On the Embodiment of Social Cognition Skills: The Inner and Outer Body Processing Differently Contributes to the Affective and Cognitive Theory of Mind
Abstract
1. Introduction
1.1. Higher-Order Body Representations and Social Cognition
1.2. Interoception and Social Cognition
1.3. The Present Study
2. Materials and Methods
2.1. Participants
2.2. Behavioral Testing
2.2.1. Procedure
2.2.2. Assessment of the Interoceptive Sensibility
2.2.3. Assessment of Body Representations
2.2.4. Assessment of Affective and Cognitive ToM
2.3. Statistical Analyses
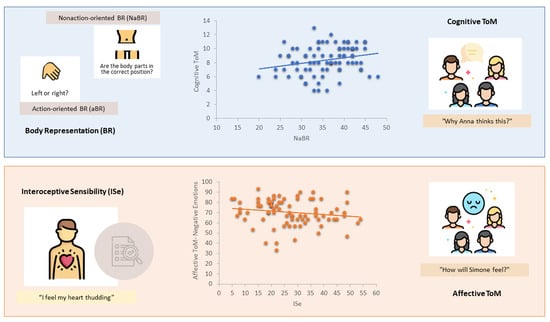
3. Results
4. Discussion
5. Conclusions
6. Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Q.; Ping, X.; Chen, W. Body Influences on Social Cognition through Interoception. Front. Psychol. 2019, 10, 2066. [Google Scholar] [CrossRef] [PubMed]
- Happé, F.; Cook, J.L.; Bird, G. The Structure of Social Cognition: In(Ter)Dependence of Sociocognitive Processes. Annu. Rev. Psychol. 2017, 68, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Baiano, C.; Job, X.; Santangelo, G.; Auvray, M.; Kirsch, L.P. Interactions between Interoception and Perspective-Taking: Current State of Research and Future Directions. Neurosci. Biobehav. Rev. 2021, 130, 252–262. [Google Scholar] [CrossRef]
- Healey, M.L.; Grossman, M. Cognitive and Affective Perspective-Taking: Evidence for Shared and Dissociable Anatomical Substrates. Front. Neurol. 2018, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Aharon-Peretz, J. Dissociable Prefrontal Networks for Cognitive and Affective Theory of Mind: A Lesion Study. Neuropsychologia 2007, 45, 3054–3067. [Google Scholar] [CrossRef] [PubMed]
- Corradi-Dell’Acqua, C.; Ronchi, R.; Thomasson, M.; Bernati, T.; Saj, A.; Vuilleumier, P. Deficits in Cognitive and Affective Theory of Mind Relate to Dissociated Lesion Patterns in Prefrontal and Insular Cortex. Cortex 2020, 128, 218–233. [Google Scholar] [CrossRef]
- Di Tella, M.; Ardito, R.B.; Dutto, F.; Adenzato, M. On the (Lack of) Association between Theory of Mind and Executive Functions: A Study in a Non-Clinical Adult Sample. Sci. Rep. 2020, 10, 17283. [Google Scholar] [CrossRef]
- Wade, M.; Prime, H.; Jenkins, J.M.; Yeates, K.O.; Williams, T.; Lee, K. On the Relation between Theory of Mind and Executive Functioning: A Developmental Cognitive Neuroscience Perspective. Psychon. Bull. Rev. 2018, 25, 2119–2140. [Google Scholar] [CrossRef]
- Damasio, A. The Feeling of Things: Body and Emotion in the Making of Consciousness; Harvest: New York, NY, USA, 2000. [Google Scholar]
- Havas, D.A.; Glenberg, A.M.; Gutowski, K.A.; Lucarelli, M.J.; Davidson, R.J. Cosmetic Use of Botulinum Toxin-A Affects Processing of Emotional Language. Psychol. Sci. 2010, 21, 895–900. [Google Scholar] [CrossRef]
- Wollmer, M.A.; de Boer, C.; Kalak, N.; Beck, J.; Götz, T.; Schmidt, T.; Hodzic, M.; Bayer, U.; Kollmann, T.; Kollewe, K. Facing Depression with Botulinum Toxin: A Randomized Controlled Trial. J. Psychiatr. Res. 2012, 46, 574–581. [Google Scholar] [CrossRef]
- Carr, L.; Iacoboni, M.; Dubeau, M.-C.; Mazziotta, J.C.; Lenzi, G.L. Neural Mechanisms of Empathy in Humans: A Relay from Neural Systems for Imitation to Limbic Areas. Proc. Natl. Acad. Sci. USA 2003, 100, 5497–5502. [Google Scholar] [CrossRef]
- Gallese, V.; Goldman, A. Mirror Neurons and the Simulation Theory of Mind-Reading. Trends Cogn. Sci. 1998, 2, 493–501. [Google Scholar] [CrossRef]
- Gallese, V. The Roots of Empathy: The Shared Manifold Hypothesis and the Neural Basis of Intersubjectivity. Psychopathology 2003, 36, 171–180. [Google Scholar] [CrossRef]
- Dijkerman, C.; Lenggenhager, B. The Body and Cognition: The Relation between Body Representations and Higher Level Cognitive and Social Processes. Cortex 2018, 104, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.; de Vignemont, F. Is Social Cognition Embodied? Trends Cogn. Sci. 2009, 13, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Raimo, S.; Martini, M.; Guariglia, C.; Santangelo, G.; Trojano, L.; Palermo, L. Editorial: Body Representation and Interoceptive Awareness: Cognitive, Affective, and Social Implications. Front. Psychol. 2022, 13, 928952. [Google Scholar] [CrossRef]
- Haggard, P.; Wolpert, D.M. Disorders of Body Scheme. In Higher-Order Motor Disorders; Freund, H.J., Jeannerod, M., Hallett, M., Leiguarda, R., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 261–271. [Google Scholar]
- De Vignemont, F. Embodiment, Ownership and Disownership. Conscious. Cogn. 2011, 20, 82–93. [Google Scholar] [CrossRef]
- Di Vita, A.; Boccia, M.; Palermo, L.; Guariglia, C. To Move or Not to Move, That Is the Question! Body Schema and Non-Action Oriented Body Representations: An FMRI Meta-Analytic Study. Neurosci. Biobehav. Rev. 2016, 68, 37–46. [Google Scholar] [CrossRef]
- Pitron, V.; de Vignemont, F. Beyond Differences between the Body Schema and the Body Image: Insights from Body Hallucinations. Conscious. Cogn. 2017, 53, 115–121. [Google Scholar] [CrossRef]
- Boccia, M.; Di Vita, A.; Palermo, L.; Nemmi, F.; Traballesi, M.; Brunelli, S.; De Giorgi, R.; Galati, G.; Guariglia, C. Neural Modifications in Lower Limb Amputation: An FMRI Study on Action and Non-Action Oriented Body Representations. Brain Imaging Behav. 2020, 14, 416–425. [Google Scholar] [CrossRef]
- Gunia, A.; Moraresku, S.; Vlček, K. Brain Mechanisms of Visuospatial Perspective-Taking in Relation to Object Mental Rotation and the Theory of Mind. Behav. Brain Res. 2021, 407, 113247. [Google Scholar] [CrossRef] [PubMed]
- Perner, J.; Roessler, J. From Infants’ to Children’s Appreciation of Belief. Trends Cogn. Sci. 2012, 16, 519–525. [Google Scholar] [CrossRef]
- Xie, J.; Cheung, H.; Shen, M.; Wang, R. Mental Rotation in False Belief Understanding. Cogn. Sci. 2018, 42, 1179–1206. [Google Scholar] [CrossRef]
- Lehmann, J.; Jansen, P. The Relationship between Theory of Mind and Mental Rotation Ability in Preschool-Aged Children. Cogent Psychol. 2019, 6, 1582127. [Google Scholar] [CrossRef]
- Schwoebel, J.; Coslett, H.B. Evidence for Multiple, Distinct Representations of the Human Body. J. Cogn. Neurosci. 2005, 17, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Raimo, S.; Iona, T.; Di Vita, A.; Boccia, M.; Buratin, S.; Ruggeri, F.; Iosa, M.; Guariglia, C.; Grossi, D.; Palermo, L. The Development of Body Representations in School-Aged Children. Appl. Neuropsychol. Child 2021, 10, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Decety, J.; Sommerville, J.A. Shared Representations between Self and Other: A Social Cognitive Neuroscience View. Trends Cogn. Sci. 2003, 7, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Von Mohr, M.; Finotti, G.; Villani, V.; Tsakiris, M. Taking the Pulse of Social Cognition: Cardiac Afferent Activity and Interoceptive Accuracy Modulate Emotional Egocentricity Bias. Cortex 2021, 145, 327–340. [Google Scholar] [CrossRef]
- Tsakiris, M. My Body in the Brain: A Neurocognitive Model of Body-Ownership. Neuropsychologia 2010, 48, 703–712. [Google Scholar] [CrossRef]
- Asai, T.; Mao, Z.; Sugimori, E.; Tanno, Y. Rubber Hand Illusion, Empathy, and Schizotypal Experiences in Terms of Self-Other Representations. Conscious. Cogn. 2011, 20, 1744–1750. [Google Scholar] [CrossRef]
- Cascio, C.J.; Foss-Feig, J.H.; Burnette, C.P.; Heacock, J.L.; Cosby, A.A. The Rubber Hand Illusion in Children with Autism Spectrum Disorders: Delayed Influence of Combined Tactile and Visual Input on Proprioception. Autism 2012, 16, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Celume, M.; Denis, L.; Motillon, T.; Keromnes, G. Reframing Schizophrenia and Autism as Bodily Self-Consciousness Disorders Leading to a Deficit of Theory of Mind and Empathy with Social Communication Impairments. Neurosci. Biobehav. Rev. 2019, 103, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. How Do You Feel? Interoception: The Sense of the Physiological Condition of the Body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Critchley, H.D. Interoception, Emotion and Brain: New Insights Link Internal Physiology to Social Behaviour. Commentary on: “Anterior Insular Cortex Mediates Bodily Sensibility and Social Anxiety” by Terasawa et al. (2012). Soc. Cogn. Affect. Neurosci. 2013, 8, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Seth, A.K.; Barrett, A.B.; Suzuki, K.; Critchley, H.D. Knowing Your Own Heart: Distinguishing Interoceptive Accuracy from Interoceptive Awareness. Biol. Psychol. 2015, 104, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M.; Tajadura-Jiménez, A.; Costantini, M. Just a Heartbeat Away from One’s Body: Interoceptive Sensitivity Predicts Malleability of Body-Representations. Proc. R. Soc. B 2011, 278, 2470–2476. [Google Scholar] [CrossRef]
- Tajadura-Jiménez, A.; Tsakiris, M. Balancing the “Inner” and the “Outer” Self: Interoceptive Sensitivity Modulates Self–Other Boundaries. J. Exp. Psychol. Gen. 2014, 143, 736–744. [Google Scholar] [CrossRef]
- Fukushima, H.; Terasawa, Y.; Umeda, S. Association between Interoception and Empathy: Evidence from Heartbeat-Evoked Brain Potential. Int. J. Psychophysiol. 2011, 79, 259–265. [Google Scholar] [CrossRef]
- Grynberg, D.; Pollatos, O. Perceiving One’s Body Shapes Empathy. Physiol. Behav. 2015, 140, 54–60. [Google Scholar] [CrossRef]
- Mul, C.; Stagg, S.D.; Herbelin, B.; Aspell, J.E. The Feeling of Me Feeling for You: Interoception, Alexithymia and Empathy in Autism. J. Autism Dev. Disord. 2018, 48, 2953–2967. [Google Scholar] [CrossRef]
- Raimo, S.; Boccia, M.; Gaita, M.; Canino, S.; Torchia, V.; Vetere, M.A.; Di Vita, A.; Palermo, L. The Bodily Fundament of Empathy: The Role of Action, Nonaction-Oriented and Interoceptive Body Representations. Psychon. Bull. Rev. 2022; submitted. [Google Scholar]
- Ainley, V.; Maister, L.; Tsakiris, M. Heartfelt Empathy? No Association between Interoceptive Awareness, Questionnaire Measures of Empathy, Reading the Mind in the Eyes Task or the Director Task. Front. Psychol. 2015, 6, 554. [Google Scholar] [CrossRef]
- Shah, P.; Catmur, C.; Bird, G. From Heart to Mind: Linking Interoception, Emotion, and Theory of Mind. Cortex 2017, 93, 220–223. [Google Scholar] [CrossRef]
- Schandry, R. Heart Beat Perception and Emotional Experience. Psychophysiology 1981, 18, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Dziobek, I.; Fleck, S.; Kalbe, E.; Rogers, K.; Hassenstab, J.; Brand, M.; Kessler, J.; Woike, J.K.; Wolf, O.T.; Convit, A. Introducing MASC: A Movie for the Assessment of Social Cognition. J. Autism Dev. Disord. 2006, 36, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Longarzo, M.; D’Olimpio, F.; Chiavazzo, A.; Santangelo, G.; Trojano, L.; Grossi, D. The Relationships between Interoception and Alexithymic Trait. The Self-Awareness Questionnaire in Healthy Subjects. Front. Psychol. 2015, 6, 1149. [Google Scholar] [CrossRef]
- Parsons, L.M. Imagined Spatial Transformations of One’s Hands and Feet. Cogn. Psychol. 1987, 19, 178–241. [Google Scholar] [CrossRef]
- Daurat-Hmeljiak, C.; Stambak, M.; Berges, J. Il Test dello Schema Corporeo. Una Prova di Conoscenza e Costruzione Dell’immagine del Corpo; Organizzazioni Speciali: Florence, Italy, 1978. [Google Scholar]
- Pino, M.C.; Pettinelli, M.; Clementi, D.; Gianfelice, C.; Mazza, M. Improvement in cognitive and affective theory of mind with observation and imitation treatment in subjects with schizophrenia. Clin. Neuropsychiatry 2015, 12, 64–72. [Google Scholar]
- Raimo, S.; Trojano, L.; Pappacena, S.; Alaia, R.; Spitaleri, D.; Grossi, D.; Santangelo, G. Neuropsychological Correlates of Theory of Mind Deficits in Patients with Multiple Sclerosis. Neuropsychology 2017, 31, 811–821. [Google Scholar] [CrossRef]
- Raimo, S.; Cropano, M.; Roldán-Tapia, M.D.; Ammendola, L.; Malangone, D.; Santangelo, G. Cognitive and Affective Theory of Mind across Adulthood. Brain Sci. 2022, 12, 899. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.J.; Cipolotti, L. Impaired Social Response Reversal: A Case of ‘Acquired Sociopathy’. Brain 2000, 123, 1122–1141. [Google Scholar] [CrossRef] [PubMed]
- Happé, F.G. An Advanced Test of Theory of Mind: Understanding of Story Characters’ Thoughts and Feelings by Able Autistic, Mentally Handicapped, and Normal Children and Adults. J. Autism Dev. Disord. 1994, 24, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Prior, M.; Marchi, S.; Sartori, G. Social Cognition and Behavior. A Tool for Assessment; Upsel Domenighini Editore: Padova, Italy, 2003. [Google Scholar]
- Iman, R.L.; Conover, W.J. The Use of the Rank Transform in Regression. Technometrics 1979, 21, 499–509. [Google Scholar] [CrossRef]
- Gabriel, E.T.; Oberger, R.; Schmoeger, M.; Deckert, M.; Vockh, S.; Auff, E.; Willinger, U. Cognitive and Affective Theory of Mind in Adolescence: Developmental Aspects and Associated Neuropsychological Variables. Psychol. Res. 2021, 85, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Nejati, V.; Moradkhani, L.; Suggate, S.; Jansen, P. The Impact of Visual-Spatial Abilities on Theory of Mind in Children and Adolescents with Autism Spectrum Disorder. Res. Dev. Disabil. 2021, 114, 103960. [Google Scholar] [CrossRef]
- Adenzato, M.; Brambilla, M.; Manenti, R.; De Lucia, L.; Trojano, L.; Garofalo, S.; Enrici, I.; Cotelli, M. Gender Differences in Cognitive Theory of Mind Revealed by Transcranial Direct Current Stimulation on Medial Prefrontal Cortex. Sci. Rep. 2017, 7, 41219. [Google Scholar] [CrossRef]
- Russell, T.A.; Tchanturia, K.; Rahman, Q.; Schmidt, U. Sex Differences in Theory of Mind: A Male Advantage on Happé’s “Cartoon” Task. Cogn. Emot. 2007, 21, 1554–1564. [Google Scholar] [CrossRef]
- Spenser, K.A.; Bull, R.; Betts, L.; Winder, B. Gender Differences in Theory of Mind, Empathic Understanding, and Moral Reasoning in an Offending and a Matched Non-Offending Population. Int. J. Offender Ther. Comp. Criminol. 2022, 66, 587–603. [Google Scholar] [CrossRef]
- Palser, E.R.; Palmer, C.E.; Galvez-Pol, A.; Hannah, R.; Fotopoulou, A.; Kilner, J.M. Alexithymia mediates the relationship between interoceptive sensibility and anxiety. PLoS ONE 2018, 13, e0203212. [Google Scholar] [CrossRef]
- Kano, M.; Fukudo, S. The Alexithymic Brain: The Neural Pathways Linking Alexithymia to Physical Disorders. BioPsychoSocial Med. 2013, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, D.; Chang, B.; Corneille, O.; Maurage, P.; Vermeulen, N.; Berthoz, S.; Luminet, O. Alexithymia and the Processing of Emotional Facial Expressions (EFEs): Systematic Review, Unanswered Questions and Further Perspectives. PLoS ONE 2012, 7, e42429. [Google Scholar] [CrossRef] [PubMed]
- Scarpazza, C.; di Pellegrino, G.; Ladavas, E. Emotional Modulation of Touch in Alexithymia. Emotion 2014, 14, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Scarpazza, C.; Zangrossi, A.; Huang, Y.-C.; Sartori, G.; Massaro, S. Disentangling Interoceptive Abilities in Alexithymia. Psychol. Res. 2022, 86, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Tiley, C.; O’Keeffe, S.; Harrison, N.A.; Seth, A.K.; Critchley, H.D. Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biol. Psychol. 2016, 114, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.T.; Badoud, D.; Tsakiris, M. Afferent Cardiac Signals Modulate Attentional Engagement to Low Spatial Frequency Fearful Faces. Cortex 2018, 104, 232–240. [Google Scholar] [CrossRef]
- Crespi, B.; Dinsdale, N. Autism and Psychosis as Diametrical Disorders of Embodiment. Evol. Med. Public Health 2019, 2019, 121–138. [Google Scholar] [CrossRef]
- Desmedt, O.; Heeren, A.; Corneille, O.; Luminet, O. What do measures of self-report interoception measure? Insights from a systematic review, latent factor analysis, and network approach. Biol. Psychol 2022, 169, 108289. [Google Scholar] [CrossRef]
- Crucianelli, L.; Enmalm, A.; Ehrsson, H.H. Interoception as independent cardiac, thermosensory, nociceptive, and affective touch perceptual submodalities. Biol. Psychol. 2022, 172, 108355. [Google Scholar] [CrossRef]
| ISe | aBR | NaBR | Affective ToM (% Correct) | Cognitive ToM | ||
|---|---|---|---|---|---|---|
| SAQ | HLT | FBE | negEAT | posEAT | ATT | |
| Mean (SD) | 27.4 (11.9) | 45.3 (5.78) | 35.8 (6.06) | 70.2 (12.5) | 80.0 (19.1) | 8.38 (2.03) |
| Min–Max | 5–54 | 24–48 | 20–48 | 33.3–93.3 | 20–100 | 4–13 |
| ISe | aBR | NaBR | |||
|---|---|---|---|---|---|
| SAQ | HLT * | FBE # | |||
| Affective ToM | negEAT | rrho p | −0.22 0.04 | −0.02 0.85 | 0.16 0.16 |
| posEAT | rrho p | −0.05 0.69 | 0.02 0.86 | 0.09 0.41 | |
| Cognitive ToM | ATT | rrho p | −0.04 0.72 | 0.21 0.03 | 0.27 <0.01 |
| Predictor | Beta | t | p | |
|---|---|---|---|---|
| Affective ToM | ||||
| negEAT | SAQ | −0.22 | −2.05 | 0.04 |
| Excluded variables | ||||
| HLT | 0.07 | 0.68 | 0.50 | |
| FBE | 0.17 | 1.54 | 0.13 | |
| Cognitive ToM | ||||
| ATT | FBE | 0.26 | 2.43 | 0.02 |
| Excluded variables | ||||
| HLT | 0.17 | 1.48 | 0.14 | |
| SAQ | −0.06 | −0.57 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canino, S.; Raimo, S.; Boccia, M.; Di Vita, A.; Palermo, L. On the Embodiment of Social Cognition Skills: The Inner and Outer Body Processing Differently Contributes to the Affective and Cognitive Theory of Mind. Brain Sci. 2022, 12, 1423. https://doi.org/10.3390/brainsci12111423
Canino S, Raimo S, Boccia M, Di Vita A, Palermo L. On the Embodiment of Social Cognition Skills: The Inner and Outer Body Processing Differently Contributes to the Affective and Cognitive Theory of Mind. Brain Sciences. 2022; 12(11):1423. https://doi.org/10.3390/brainsci12111423
Chicago/Turabian StyleCanino, Silvia, Simona Raimo, Maddalena Boccia, Antonella Di Vita, and Liana Palermo. 2022. "On the Embodiment of Social Cognition Skills: The Inner and Outer Body Processing Differently Contributes to the Affective and Cognitive Theory of Mind" Brain Sciences 12, no. 11: 1423. https://doi.org/10.3390/brainsci12111423
APA StyleCanino, S., Raimo, S., Boccia, M., Di Vita, A., & Palermo, L. (2022). On the Embodiment of Social Cognition Skills: The Inner and Outer Body Processing Differently Contributes to the Affective and Cognitive Theory of Mind. Brain Sciences, 12(11), 1423. https://doi.org/10.3390/brainsci12111423