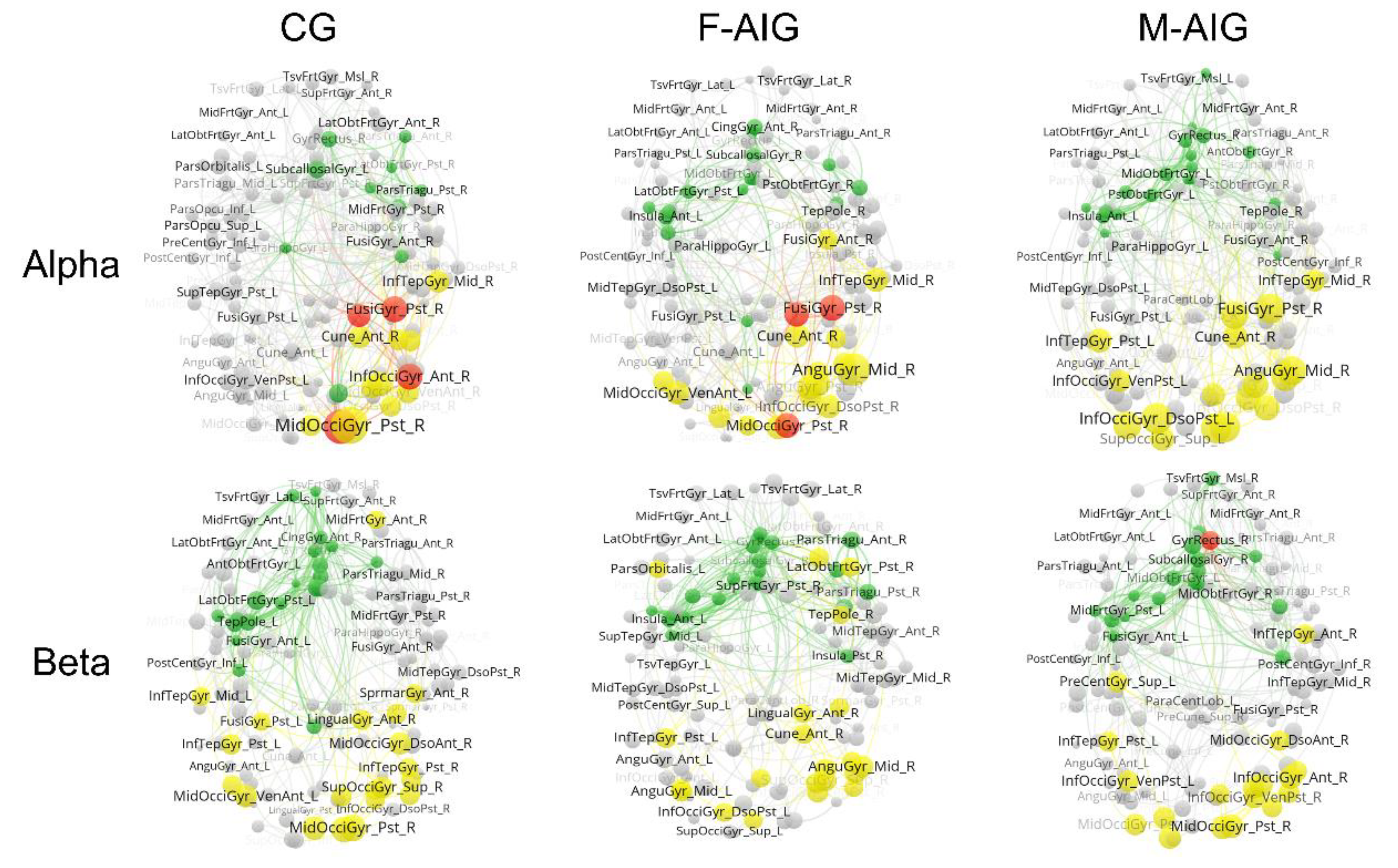
3.5. Comparison of Functional Brain Networks among Three Groups
4. Discussion
4.1. ERS Pattern of MI-SW
4.2. M-AIG and F-AIG versus CG: The Comparisons of Brain Activation
4.3. M-AIG and F-AIG versus CG: Functional Brain Network Connectivity Analysis
4.4. M-AIG versus F-AIG: The Comparisons of Brain Activation
5. Limitations and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MI | Motor imagery |
| AO | Action observation |
| MI-SW | Motor imagery of swallowing |
| MEG | Magnetoencephalography |
| MMSE | Mini-mental state examination |
| ERSPs | Event-related spectral perturbations |
| ERD | Event-related desynchronization |
| ERS | Event-related synchronization |
| PLV | Phase locking value |
| ROI | Regions of interest |
| CG | Control group |
| M-AIG | Male action observation-induced group |
| F-AIG | Female action observation-induced group |
References
- Rommel, N.; Hamdy, S. Oropharyngeal Dysphagia: Manifestations and Diagnosis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bhidayasiri, R.; Phuenpathom, W.; Tan, A.H.; Leta, V.; Phumphid, S.; Chaudhuri, K.R.; Pal, P.K. Management of Dysphagia and Gastroparesis in Parkinson’s Disease in Real-World Clinical Practice-Balancing Pharmacological and Non-Pharmacological Approaches. Front. Aging Neurosci. 2022, 14, 979826. [Google Scholar] [CrossRef]
- McCarty, E.B.; Chao, T.N. Dysphagia and Swallowing Disorders. Med. Clin. N. Am. 2021, 105, 939–954. [Google Scholar] [CrossRef]
- Terré, R. Oropharyngeal dysphagia in stroke: Diagnostic and therapeutic aspects. Rev. Neurol. 2020, 70, 444–452. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wu, L.; Ni, F.; Li, X.; Liang, K.; Ma, R. Efficacy and Safety of Mirror Therapy for Post-Stroke Dysphagia: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 874994. [Google Scholar] [CrossRef] [PubMed]
- Kilner, J.M.; Lemon, R.N. What We Know Currently about Mirror Neurons. Curr. Biol. 2013, 23, R1057–R1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, Y.; Lin, T.; Li, W.; Wu, C.; Li, X.; Ding, Q.; Wu, M.; Xu, G.; Lan, Y. Comparison of Activation Patterns in Mirror Neurons and the Swallowing Network During Action Observation and Execution: A Task-Based FMRI Study. Front. Neurosci. 2020, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Kober, S.E.; Grössinger, D.; Wood, G. Effects of Motor Imagery and Visual Neurofeedback on Activation in the Swallowing Network: A Real-Time FMRI Study. Dysphagia 2019, 34, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Kim, J.-H.; Yang, Y.-J. Mental Practice for Upper Limb Rehabilitation after Stroke: A Systematic Review and Meta-Analysis. Int. J. Rehabil. Res. 2018, 41, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Romkema, S.; Bongers, R.M.; van der Sluis, C.K. Influence of Mirror Therapy and Motor Imagery on Intermanual Transfer Effects in Upper-Limb Prosthesis Training of Healthy Participants: A Randomized Pre-Posttest Study. PLoS ONE 2018, 13, e0204839. [Google Scholar] [CrossRef]
- Crajé, C.; van der Graaf, C.; Lem, F.C.; Geurts, A.C.H.; Steenbergen, B. Determining Specificity of Motor Imagery Training for Upper Limb Improvement in Chronic Stroke Patients: A Training Protocol and Pilot Results. Int. J. Rehabil. Res. 2010, 33, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Rannaud Monany, D.; Papaxanthis, C.; Guillot, A.; Lebon, F. Motor Imagery and Action Observation Following Immobilization-Induced Hypoactivity: A Narrative Review. Ann. Phys. Rehabil. Med. 2021, 65, 101541. [Google Scholar] [CrossRef]
- Scott, M.W.; Wood, G.; Holmes, P.S.; Williams, J.; Marshall, B.; Wright, D.J. Combined Action Observation and Motor Imagery: An Intervention to Combat the Neural and Behavioural Deficits Associated with Developmental Coordination Disorder. Neurosci. Biobehav. Rev. 2021, 127, 638–646. [Google Scholar] [CrossRef]
- Szynkiewicz, S.H.; Kamarunas, E.; Drulia, T.; Nobriga, C.V.; Griffin, L.; O’Donoghue, C.R. A Randomized Controlled Trial Comparing Physical and Mental Lingual Exercise for Healthy Older Adults. Dysphagia 2020, 36, 474–482. [Google Scholar] [CrossRef]
- Rovner, B.W.; Folstein, M.F. Mini-Mental State Exam in Clinical Practice. Hosp. Pract. (Off. Ed.) 1987, 22, 99, 103, 106, 110. [Google Scholar] [PubMed]
- Malouin, F.; Richards, C.L.; Jackson, P.L.; Lafleur, M.F.; Durand, A.; Doyon, J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for Assessing Motor Imagery in Persons with Physical Disabilities: A Reliability and Construct Validity Study. J. Neurol. Phys. Ther. 2007, 31, 20–29. [Google Scholar] [CrossRef]
- Ahmed, M.Z.I.; Sinha, N.; Phadikar, S.; Ghaderpour, E. Automated Feature Extraction on As Map for Emotion Classification Using EEG. Sensors 2022, 22, 2346. [Google Scholar] [CrossRef] [PubMed]
- Grobbelaar, M.; Phadikar, S.; Ghaderpour, E.; Struck, A.F.; Sinha, N.; Ghosh, R.; Ahmed, M.Z.I. A Survey on Denoising Techniques of Electroencephalogram Signals Using Wavelet Transform. Signals 2022, 3, 577–586. [Google Scholar] [CrossRef]
- Yang, H.; Guan, C.; Chua, K.S.G.; Chok, S.S.; Wang, C.C.; Soon, P.K.; Tang, C.K.Y.; Ang, K.K. Detection of Motor Imagery of Swallow EEG Signals Based on the Dual-Tree Complex Wavelet Transform and Adaptive Model Selection. J. Neural. Eng. 2014, 11, 035016. [Google Scholar] [CrossRef] [PubMed]
- Dinga, S.; Wu, D.; Huang, S.; Wu, C.; Wang, X.; Shi, J.; Hu, Y.; Liang, C.; Zhang, F.; Lu, M.; et al. Neuromagnetic Correlates of Audiovisual Word Processing in the Developing Brain. Int. J. Psychophysiol. 2018, 128, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guan, C.; Wang, C.C.; Ang, K.K.; Phua, K.S.; Chok, S.S.; Tang, C.K.Y.; Chua, K.S.G. On the Correlations of Motor Imagery of Swallow with Motor Imagery of Tongue Movements and Actual Swallow. In Advances in Cognitive Neurodynamics (V); Wang, R., Pan, X., Eds.; Springer: Singapore, 2016; pp. 397–404. ISBN 978-981-10-0205-2. [Google Scholar]
- Kober, S.E.; Wood, G. Changes in Hemodynamic Signals Accompanying Motor Imagery and Motor Execution of Swallowing: A near-Infrared Spectroscopy Study. NeuroImage 2014, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sanjo, Y.; Watanabe, T.; Ushioda, T. Visual Stimuli Associated with Swallowing Activate Mirror Neurons: An FMRI Study. Clin. Dent. Res. 2011, 35, 3–16. [Google Scholar]
- Kawai, T.; Watanabe, Y.; Tonogi, M.; Yamane, G.; Abe, S.; Yamada, Y.; Callan, A. Visual and Auditory Stimuli Associated with Swallowing: An FMRI Study. Bull. Tokyo Dent. Coll. 2009, 50, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Ushioda, T.; Watanabe, Y.; Sanjo, Y.; Yamane, G.; Abe, S.; Tsuji, Y.; Ishiyama, A. Visual and Auditory Stimuli Associated with Swallowing Activate Mirror Neurons: A Magnetoencephalography Study. Dysphagia 2012, 27, 504–513. [Google Scholar] [CrossRef]
- Ogura, M.; Watanabe, Y.; Sanjo, Y.; Edahiro, A.; Sato, K.; Katakura, A. Mirror Neurons Activated during Swallowing and Finger Movements: An FMRI Study. J. Oral Maxillofac. Surg. Med. Pathol. 2014, 26, 188–197. [Google Scholar] [CrossRef]
- Joshi, A.A.; Choi, S.; Sonkar, G.; Chong, M.; Gonzalez-Martinez, J.; Nair, D.; Shattuck, D.W.; Damasio, H.; Leahy, R.M. A Whole Brain Atlas with Sub-Parcellation of Cortical Gyri Using Resting FMRI. In Proceedings of the SPIE Medical Imaging 2017: Image Processing, Orlando, FL, USA, 11–16 February 2017; Styner, M.A., Angelini, E.D., Eds.; 2 March 2017; Volume 10133, pp. 186–194. [Google Scholar]
- Tadel, F.; Bock, E.; Niso, G.; Mosher, J.C.; Cousineau, M.; Pantazis, D.; Leahy, R.M.; Baillet, S. MEG/EEG Group Analysis With Brainstorm. Front. Neurosci. 2019, 13, 76. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Pavon, J.C.; Kugiumtzis, D.; Zrenner, C.; Kimiskidis, V.K.; Metsomaa, J. Removing Artifacts from TMS-Evoked EEG: A Methods Review and a Unifying Theoretical Framework. J. Neurosci. Methods 2022, 376, 109591. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-H.; Kim, B.; Kim, H.-S.; Gim, S.-Y.; Kim, W.-R.; Chung, S.-C. Perceptual Threshold Level for the Tactile Stimulation and Response Features of ERD/ERS-Based Specific Indices Upon Changes in High-Frequency Vibrations. Front. Hum. Neurosci. 2017, 11, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuellar, M.; Harkrider, A.W.; Jenson, D.; Thornton, D.; Bowers, A.; Saltuklaroglu, T. Time–Frequency Analysis of the EEG Mu Rhythm as a Measure of Sensorimotor Integration in the Later Stages of Swallowing. Clin. Neurophysiol. 2016, 127, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. Alpha-Band Oscillations, Attention, and Controlled Access to Stored Information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grazia, A.; Wimmer, M.; Müller-Putz, G.R.; Wriessnegger, S.C. Neural Suppression Elicited During Motor Imagery Following the Observation of Biological Motion from Point-Light Walker Stimuli. Front. Hum. Neurosci. 2021, 15, 788036. [Google Scholar] [CrossRef]
- Düzel, E.; Habib, R.; Schott, B.; Schoenfeld, A.; Lobaugh, N.; McIntosh, A.R.; Scholz, M.; Heinze, H.J. A Multivariate, Spatiotemporal Analysis of Electromagnetic Time-Frequency Data of Recognition Memory. NeuroImage 2003, 18, 185–197. [Google Scholar] [CrossRef]
- Muldoon, S.F.; Pasqualetti, F.; Gu, S.; Cieslak, M.; Grafton, S.T.; Vettel, J.M.; Bassett, D.S. Stimulation-Based Control of Dynamic Brain Networks. PLoS Comput. Biol. 2016, 12, e1005076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Nooy, W.; Mrvar, A.; Batagelj, V. Exploratory Social Network Analysis with Pajek, 3rd ed.; Cambridge University Press: New York, NY, USA, 2018. [Google Scholar]
- Van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Gonuguntla, V.; Wang, Y.; Veluvolu, K.C. Event-Related Functional Network Identification: Application to EEG Classification. IEEE J. Sel. Top. Signal Process. 2016, 10, 1284–1294. [Google Scholar] [CrossRef]
- Lin, F.; Cheng, S.-Q.; Qi, D.-Q.; Jiang, Y.-E.; Lyu, Q.-Q.; Zhong, L.-J.; Jiang, Z.-L. Brain Hothubs and Dark Functional Networks: Correlation Analysis between Amplitude and Connectivity for Broca’s Aphasia. PeerJ 2020, 8, e10057. [Google Scholar] [CrossRef]
- Bishara, A.J.; Hittner, J.B. Testing the Significance of a Correlation with Nonnormal Data: Comparison of Pearson, Spearman, Transformation, and Resampling Approaches. Psychol. Methods 2012, 17, 399–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Emerson, J.R.; Binks, J.A.; Scott, M.W.; Kenny, R.P.W.; Eaves, D.L. Combined Action Observation and Motor Imagery Therapy: A Novel Method for Post-Stroke Motor Rehabilitation. AIMS Neurosci. 2018, 5, 236–252. [Google Scholar] [CrossRef]
- Romano Smith, S.; Wood, G.; Coyles, G.; Roberts, J.W.; Wakefield, C.J. The Effect of Action Observation and Motor Imagery Combinations on Upper Limb Kinematics and EMG during Dart-throwing. Scand. J. Med. Sci. Sports 2019, 29, 1917–1929. [Google Scholar] [CrossRef]
- Villiger, M.; Estévez, N.; Hepp-Reymond, M.-C.; Kiper, D.; Kollias, S.S.; Eng, K.; Hotz-Boendermaker, S. Enhanced Activation of Motor Execution Networks Using Action Observation Combined with Imagination of Lower Limb Movements. PLoS ONE 2013, 8, e72403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R.E.; Sessle, B.J. The Role of the Cerebral Cortex in Swallowing. Dysphagia 1993, 8, 195–202. [Google Scholar] [CrossRef]
- Hamdy, S.; Mikulis, D.J.; Crawley, A.; Xue, S.; Lau, H.; Henry, S.; Diamant, N.E. Cortical Activation during Human Volitional Swallowing: An Event-Related FMRI Study. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 277, G219–G225. [Google Scholar] [CrossRef]
- Kern, M.K.; Jaradeh, S.; Arndorfer, R.C.; Shaker, R. Cerebral Cortical Representation of Reflexive and Volitional Swallowing in Humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G354–G360. [Google Scholar] [CrossRef]
- Martin, R.E.; Goodyear, B.G.; Gati, J.S.; Menon, R.S. Cerebral Cortical Representation of Automatic and Volitional Swallowing in Humans. J. Neurophysiol. 2001, 85, 938–950. [Google Scholar] [CrossRef]
- Martin, R.E.; MacIntosh, B.J.; Smith, R.C.; Barr, A.M.; Stevens, T.K.; Gati, J.S.; Menon, R.S. Cerebral Areas Processing Swallowing and Tongue Movement Are Overlapping but Distinct: A Functional Magnetic Resonance Imaging Study. J. Neurophysiol. 2004, 92, 2428–2443. [Google Scholar] [CrossRef] [Green Version]
- Toogood, J.A.; Smith, R.C.; Stevens, T.K.; Gati, J.S.; Menon, R.S.; Theurer, J.; Weisz, S.; Affoo, R.H.; Martin, R.E. Swallowing Preparation and Execution: Insights from a Delayed-Response Functional Magnetic Resonance Imaging (FMRI) Study. Dysphagia 2017, 32, 526–541. [Google Scholar] [CrossRef]
- Hashimoto, H.; Takahashi, K.; Kameda, S.; Yoshida, F.; Maezawa, H.; Oshino, S.; Tani, N.; Khoo, H.M.; Yanagisawa, T.; Yoshimine, T.; et al. Swallowing-related Neural Oscillation: An Intracranial EEG Study. Ann. Clin. Transl. Neurol. 2021, 8, 1224–1238. [Google Scholar] [CrossRef]
- Babaei, A.; Ward, B.D.; Siwiec, R.M.; Ahmad, S.; Kern, M.; Nencka, A.; Li, S.-J.; Shaker, R. Functional Connectivity of the Cortical Swallowing Network in Humans. NeuroImage 2013, 76, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Jestrović, I.; Coyle, J.L.; Perera, S.; Sejdić, E. Functional Connectivity Patterns of Normal Human Swallowing: Difference among Various Viscosity Swallows in Normal and Chin-Tuck Head Positions. Brain Res. 2016, 1652, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Bülow, M.; Olsson, R.; Ekberg, O. Videomanometric Analysis of Supraglottic Swallow, Effortful Swallow, and Chin Tuck in Healthy Volunteers. Dysphagia 1999, 14, 67–72. [Google Scholar] [CrossRef]
- Lewin, J.S.; Hebert, T.M.; Putnam, J.B.; DuBrow, R.A. Experience with the Chin Tuck Maneuver in Postesophagectomy Aspirators. Dysphagia 2001, 16, 216–219. [Google Scholar] [CrossRef]
- Nam, C.S.; Jeon, Y.; Kim, Y.-J.; Lee, I.; Park, K. Movement Imagery-Related Lateralization of Event-Related (de)Synchronization (ERD/ERS): Motor-Imagery Duration Effects. Clin. Neurophysiol. 2011, 122, 567–577. [Google Scholar] [CrossRef]
- Tariq, M.; Trivailo, P.M.; Simic, M. Mu-Beta Event-Related (de)Synchronization and EEG Classification of Left-Right Foot Dorsiflexion Kinaesthetic Motor Imagery for BCI. PLoS ONE 2020, 15, e0230184. [Google Scholar] [CrossRef]
- Kober, S.E.; Bauernfeind, G.; Woller, C.; Sampl, M.; Grieshofer, P.; Neuper, C.; Wood, G. Hemodynamic Signal Changes Accompanying Execution and Imagery of Swallowing in Patients with Dysphagia: A Multiple Single-Case Near-Infrared Spectroscopy Study. Front. Neurol. 2015, 6, 151. [Google Scholar] [CrossRef] [Green Version]
- Zannino, G.D.; Barban, F.; Macaluso, E.; Caltagirone, C.; Carlesimo, G.A. The Neural Correlates of Object Familiarity and Domain Specificity in the Human Visual Cortex: An FMRI Study. J. Cogn. Neurosci. 2011, 23, 2878–2891. [Google Scholar] [CrossRef]
- Kim, H. Dissociating the Roles of the Default-Mode, Dorsal, and Ventral Networks in Episodic Memory Retrieval. NeuroImage 2010, 50, 1648–1657. [Google Scholar] [CrossRef]
- Jackson, P.L.; Lafleur, M.F.; Malouin, F.; Richards, C.; Doyon, J. Potential Role of Mental Practice Using Motor Imagery in Neurologic Rehabilitation. Arch. Phys. Med. Rehabil. 2001, 82, 1133–1141. [Google Scholar] [CrossRef]
- Neuper, C.; Scherer, R.; Reiner, M.; Pfurtscheller, G. Imagery of Motor Actions: Differential Effects of Kinesthetic and Visual–Motor Mode of Imagery in Single-Trial EEG. Cogn. Brain Res. 2005, 25, 668–677. [Google Scholar] [CrossRef]
- Stinear, C.M.; Byblow, W.D.; Steyvers, M.; Levin, O.; Swinnen, S.P. Kinesthetic, but Not Visual, Motor Imagery Modulates Corticomotor Excitability. Exp. Brain Res. 2006, 168, 157–164. [Google Scholar] [CrossRef]
- Posner, M.I.; Rothbart, M.K.; Voelker, P. Developing Brain Networks of Attention. Curr. Opin. Pediatr. 2016, 28, 720–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medaglia, J.D. Functional Neuroimaging in Traumatic Brain Injury: From Nodes to Networks. Front. Neurol. 2017, 8, 407. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, H.; Chen, J.-J.; Gikaro, J.M.; Wang, C.-G.; Lin, F. Activation Patterns of Functional Brain Network in Response to Action Observation-Induced and Non-Induced Motor Imagery of Swallowing: A Pilot Study. Brain Sci. 2022, 12, 1420. https://doi.org/10.3390/brainsci12101420
Xiong H, Chen J-J, Gikaro JM, Wang C-G, Lin F. Activation Patterns of Functional Brain Network in Response to Action Observation-Induced and Non-Induced Motor Imagery of Swallowing: A Pilot Study. Brain Sciences. 2022; 12(10):1420. https://doi.org/10.3390/brainsci12101420
Chicago/Turabian StyleXiong, Hao, Jin-Jin Chen, John M. Gikaro, Chen-Guang Wang, and Feng Lin. 2022. "Activation Patterns of Functional Brain Network in Response to Action Observation-Induced and Non-Induced Motor Imagery of Swallowing: A Pilot Study" Brain Sciences 12, no. 10: 1420. https://doi.org/10.3390/brainsci12101420




