HPA Axis Responsiveness Associates with Central Serotonin Transporter Availability in Human Obesity and Non-Obesity Controls
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Procedures
5-HTTLPR Genotyping
2.3. Questionnaires Assessing the Behavioral Inhibition System (BIS)/Behavioral Activation System (BAS), Depression and Anxiety
3. Results
3.1. Study Population Characteristics
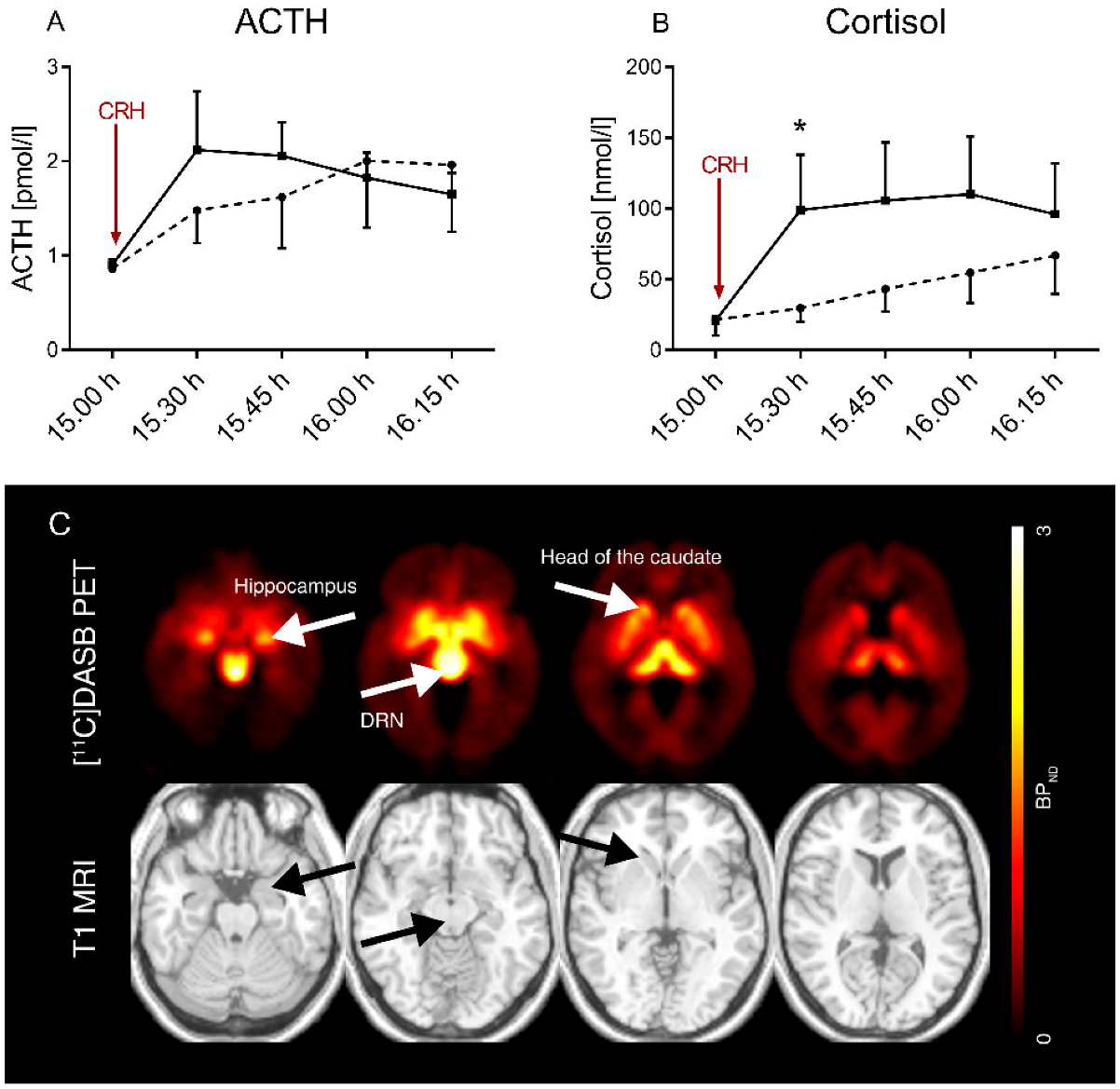
3.2. Individuals with Obesity Tend to Have a Higher HPA Axis Responsiveness and a Higher Adrenal Sensitivity to ACTH
3.3. HPA Axis Responsiveness Differentially Relates to 5-HTT Availability between OB and NO
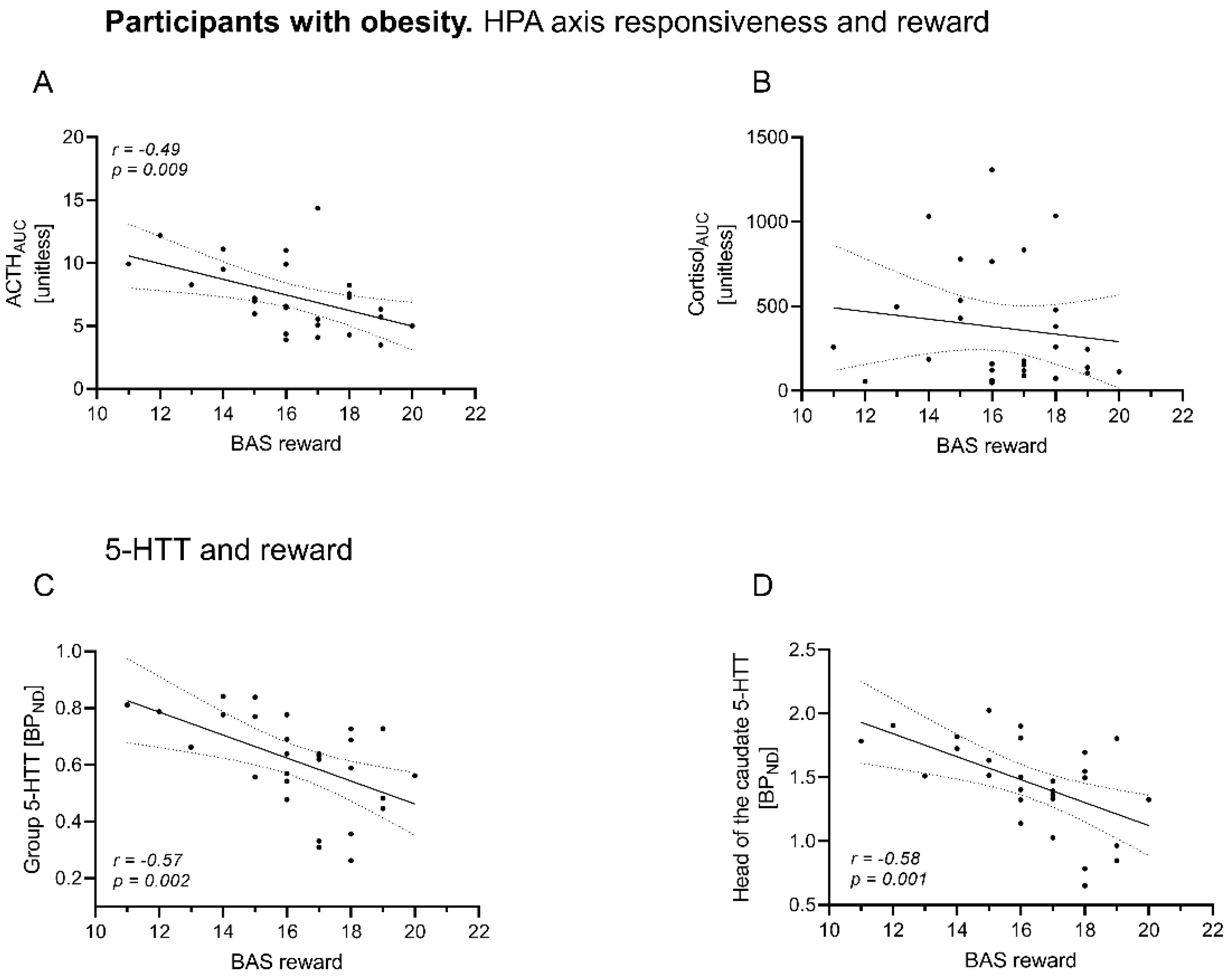
3.4. BAS Reward Scores Relate Differentially to HPA Responsiveness in OB vs. NO
3.5. In Obesity, BAS Reward Scores Are Associated with Overall 5-HTT BPND and Caudate Nucleus 5-HTT BPND
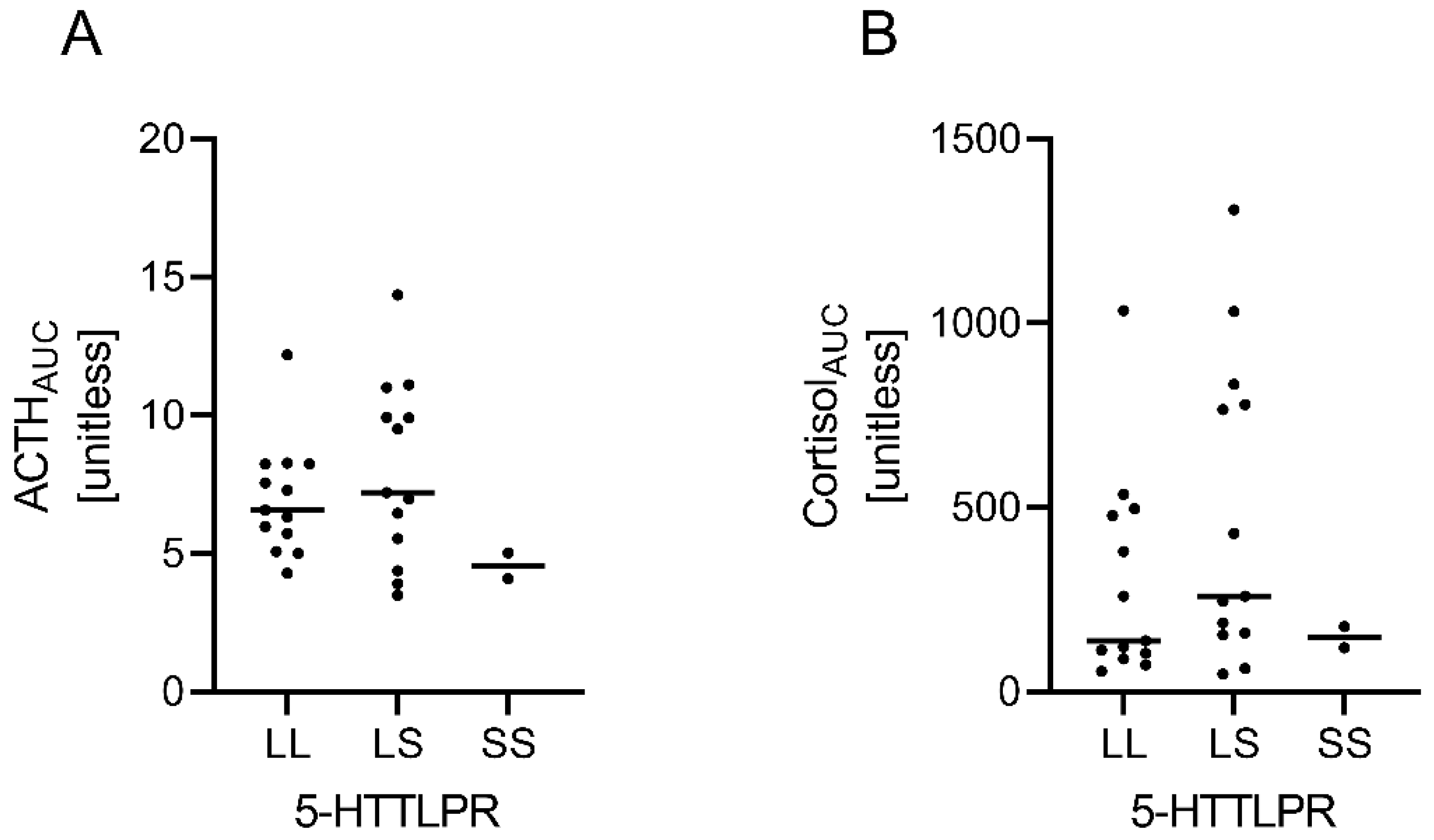
3.6. Explorative Analyses of HPA Axis Responsiveness according to 5-HTTLPR Genotype Does Not Point towards Substantial Differences between the S/S, S/L and L/L Allele Carriers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Obesity, Metabolic Syndrome, and Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef]
- Hemmingsson, E. A new model of the role of psychological and emotional distress in promoting obesity: Conceptual review with implications for treatment and prevention. Obes. Rev. 2014, 15, 769–779. [Google Scholar] [CrossRef]
- Dutton, G.R.; Lewis, T.T.; Durant, N.; Halanych, J.; Kiefe, C.I.; Sidney, S.; Kim, Y.; Lewis, C.E. Perceived weight discrimination in the CARDIA study: Differences by race, sex, and weight status. Obesity 2014, 22, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira-Miranda, E.; Costa, P.R.F.; Queiroz, V.A.O.; Pereira-Santos, M.; Santana, M.L.P. Overweight and Obesity Associated with Higher Depression Prevalence in Adults: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2017, 36, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Björntorp, P.; Rosmond, R. Neuroendocrine abnormalities in visceral obesity. Int. J. Obes. 2000, 24, S80–S85. [Google Scholar] [CrossRef] [Green Version]
- Galen, K.A.; Horst, K.W.; Serlie, M.J. Serotonin, food intake, and obesity. Obes. Rev. 2021, 22, e13210. [Google Scholar] [CrossRef]
- Schinke, C.; Hesse, S.; Stoppe, M.; Meyer, K.; Schmidt, E.; Orthgiess, J.; Bechmann, L.; Bresch, A.; Rullmann, M.; Luthardt, J.; et al. Post-dexamethasone serum copeptin corresponds to HPA axis responsiveness in human obesity. Psychoneuroendocrinology 2017, 78, 39–47. [Google Scholar] [CrossRef]
- Incollingo Rodriguez, A.C.; Epel, E.S.; White, M.L.; Standen, E.C.; Seckl, J.R.; Tomiyama, A.J. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology 2015, 62, 301–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Valk, E.S.; Savas, M.; Van Rossum, E.F.C. Stress and Obesity: Are There More Susceptible Individuals? Curr. Obes. Rep. 2018, 7, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavagnini, F.; Croci, M.; Putignano, P.; Petroni, M.; Invitti, C. Glucocorticoids and neuroendocrine function. Int. J. Obes. 2000, 24, S77–S79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loriaux, D.L. Diagnosis and Differential Diagnosis of Cushing’s Syndrome. N. Engl. J. Med. 2017, 376, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Rowland, N.E.; Antelman, S.M. Stress-induced hyperphagia and obesity in rats: A possible model for understanding human obesity. Science 1976, 191, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Muscat, R.; Papp, M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef]
- Chandola, T.; Brunner, E.; Marmot, M. Chronic stress at work and the metabolic syndrome: Prospective study. BMJ 2006, 332, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Tafet, G.E.; Nemeroff, C.B. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Blackmore, E.R.; Stansfeld, S.A.; Weller, I.; Munce, S.; Zagorski, B.M.; Stewart, D.E. Major Depressive Episodes and Work Stress: Results From a National Population Survey. Am. J. Public Health 2007, 97, 2088–2093. [Google Scholar] [CrossRef]
- Epel, E.; Lapidus, R.; McEwen, B.; Brownell, K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 2001, 26, 37–49. [Google Scholar] [CrossRef]
- Herhaus, B.; Ullmann, E.; Chrousos, G.; Petrowski, K. High/low cortisol reactivity and food intake in people with obesity and healthy weight. Transl. Psychiatry 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heisler, L.K.; Pronchuk, N.; Nonogaki, K.; Zhou, L.; Raber, J.; Tung, L.; Yeo, G.S.H.; O’Rahilly, S.; Colmers, W.F.; Elmquist, J.K.; et al. Serotonin Activates the Hypothalamic-Pituitary-Adrenal Axis via Serotonin 2C Receptor Stimulation. J. Neurosci. 2007, 27, 6956–6964. [Google Scholar] [CrossRef] [PubMed]
- Lowry, C.A. Functional Subsets of Serotonergic Neurones: Implications for Control of the Hypothalamic-Pituitary-Adrenal Axis. J. Neuroendocrinol. 2002, 14, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Breisch, S.T.; Zemlan, F.P.; Hoebel, B.G. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science 1976, 192, 382–385. [Google Scholar] [CrossRef]
- Saller, C.F.; Stricker, E.M. Hyperphagia and increased growth in rats after intraventricular injection of 5,7-dihydroxytryptamine. Science 1976, 192, 385–387. [Google Scholar] [CrossRef]
- Pollock, J.D.; Rowland, N. Peripherally administered serotonin decreases food intake in rats. Pharm. Biochem. Behav. 1981, 15, 179–183. [Google Scholar] [CrossRef]
- Hainer, V.; Kabrnova, K.; Aldhoon, B.; Kunesova, M.; Wagenknecht, M. Serotonin and norepinephrine reuptake inhibition and eating behavior. Ann. N. Y. Acad. Sci. 2006, 1083, 252–269. [Google Scholar] [CrossRef]
- Thomsen, W.J.; Grottick, A.J.; Menzaghi, F.; Reyes-Saldana, H.; Espitia, S.; Yuskin, D.; Whelan, K.; Martin, M.; Morgan, M.; Chen, W.; et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: In vitro and in vivo pharmacological characterization. J. Pharm. Exp. 2008, 325, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, E.E. Monoamine transporters: Structure, regulation, and clinical implications. Neurology 2013, 81, 761–768. [Google Scholar] [CrossRef]
- Erritzoe, D.; Frokjaer, V.G.; Haahr, M.T.; Kalbitzer, J.; Svarer, C.; Holst, K.K.; Hansen, D.L.; Jernigan, T.L.; Lehel, S.; Knudsen, G.M. Cerebral serotonin transporter binding is inversely related to body mass index. NeuroImage 2010, 52, 284–289. [Google Scholar] [CrossRef]
- Hesse, S.; Rullmann, M.; Luthardt, J.; Winter, K.; Hankir, M.K.; Becker, G.-A.; Zientek, F.; Reissig, G.; Regenthal, R.; Drabe, M.; et al. Central serotonin transporter availability in highly obese individuals compared with non-obese controls: A [11C] DASB positron emission tomography study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1096–1104. [Google Scholar] [CrossRef]
- Drabe, M.; Rullmann, M.; Luthardt, J.; Boettcher, Y.; Regenthal, R.; Ploetz, T.; Becker, G.A.; Patt, M.; Schinke, C.; Bergh, F.T.; et al. Serotonin transporter gene promoter methylation status correlates with in vivo prefrontal 5-HTT availability and reward function in human obesity. Transl. Psychiatry 2017, 7, e1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfumey, L.; Mongeau, R.; Cohen-Salmon, C.; Hamon, M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci. Biobehav. Rev. 2008, 32, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Wankerl, M.; Stalder, T.; Kirschbaum, C.; Alexander, N. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cortisol stress reactivity: A meta-analysis. Mol. Psychiatry 2013, 18, 1018–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nothdurfter, C.; Schmotz, C.; Sarubin, N.; Baghai, T.C.; Laenger, A.; Lieb, M.; Bondy, B.; Rupprecht, R.; Schüle, C. Effects of escitalopram/quetiapine combination therapy versus escitalopram monotherapy on hypothalamic-pituitary-adrenal-axis activity in relation to antidepressant effectiveness. J. Psychiatr. Res. 2014, 52, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Reimold, M.; Knobel, A.; Rapp, M.A.; Batra, A.; Wiedemann, K.; Ströhle, A.; Zimmer, A.; Schönknecht, P.; Smolka, M.N.; Weinberger, D.R.; et al. Central serotonin transporter levels are associated with stress hormone response and anxiety. Psychopharmacology 2011, 213, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, L.J.; Rubin-Falcone, H.; Galfalvy, H.C.; Kaufman, J.; Miller, J.M.; Sublette, M.E.; Cooper, T.B.; Min, E.; Keilp, J.G.; Stanley, B.H.; et al. Cortisol Stress Response and in Vivo PET Imaging of Human Brain Serotonin 1A Receptor Binding. Int. J. Neuropsychopharmacol. 2019, 22, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Bergh, F.T.; Kümpfel, T.; Grasser, A.; Rupprecht, R.; Holsboer, F.; Trenkwalder, C. Combined Treatment with Corticosteroids and Moclobemide Favors Normalization of Hypothalamo-Pituitary-Adrenal Axis Dysregulation in Relapsing-Remitting Multiple Sclerosis: A Randomized, Double Blind Trial. J. Clin. Endocrinol. Metab. 2001, 86, 1610–1615. [Google Scholar] [CrossRef]
- Schüle, C.; Baghai, T.C.; Eser, D.; Zwanzger, P.; Jordan, M.; Buechs, R.; Rupprecht, R. Time course of hypothalamic-pituitary-adrenocortical axis activity during treatment with reboxetine and mirtazapine in depressed patients. Psychopharmacology 2006, 186, 601–611. [Google Scholar] [CrossRef]
- Heuser, I.; Yassouridis, A.; Holsboer, F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. J. Psychiatr. Res. 1994, 28, 341–356. [Google Scholar] [CrossRef]
- Schinke, C.; Hesse, S.; Rullmann, M.; Becker, G.-A.; Luthardt, J.; Zientek, F.; Patt, M.; Stoppe, M.; Schmidt, E.; Meyer, K.; et al. Central noradrenaline transporter availability is linked with HPA axis responsiveness and copeptin in human obesity and non-obese controls. Stress 2019, 22, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hautzinger, M. The Beck Depression Inventory in clinical practice. Nervenarzt 1991, 62, 689–696. [Google Scholar] [PubMed]
- Wilson, A.A.; Ginovart, N.; Hussey, D.; Meyer, J.; Houle, S. In vitro and in vivo characterisation of [11C]-DASB: A probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl. Med. Biol. 2002, 29, 509–515. [Google Scholar] [CrossRef]
- Ichise, M.; Liow, J.-S.; Lu, J.-Q.; Takno, A.; Model, K.; Toyama, H.; Suhara, T.; Suzuki, K.; Innis, R.B.; Carson, R.E. Linearized Reference Tissue Parametric Imaging Methods: Application to [11C]DASB Positron Emission Tomography Studies of the Serotonin Transporter in Human Brain. J. Cereb. Blood Flow Metab. 2003, 23, 1096–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heils, A.; Teufel, A.; Petri, S.; Stöber, G.; Riederer, P.; Bengel, D.; Lesch, K.P. Allelic Variation of Human Serotonin Transporter Gene Expression. J. Neurochem. 2002, 66, 2621–2624. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Federbusch, M.; Grellmann, C.; Villringer, A.; Horstmann, A. Body weight status, eating behavior, sensitivity to reward/punishment, and gender: Relationships and interdependencies. Front. Psychol. 2014, 5, 1073. [Google Scholar] [CrossRef] [Green Version]
- Törk, I. Anatomy of the Serotonergic System. Ann. N. Y. Acad. Sci. 1990, 600, 9–34. [Google Scholar] [CrossRef]
- Chaouloff, F. Serotonin, stress and corticoids. J. Psychopharmacol. 2000, 14, 139–151. [Google Scholar] [CrossRef]
- Sarubin, N.; Nothdurfter, C.; Schmotz, C.; Wimmer, A.M.; Trummer, J.; Lieb, M.; Uhr, M.; Baghai, T.C.; Wetter, T.C.; Buhner, M.; et al. Impact on cortisol and antidepressant efficacy of quetiapine and escitalopram in depression. Psychoneuroendocrinology 2014, 39, 141–151. [Google Scholar] [CrossRef]
- Frokjaer, V.G.; Erritzoe, D.; Holst, K.K.; Jensen, P.S.; Rasmussen, P.M.; Fisher, P.M.; Baare, W.; Madsen, K.S.; Madsen, J.; Svarer, C.; et al. Prefrontal serotonin transporter availability is positively associated with the cortisol awakening response. Eur. Neuropsychopharmacol. 2013, 23, 285–294. [Google Scholar] [CrossRef]
- Bombardi, C.; Grandis, A.; Pivac, N.; Sagud, M.; Lucas, G.; Chagraoui, A.; Lemaire-Mayo, V.; De Deurwaerdere, P.; Di Giovanni, G. Serotonin modulation of hippocampal functions: From anatomy to neurotherapeutics. Prog. Brain Res. 2021, 261, 83–158. [Google Scholar] [PubMed]
- Tafet, G.E.; Idoyaga-Vargas, V.P.; Abulafia, D.P.; Calandria, J.M.; Roffman, S.S.; Chiovetta, A.; Shinitzky, M. Correlation between cortisol level and serotonin uptake in patients with chronic stress and depression. Cogn. Affect. Behav. Neurosci. 2001, 1, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatz, K.; Mossner, R.; Heils, A.; Lesch, K.P. Glucocorticoid-regulated human serotonin transporter (5-HTT) expression is modulated by the 5-HTT gene-promotor-linked polymorphic region. J. Neurochem. 2003, 86, 1072–1078. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Kreider, M.L.; Tate, C.A.; Seidler, F.J. Critical Prenatal and Postnatal Periods for Persistent Effects of Dexamethasone on Serotonergic and Dopaminergic Systems. Neuropsychopharmacology 2006, 31, 904–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, H.B.; Wild, S.H.; Postle, A.D.; Zhang, J.; Koster, G.; Umpleby, M.; Shojaee-Moradie, F.; Dewbury, K.; Wood, P.J.; Phillips, D.I.; et al. Cortisol clearance and associations with insulin sensitivity, body fat and fatty liver in middle-aged men. Diabetologia 2007, 50, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Andrew, R.; Phillips, D.I.W.; Walker, B.R. Obesity and Gender Influence Cortisol Secretion and Metabolism in Man. J. Clin. Endocrinol. Metab. 1998, 83, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.; Sapolsky, R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991, 12, 118–134. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar]
- Green, E.; Jacobson, A.; Haase, L.; Murphy, C. Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Res. 2011, 1386, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Royce, G.J.; Laine, E.J. Efferent connections of the caudate nucleus, including cortical projections of the striatum and other basal ganglia: An autoradiographic and horseradish peroxidase investigation in the cat. J. Comp. Neurol. 1984, 226, 28–49. [Google Scholar] [CrossRef]
- Robinson, J.L.; Laird, A.R.; Glahn, D.C.; Blangero, J.; Sanghera, M.K.; Pessoa, L.; Fox, P.M.; Uecker, A.; Friehs, G.; Young, K.A.; et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage 2012, 60, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Fox, J. Sensitivity to reward and body mass index (BMI): Evidence for a non-linear relationship. Appetite 2008, 50, 43–49. [Google Scholar] [CrossRef]
- Hori, H.; Teraishi, T.; Sasayama, D.; Hattori, K.; Hashikura, M.; Higuchi, T.; Kunugi, H. Relationship of temperament and character with cortisol reactivity to the combined dexamethasone/CRH test in depressed outpatients. J. Affect. Disord. 2013, 147, 128–136. [Google Scholar] [CrossRef]
- Stice, E.; Spoor, S.; Bohon, C.; Small, D.M. Relation Between Obesity and Blunted Striatal Response to Food Is Moderated by Taq IA A1 Allele. Science 2008, 322, 449–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babbs, R.K.; Sun, X.; Felsted, J.; Chouinard-Decorte, F.; Veldhuizen, M.G.; Small, D.M. Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol. Behav. 2013, 121, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, M.J.; Dixon, J.B.; Dixon, M.E.; O’Brien, P.E. Confirmatory Factor Analysis of the Beck Depression Inventory in Obese Individuals Seeking Surgery. Obes. Surg. 2010, 20, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.; Small, H.; Yoong, S.L.; Boyes, A.; Bisquera, A.; Sanson-Fisher, R. Prevalence of comorbid depression and obesity in general practice: A cross-sectional survey. Br. J. Gen. Pract. 2014, 64, e122–e127. [Google Scholar] [CrossRef] [Green Version]
- Noskova, T.; Pivac, N.; Nedic, G.; Kazantseva, A.; Gaysina, D.; Faskhutdinova, G.; Gareeva, A.; Khalilova, Z.; Khusnutdinova, E.; Kovacic, D.K.; et al. Ethnic differences in the serotonin transporter polymorphism (5-HTTLPR) in several European populations. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1735–1739. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Horwood, L.J.; Miller, A.L.; Kennedy, M.A. Life stress, 5-HTTLPR and mental disorder: Findings from a 30-year longitudinal study. Br. J. Psychiatry 2011, 198, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Karg, K.; Burmeister, M.; Shedden, K.; Sen, S. The Serotonin Transporter Promoter Variant (5-HTTLPR), Stress, and Depression Meta-analysis Revisited. Arch. Gen. Psychiatry 2011, 68, 444. [Google Scholar] [CrossRef] [PubMed]
- Culverhouse, R.C.; Saccone, N.L.; Horton, A.C.; Ma, Y.; Anstey, K.J.; Banaschewski, T.; Burmeister, M.; Cohen-Woods, S.; Etain, B.; Fisher, H.L.; et al. Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol. Psychiatry 2018, 23, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welper, H.; Aller, A.; Guttenthaler, V.; Höfels, S.; Lennertz, L.; Pfeiffer, U.; Schwab, S.G.; Zobel, A. Serotonintransportergen und Stressreagibilität bei unipolarer Depression. Der. Nervenarzt. 2014, 85, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Wust, S.; Kumsta, R.; Treutlein, J.; Frank, J.; Entringer, S.; Schulze, T.G.; Rietschel, M. Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology 2009, 34, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, I.H.; Joormann, J.; Minor, K.L.; Hallmayer, J. HPA Axis Reactivity: A Mechanism Underlying the Associations Among 5-HTTLPR, Stress, and Depression. Biol. Psychiatry 2008, 63, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Lesch, K.P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Alexander, N.; Illius, S.; Stalder, T.; Wankerl, M.; Muehlhan, M.; Kirschbaum, C. Serotonin transporter gene methylation predicts long-term cortisol concentrations in hair. Psychoneuroendocrinology 2019, 106, 179–182. [Google Scholar] [CrossRef]
- Kumpfel, T.; Schwan, M.; Weber, F.; Holsboer, F.; Trenkwalder, C.; Then Bergh, F. Hypothalamo-pituitary-adrenal axis activity evolves differentially in untreated versus treated multiple sclerosis. Psychoneuroendocrinology 2014, 45, 87–95. [Google Scholar] [CrossRef]
- Pasquali, R.; Ambrosi, B.; Armanini, D.; Cavagnini, F.; Uberti, E.D.; Del Rio, G.; De Pergola, G.; Maccario, M.; Mantero, F.; Marugo, M.; et al. Cortisol and ACTH Response to Oral Dexamethasone in Obesity and Effects of Sex, Body Fat Distribution, and Dexamethasone Concentrations: A Dose-Response Study. J. Clin. Endocrinol. Metab. 2002, 87, 166–175. [Google Scholar] [CrossRef]
- Åsvold, B.O.; Grill, V.; Thorstensen, K.; Bjørgaas, M.R. Association between posttest dexamethasone and cortisol concentrations in the 1 mg overnight dexamethasone suppression test. Endocr. Connect. 2012, 1, 62–67. [Google Scholar] [CrossRef]
- Schneider, K.L.; Busch, A.M.; Whited, M.C.; Appelhans, B.M.; Waring, M.E.; Pagoto, S.L. Assessing depression in obese women: An examination of two commonly-used measures. J. Psychosom. Res. 2013, 75, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Oroszi, G.; Chun, J.; Smith, T.L.; Goldman, D.; Schuckit, M.A. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol. Clin. Exp. Res. 2005, 29, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Houtepen, L.C.; Vinkers, C.H.; Carrillo-Roa, T.; Hiemstra, M.; Van Lier, P.A.; Meeus, W.; Branje, S.; Heim, C.M.; Nemeroff, C.B.; Mill, J.; et al. Genome-wide DNA methylation levels and altered cortisol stress reactivity following childhood trauma in humans. Nat. Commun. 2016, 7, 10967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modell, M.D.S. Hormonal Response Pattern in the Combined DEX-CRH Test Is Stable over Time in Subjects at High Familial Risk for Affective Disorders. Neuropsychopharmacology 1998, 18, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.E.; Svanborg, C.; Plavén-Sigray, P.; Kaldo, V.; Halldin, C.; Schain, M.; Lundberg, J. Serotonin transporter availability increases in patients recovering from a depressive episode. Transl. Psychiatry 2021, 11, 264. [Google Scholar] [CrossRef]


| Obesity Group | Non-Obesity Controls | p-Value | |
|---|---|---|---|
| Number of participants (female) | 28 | 12 | |
| Sex, male/female | 7/21 | 4/8 | 0.70 c |
| Age (years) | 36.6 ± 10.6 | 35.8 ± 7.4 | 0.81 a |
| BMI (kg/m2) | 41.2 ± 5.1 | 22.4 ± 2.3 | <0.0001 a |
| Smoking habits, # with score 0/1/2/3 | 19/0/2/7 | 10/1/0/1 | 0.48 d |
| Beck Depression Inventory | 6.5 [3–11] | 0 [0–3.3] | <0.0001 b |
| SCL-90-anxiety | 49.0 ± 7.6 | 44.8 ± 5.7 | 0.09 b |
| BAS Drive | 14 [11.25–14] | 13 [12–14] | 0.48 b |
| BAS Fun | 11.1 ± 2.0 | 12.4 ± 1.7 | 0.028 b |
| BAS Reward | 16.3 ± 2.2 | 17.3 ± 1.9 | 0.22 b |
| BIS | 19.0 ± 3.8 | 18.6 ± 2.7 | 0.85 b |
| Injected activity (MBq) | 481.3 ± 10.9 | 487.5 ± 6.4 | 0.08 a |
| Obesity Group (n = 28) | Non-Obesity Controls (n = 12) | p-Value | |
|---|---|---|---|
| ACTH1500h | <0.84 (<0.84–<0.87) | <0.84 (<0.84–<0.84) | 0.42 |
| ACTHpostCRH | 1.66 (1.21–2.42) | 1.48 (0.91–1.82) | 0.17 |
| ACTHMAX | 2.10 (1.57–2.98) | 1.85 (1.34–3.06) | 0.46 |
| ACTHAUC | 6.78 (5.03–9.21) | 5.53 (4.05–8.82) | 0.33 |
| Cortisol1500h | 20.0 (15.2–23.3) | 15.8 (11.0–24.2) | 0.29 |
| CortisolpostCRH | 48.9 (32.6–154.1) | 25.5 (20.1–38.1) | 0.01 |
| CortisolMAX | 74.4 (38.6–170.5) | 66.4 (25.9–100.7) | 0.29 |
| CortisolAUC | 216.0 (114.2–525.5) | 165.4 (87.8–262.5) | 0.22 |
| ACTH/cortisolpostCRH | 0.029 (0.013–0.050) | 0.059 (0.034–0.097) | 0.02 |
| ACTH/cortisolMAX | 0.023 (0.013–0.047) | 0.038 (0.021–0.062) | 0.22 |
| ACTH/cortisolAUC | 0.025 (0.014–0.053) | 0.043 (0.027–0.066) | 0.19 |
| Participants with Obesity (n = 28) | Non-Obesity Controls (n = 12) | |||
|---|---|---|---|---|
| ACTHAUC | CortisolAUC | ACTHAUC | CortisolAUC | |
| Group | 0.39 (0.04) | 0.09 (0.65) | 0.05 (0.88) | 0.13 (0.68) |
| FC | 0.29 (0.14) | 0.00 (0.98) | 0.23 (0.47) | 0.56 (0.06) |
| OFC/vmPFC | 0.35 (0.07) | 0.01 (0.96) | 0.09 (0.78) | 0.08 (0.81) |
| dlPFC | 0.33 (0.09) | −0.11 (0.58) | 0.13 (0.68) | 0.50 (0.10) |
| ACC | 0.30 (0.12) | 0.08 (0.68) | −0.17 (0.60) | 0.20 (0.53) |
| Insula | 0.20 (0.30) | 0.06 (0.75) | 0.23 (0.47) | 0.36 (0.25) |
| Hippocampus | 0.05 (0.81) | −0.07 (0.71) | 0.55 (0.07) | 0.59 (0.04) |
| Amygdala | 0.24 (0.22) | −0.08 (0.68) | −0.38 (0.23) | −0.39 (0.21) |
| NAcc | 0.29 (0.13) | 0.05 (0.81) | −0.29 (0.37) | 0.14 (0.66) |
| Head of the caudate | 0.54 (0.003) | 0.15 (0.45) | −0.18 (0.57) | −0.34 (0.29) |
| Putamen | 0.24 (0.23) | −0.02 (0.93) | −0.03 (0.93) | −0.11 (0.73) |
| Thalamus | 0.03 (0.89) | −0.18 (0.37) | 0.32 (0.31) | 0.24 (0.44) |
| Hypothalamus | 0.12 (0.53) | −0.14 (0.49) | 0.15 (0.63) | 0.17 (0.59) |
| Substantia nigra/VTA | 0.19 (0.34) | −0.22 (0.26) | 0.15 (0.65) | 0.13 (0.68) |
| Midbrain | 0.22 (0.27) | −0.22 (0.27) | 0.16 (0.62) | 0.03 (0.91) |
| Pons | 0.04 (0.82) | −0.18 (0.37) | −0.33 (0.30) | 0.32 (0.31) |
| Dorsal raphe nuclei | −0.07 (0.71) | −0.08 (0.70) | 0.03 (0.93) | 0.14 (0.67) |
| Obesity Group (n = 28) | Non-Obesity Controls (n = 12) | |||
|---|---|---|---|---|
| ACTHAUC | CortisolAUC | ACTHAUC | CortisolAUC | |
| Beck Depression Inventory | 0.13 (0.51) | 0.13 (0.53) | −0.03 (0.94) | 0.13 (0.70) |
| SCL-90 anxiety | 0.06 (0.79) | −0.09 (0.71) | 0.31 (0.33) | 0.58 (0.049) |
| BAS Drive | −0.19 (0.34) | −0.16 (0.43) | 0.16 (0.62) | −0.03 (0.92) |
| BAS Fun | −0.18 (0.35) | −0.07 (0.72) | 0.57 (0.05) | 0.35 (0.25) |
| BAS Reward | −0.49 (0.009) | −0.20 (0.32) | 0.52 (0.09) | 0.04 (0.90) |
| BIS | −0.25 (0.21) | −0.35 (0.07) | 0.27 (0.40) | 0.15 (0.65) |
| Obesity Group (n = 28) | ||||||
|---|---|---|---|---|---|---|
| Beck Depression Inventory | SCL-90 Anxiety | BAS Drive | BAS Fun | BAS Reward | BIS | |
| Group | 0.34 (0.07) | −0.29 (0.21) | −0.26 (0.19) | −0.19 (0.32) | −0.57 (0.002) | −0.22 (0.26) |
| Head of the caudate | 0.31 (0.11) | −0.21 (0.37) | −0.11 (0.58) | −0.15 (0.44) | −0.58 (0.001) | −0.26 (0.18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schinke, C.; Rullmann, M.; Luthardt, J.; Drabe, M.; Preller, E.; Becker, G.A.; Patt, M.; Regenthal, R.; Zientek, F.; Sabri, O.; et al. HPA Axis Responsiveness Associates with Central Serotonin Transporter Availability in Human Obesity and Non-Obesity Controls. Brain Sci. 2022, 12, 1430. https://doi.org/10.3390/brainsci12111430
Schinke C, Rullmann M, Luthardt J, Drabe M, Preller E, Becker GA, Patt M, Regenthal R, Zientek F, Sabri O, et al. HPA Axis Responsiveness Associates with Central Serotonin Transporter Availability in Human Obesity and Non-Obesity Controls. Brain Sciences. 2022; 12(11):1430. https://doi.org/10.3390/brainsci12111430
Chicago/Turabian StyleSchinke, Christian, Michael Rullmann, Julia Luthardt, Mandy Drabe, Elisa Preller, Georg A. Becker, Marianne Patt, Ralf Regenthal, Franziska Zientek, Osama Sabri, and et al. 2022. "HPA Axis Responsiveness Associates with Central Serotonin Transporter Availability in Human Obesity and Non-Obesity Controls" Brain Sciences 12, no. 11: 1430. https://doi.org/10.3390/brainsci12111430
APA StyleSchinke, C., Rullmann, M., Luthardt, J., Drabe, M., Preller, E., Becker, G. A., Patt, M., Regenthal, R., Zientek, F., Sabri, O., Then Bergh, F., & Hesse, S. (2022). HPA Axis Responsiveness Associates with Central Serotonin Transporter Availability in Human Obesity and Non-Obesity Controls. Brain Sciences, 12(11), 1430. https://doi.org/10.3390/brainsci12111430







