Eye-Movement Deficits in Seniors with Hearing Aids: Cognitive and Multisensory Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Hearing Tests
2.2.1. The Tonal Audiometry
2.2.2. The Vocal Audiometry in Silence
2.3. Oculomotor Tests
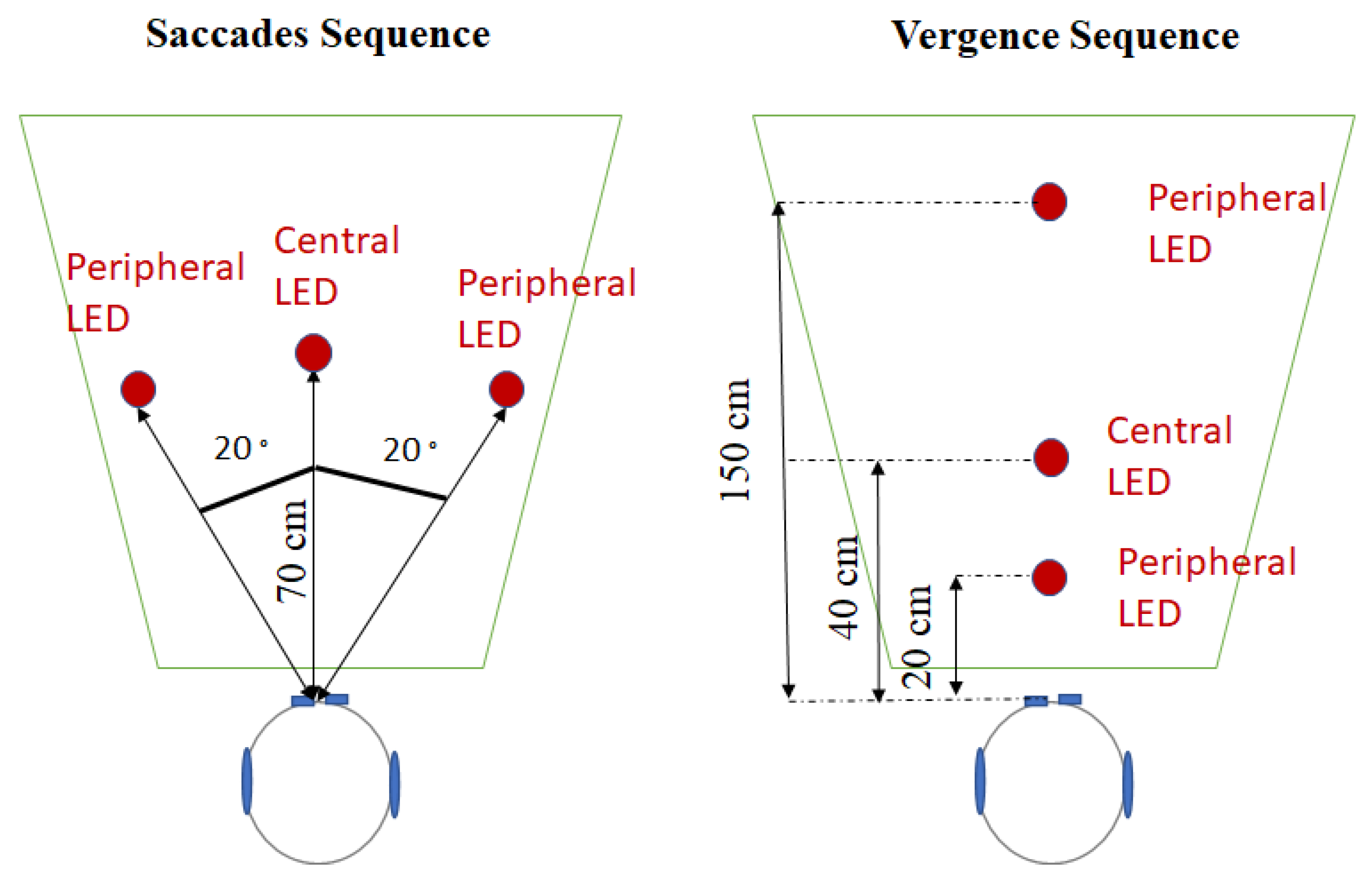
2.3.1. Saccades Sequence
2.3.2. Vergence Sequence
2.4. The Targets Modality
2.5. Eye-Movement Analysis
2.6. Stroop Test
3. Results
3.1. Auditory and Cognitive Characterizations
3.2. Oculomotor Latencies and Audiovisual Interactions
3.2.1. Influence of Hearing Aids for the Participants of the Group Senior_HA
3.2.2. Comparison between the Group Senior_HA and the Group Senior
4. Discussion
4.1. The Immediate Effect of Hearing Aids on Oculomotricity and Audiovisual Facilitation in a Presbycusis Hearing-Aided Population
4.1.1. Wearing Hearing Aids Increases Concentration
4.1.2. The Influence of Hearing Aids on Audiovisual facilitation
4.2. Audiovisual Facilitation and Vergence Slowness as Biomarkers of the Need for Hearing Aids
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yamasoba, T.; Lin, F.R.; Someya, S.; Kashio, A.; Sakamoto, T.; Kondo, K. Current concepts in age-related hearing loss: Epidemiology and mechanistic pathways. Hear. Res. 2013, 303, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Thorpe, R.; Gordon-Salant, S.; Ferrucci, L. Hearing loss prevalence and risk factors among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, G.M.; Riechelmann, H. Current Trends in Treating Hearing Loss in Elderly People: A Review of the Technology and Treatment Options—A Mini-Review. Gerontology 2010, 56, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing Loss and Incident Dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Gallacher, J.; Ilubaera, V.; Ben-Shlomo, Y.; Bayer, A.; Fish, M.; Babisch, W.; Elwood, P. Auditory threshold, phonologic demand, and incident dementia. Neurology 2012, 79, 1583–1590. [Google Scholar] [CrossRef]
- Lin, F.R.; Ferrucci, L.; Metter, E.J.; An, Y.; Zonderman, A.B.; Resnick, S.M. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 2011, 25, 763–770. [Google Scholar] [CrossRef]
- Amieva, H.; Ouvrard, C.; Giulioli, C.; Meillon, C.; Rullier, L.; Dartigues, J.-F. Self-reported hearing loss, hearing aids, and cognitive decline in elderly adults: A 25-year study. J. Am. Geriatr. Soc. 2015, 63, 2099–2104. [Google Scholar] [CrossRef]
- Curhan, S.G.; Willett, W.; Grodstein, F.; Curhan, G. Longitudinal study of hearing loss and subjective cognitive function decline in men. Alzheimers Dement. 2019, 15, 525–533. [Google Scholar] [CrossRef]
- Tun, P.A.; McCoy, S.; Wingfield, A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol. Aging 2009, 24, 761. [Google Scholar] [CrossRef]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.-L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M.; et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013, 173, 293–299. [Google Scholar] [CrossRef]
- Hopkins, K. Chapter 27—Deafness in cochlear and auditory nerve disorders. In Handbook of Clinical Neurology; The Human Auditory System; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 129, pp. 479–494. [Google Scholar]
- Gates, G.A.; Mills, J.H. Presbycusis. Lancet 2005, 366, 1111–1120. [Google Scholar] [CrossRef]
- Dawes, P.; Emsley, R.; Cruickshanks, K.J.; Moore, D.R.; Fortnum, H.; Edmondson-Jones, M.; McCormack, A.; Munro, K.J. Hearing Loss and Cognition: The Role of Hearing Aids, Social Isolation and Depression. PLoS ONE 2015, 10, e0119616. [Google Scholar] [CrossRef]
- Maharani, A.; Dawes, P.; Nazroo, J.; Tampubolon, G.; Pendleton, N.; SENSE-Cog WP1 group. Longitudinal Relationship Between Hearing Aid Use and Cognitive Function in Older Americans. J. Am. Geriatr. Soc. 2018, 66, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, A.; Benatti, A.; Velardita, C.; Favaro, D.; Padoan, E.; Severi, D.; Pagliaro, M.; Bovo, R.; Vallesi, A.; Gabelli, C.; et al. Aging, Cognitive Decline and Hearing Loss: Effects of Auditory Rehabilitation and Training with Hearing Aids and Cochlear Implants on Cognitive Function and Depression among Older Adults. AUD 2016, 21, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Nkyekyer, J.; Meyer, D.; Pipingas, A.; Reed, N.S. The cognitive and psychosocial effects of auditory training and hearing aids in adults with hearing loss. Clin. Interv. Aging 2019, 14, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Riggio, L.; Dascola, I.; Umiltá, C. Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 1987, 25, 31–40. [Google Scholar] [CrossRef]
- Deubel, H.; Schneider, W.X. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vis. Res. 1996, 36, 1827–1837. [Google Scholar] [CrossRef]
- Carter, J.E.; Obler, L.; Woodward, S.; Albert, M.L. The Effect of Increasing Age on the Latency for Saccadic Eye Movements1. J. Gerontol. 1983, 38, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.A.; Zackon, D.H. Senescent Saccades: Effects of Aging on Their Accuracy, Latency and Velocity. Acta Oto-Laryngol. 1987, 104, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Munoz, D.P.; Broughton, J.R.; Goldring, J.E.; Armstrong, I.T. Age-related performance of human subjects on saccadic eye movement tasks. Exp. Brain Res. 1998, 121, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.C.; Rawles, J.M. The effect of age on saccadic latency and velocity. Neuro-Ophthalmology 1988, 8, 123–129. [Google Scholar] [CrossRef]
- Rambold, H.; Neumann, G.; Sander, T.; Helmchen, C. Age-related changes of vergence under natural viewing conditions. Neurobiol. Aging 2006, 27, 163–172. [Google Scholar] [CrossRef]
- Yang, Q.; Le, T.-T.; Kapoula, Z. Effects of aging on regular and express latencies of vergence. J. Eye Mov. Res. 2009, 1. [Google Scholar] [CrossRef]
- Yang, Q.; Le, T.-T.; Kapoula, Z. Aging Effects on the Visually Driven Part of Vergence Movements. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Chavant, M.; Kapoula, Z. Presbycusis and the Aging of Eye Movement: Common Attention Mechanisms. Brain Sci. 2022, 12, 107. [Google Scholar] [CrossRef]
- Chavant, M.; Kapoula, Z. Audiovisual integration for saccade and vergence eye move-ments increases with presbycusis and loss of selective attention on the Stroop test. Brain Sci. 2022, 12, 591. [Google Scholar] [CrossRef]
- Bugg, J.M.; DeLosh, E.L.; Davalos, D.B.; Davis, H.P. Age Differences in Stroop Interference: Contributions of General Slowing and Task-Specific Deficits. Aging Neuropsychol. Cogn. 2007, 14, 155–167. [Google Scholar] [CrossRef]
- Troyer, A.K.; Leach, L.; Strauss, E. Aging and Response Inhibition: Normative Data for the Victoria Stroop Test. Aging Neuropsychol. Cogn. 2006, 13, 20–35. [Google Scholar] [CrossRef]
- Bayard, S.; Erkes, J.; Moroni, C. Victoria Stroop Test: Normative Data in a Sample Group of Older People and the Study of Their Clinical Applications in the Assessment of Inhibition in Alzheimer’s Disease. Arch. Clin. Neuropsychol. 2011, 26, 653–661. [Google Scholar] [CrossRef]
- Graf, P.; Uttl, B.; Tuokko, H. Color- and picture-word stroop tests: Performance changes in old age. J. Clin. Exp. Neuropsychol. 1995, 17, 390–415. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Davis, A.C.; Hoffman, H.J. Hearing loss grades and the International classification of functioning, disability and health. Bull. World Health Organ. 2019, 97, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Lafon, J.-C. Le Test Phonétique et la Mesure de L’audition; Editions Centrex: Eindhoven, The Netherlands, 1964. [Google Scholar]
- von Noorden Gunter, K.; Emilio, C.C.; St. Louis, M. (Eds.) Binocular Vision and Ocular Motility, 6th ed.; Mosby: St. Louis, MO, USA, 2002; Volume 47, p. 393. [Google Scholar] [CrossRef]
- Hung, G.K.; Semmlow, J.L.; Ciufferda, K.J. A Dual-Mode Dynamic Model of the Vergence Eye Movement System. IEEE Trans. Biomed. Eng. 1986, BME-33, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Hung, G.K. Models of Oculomotor Control; World Scientific: Singapore, 2001. [Google Scholar]
- Semmlow, J.L.; Hung, G.K.; Horng, J.-L.; Ciuffreda, K.J. Disparity vergence eye movements exhibit preprogrammed motor control. Vis. Res. 1994, 34, 1335–1343. [Google Scholar] [CrossRef]
- Davies, D.R.; Parasuraman, R. The Psychology of Vigilance; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Poulton, E.C. Composite model for human performance in continuous noise. Psychol. Rev. 1979, 86, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Auburn, T.C.; Jones, D.M.; Chapman, A.J. Arousal and the Bakan vigilance task: The effects of noise intensity and the presence of others. Curr. Psychol. 1987, 6, 196–206. [Google Scholar] [CrossRef]
- Moradi, G.; Omidi, L.; Vosoughi, S.; Ebrahimi, H.; Alizadeh, A.; Alimohammadi, I. Effects of noise on selective attention: The role of introversion and extraversion. Appl. Acoust. 2019, 146, 213–217. [Google Scholar] [CrossRef]
- Helton, W.S.; Matthews, G.; Warm, J.S. Stress state mediation between environmental variables and performance: The case of noise and vigilance. Acta Psychol. 2009, 130, 204–213. [Google Scholar] [CrossRef]
- Kapoula, Z.; Chavant, M. Hearing Amplification Improves Eye Movements: A Case Study. CSMC 2020, 7, 93. [Google Scholar] [CrossRef]
- Corneil, B.D.; Van Wanrooij, M.; Munoz, D.P.; Van Opstal, A.J. Auditory-Visual Interactions Subserving Goal-Directed Saccades in a Complex Scene. J. Neurophysiol. 2002, 88, 438–454. [Google Scholar] [CrossRef]
- Diederich, A.; Colonius, H. Crossmodal interaction in saccadic reaction time: Separating multisensory from warning effects in the time window of integration model. Exp. Brain Res. 2008, 186, 1–22. [Google Scholar] [CrossRef]
- Rach, S.; Diederich, A.; Colonius, H. On quantifying multisensory interaction effects in reaction time and detection rate. Psychol. Res. 2011, 75, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.C.; Reuter-Lorenz, P.A.; Nozawa, G.; Fendrich, R. Visual-auditory interactions in sensorimotor processing: Saccades versus manual responses. J. Exp. Psychol. Hum. Percept. Perform. 1994, 20, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Arndt, P.A.; Colonius, H. Two stages in crossmodal saccadic integration: Evidence from a visual-auditory focused attention task. Exp. Brain Res. 2003, 150, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Colonius, H.; Diederich, A. The optimal time window of visual-auditory integration: A reaction time analysis. Front. Integr. Neurosci. 2010, 4, 11. [Google Scholar] [CrossRef]
- Colonius, H.; Arndt, P. A two-stage model for visual-auditory interaction in saccadic latencies. Percept. Psychophys. 2001, 63, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Van der Stoep, N.; Spence, C.; Nijboer, T.C.W.; Van der Stigchel, S. On the relative contributions of multisensory integration and crossmodal exogenous spatial attention to multisensory response enhancement. Acta Psychol. 2015, 162, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Perrott, D.R.; Saberi, K.; Brown, K.; Strybel, T.Z. Auditory psychomotor coordination and visual search performance. Percept. Psychophys. 1990, 48, 214–226. [Google Scholar] [CrossRef]
- Frens, M.A.; Van Opstal, A.J.; Van der Willigen, R.F. Spatial and temporal factors determine auditory-visual interactions in human saccadic eye movements. Percept. Psychophys. 1995, 57, 802–816. [Google Scholar] [CrossRef]
- Lueck, C.J.; Crawford, T.J.; Savage, C.J.; Kennard, C. Auditory-visual interaction in the generation of saccades in man. Exp. Brain Res. 1990, 82, 149–157. [Google Scholar] [CrossRef]
- Hughes, H.C.; Nelson, M.D.; Aronchick, D.M. Spatial characteristics of visual-auditory summation in human saccades. Vis. Res. 1998, 38, 3955–3963. [Google Scholar] [CrossRef]
- Van Wanrooij, M.M.; Bell, A.H.; Munoz, D.P.; Van Opstal, A.J. The effect of spatial–temporal audiovisual disparities on saccades in a complex scene. Exp. Brain Res. 2009, 198, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Tye-Murray, N.; Sommers, M.S.; Spehar, B. Audiovisual Integration and Lipreading Abilities of Older Adults with Normal and Impaired Hearing. Ear Hear. 2007, 28, 656–668. [Google Scholar] [CrossRef]
- Keidser, G.; O’Brien, A.; Carter, L.; McLelland, M.; Yeend, I. Variation in preferred gain with experience for hearing-aid users. Int. J. Audiol. 2008, 47, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Giroud, N.; Lemke, U.; Reich, P.; Matthes, K.L.; Meyer, M. The impact of hearing aids and age-related hearing loss on auditory plasticity across three months—An electrical neuroimaging study. Hear. Res. 2017, 353, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.; John, A.; Schafer, E.; Nyffeler, M.; Boretzki, M.; Caraway, T.; Hudson, M. Long-term effects of non-linear frequency compression for children with moderate hearing loss. Int. J. Audiol. 2011, 50, 396–404. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavant, M.; Kapoula, Z. Eye-Movement Deficits in Seniors with Hearing Aids: Cognitive and Multisensory Implications. Brain Sci. 2022, 12, 1425. https://doi.org/10.3390/brainsci12111425
Chavant M, Kapoula Z. Eye-Movement Deficits in Seniors with Hearing Aids: Cognitive and Multisensory Implications. Brain Sciences. 2022; 12(11):1425. https://doi.org/10.3390/brainsci12111425
Chicago/Turabian StyleChavant, Martin, and Zoï Kapoula. 2022. "Eye-Movement Deficits in Seniors with Hearing Aids: Cognitive and Multisensory Implications" Brain Sciences 12, no. 11: 1425. https://doi.org/10.3390/brainsci12111425
APA StyleChavant, M., & Kapoula, Z. (2022). Eye-Movement Deficits in Seniors with Hearing Aids: Cognitive and Multisensory Implications. Brain Sciences, 12(11), 1425. https://doi.org/10.3390/brainsci12111425




