The Role of Extra-Operative Cortical Stimulation and Mapping in the Surgical Management of Intracranial Gliomas
Abstract
1. Introduction
2. Methodology
2.1. Study Design
2.2. Eligibility Criteria
2.3. Preoperative Planning
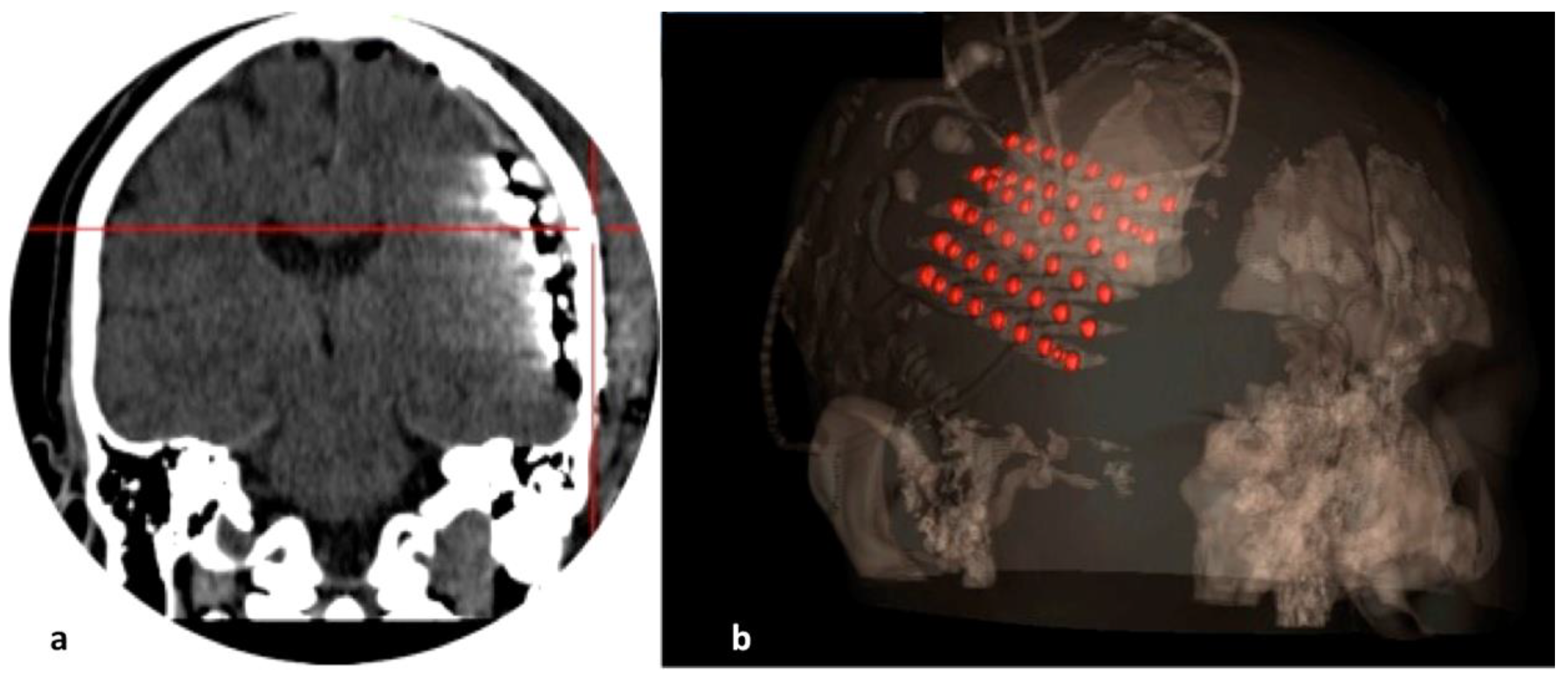
2.4. Electrode Implantation
2.5. Stimulation and Mapping Phase
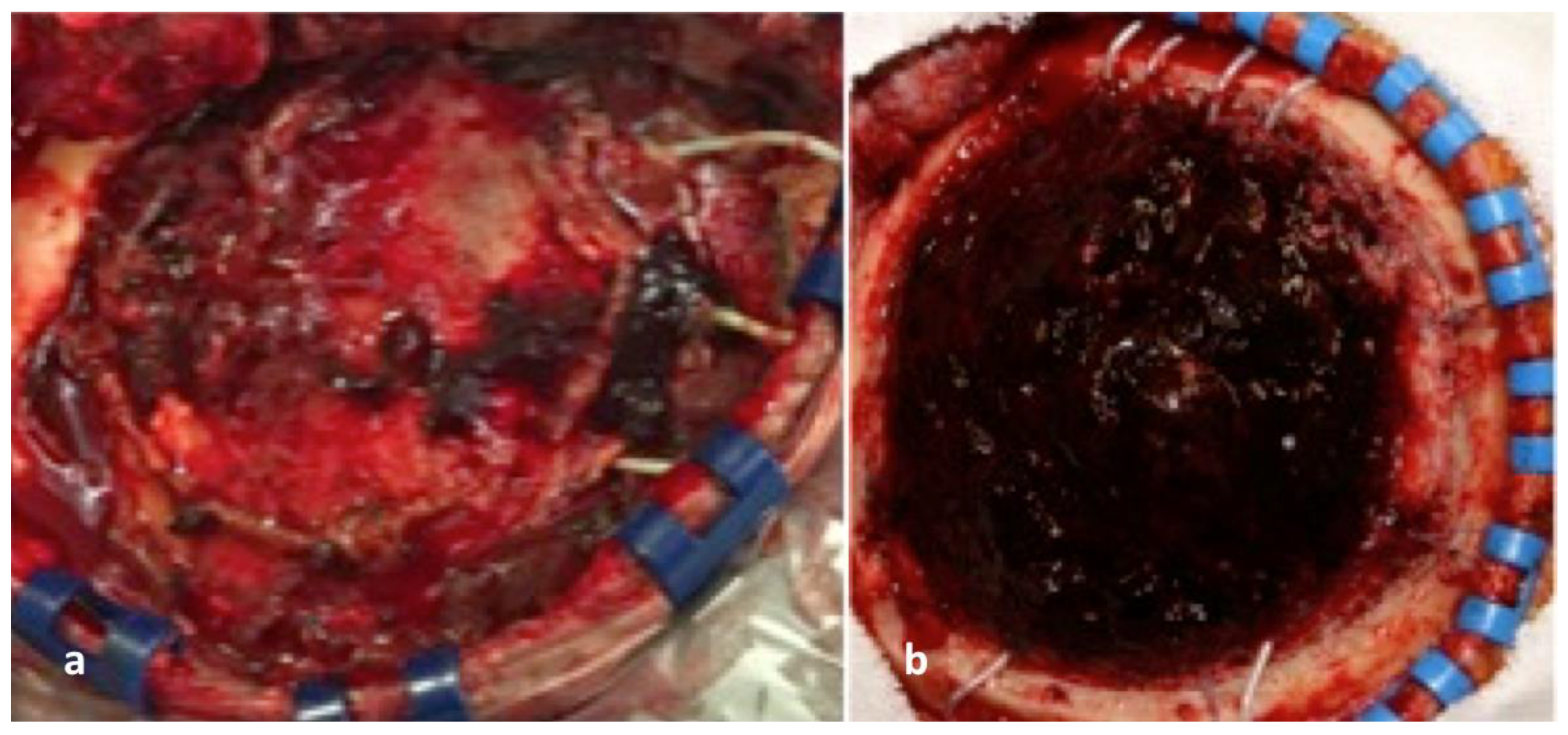
2.6. Tumour Resection Phase
2.7. Data Extraction
2.8. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Efficacy of EOCSM
3.3. Complications Associated with Electrode Implantation and Stimulation
3.4. Complications Associated with Glioma Resection
4. Discussion
4.1. Overview of Our Findings
4.2. Comparison of EOCSM with Other Non-Invasive Modalities
4.3. Comparison of EOCSM with DCS
4.4. Procedure Technical Tips & Tricks
4.5. Prognostic Factors
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hervey-Jumper, S.L.; Berger, M.S. Role of surgical resection in low- and high-grade gliomas. Curr. Treat. Options Neurol. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; Vandenberg, S.; McDermott, M.W.; Berger, M.S. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neurooncol. 2016, 130, 269–282. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Englander, Z.K.; Canoll, P.; Bruce, J.N. Extent of Resection in Glioma–A Review of the Cutting Edge. World Neurosurg. 2017, 103, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Campos, B.; Bruckner, T.; Vogt, L.; Unterberg, A.; Ahmadi, R. Evaluation of neuropsychological outcome and “quality of life” after glioma surgery. Langenbeck’s Arch. Surg. 2016, 401, 541–549. [Google Scholar] [CrossRef]
- Schucht, P.; Beck, J.; Seidel, K.; Raabe, A. Extending resection and preserving function: Modern concepts of glioma surgery. Swiss Med. Wkly. 2015, 145, w14082. [Google Scholar] [CrossRef][Green Version]
- Esquenazi, Y.; Friedman, E.; Liu, Z.; Zhu, J.-J.; Hsu, S.; Tandon, N. The Survival Advantage of “Supratotal” Resection of Glioblastoma Using Selective Cortical Mapping and the Subpial Technique. Neurosurgery 2017, 81, 275–288. [Google Scholar] [CrossRef]
- Englot, D.J.; Han, S.J.; Berger, M.S.; Barbaro, N.M.; Chang, E.F. Extent of Surgical Resection Predicts Seizure Freedom in Low-Grade Temporal Lobe Brain Tumors. Neurosurgery 2012, 70, 921–928. [Google Scholar] [CrossRef]
- Al-Holou, W.N.; Hodges, T.R.; Everson, R.G.; Freeman, J.; Zhou, S.; Suki, D.; Rao, G.; Ferguson, S.D.; Heimberger, A.B.; Mccutcheon, I.E.; et al. Perilesional Resection of Glioblastoma Is Independently Associated With Improved Outcomes. Neurosurgery 2020, 86, 112–121. [Google Scholar] [CrossRef]
- Cardinale, F.; Cossu, M.; Castana, L.; Casaceli, G.; Schiariti, M.P.; Miserocchi, A.; Fuschillo, D.; Moscato, A.; Caborni, C.; Arnulfo, G.; et al. Stereoelectroencephalography: Surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery 2013, 72, 353–366. [Google Scholar] [CrossRef]
- Sagar, S.; Rick, J.; Chandra, A.; Yagnik, G.; Aghi, M.K. Functional brain mapping: Overview of techniques and their application to neurosurgery. Neurosurg. Rev. 2019, 42, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Hervey-Jumper, S.L.; Li, J.; Lau, D.; Molinaro, A.M.; Perry, D.W.; Meng, L.; Berger, M.S. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J. Neurosurg. 2015, 123, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.; Nossek, E.; Sitt, R.; Hayat, D.; Shahar, T.; Barzilai, O.; Gonen, T.; Korn, A.; Sela, G.; Ram, Z. Outcome of elderly patients undergoing awake-craniotomy for tumor resection. Ann. Surg. Oncol. 2013, 20, 1722–1728. [Google Scholar] [CrossRef]
- Nossek, E.; Matot, I.; Shahar, T.; Barzilai, O.; Rapoport, Y.; Gonen, T.; Sela, G.; Korn, A.; Hayat, D.; Ram, Z. Failed awake craniotomy: A retrospective analysis in 424 patients undergoing craniotomy for brain tumor; Clinical article. J. Neurosurg. 2013, 118, 243–249. [Google Scholar] [CrossRef]
- Lehéricy, S.; Duffau, H.; Cornu, P.; Capelle, L.; Pidoux, B.; Carpentier, A.; Auliac, S.; Clemenceau, S.; Sichez, J.P.; Bitar, A.; et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: Comparison with intraoperative stimulation in patients with brain tumors. J. Neurosurg. 2000, 92, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Dym, R.J.; Burns, J.; Freeman, K.; Lipton, M.L. Is Functional MR Imaging Assessment of Hemispheric Language Dominance as Good as the Wada Test?: A Meta-Analysis. Radiology 2011, 261, 446–455. [Google Scholar] [CrossRef]
- FitzGerald, D.B.; Cosgrove, G.R.; Ronner, S.; Jiang, H.; Buchbinder, B.R.; Belliveau, J.W.; Rosen, B.R.; Benson, R.R. Location of language in the cortex: A comparison between functional MR imaging and electrocortical stimulation. Am. J. Neuroradiol. 1997, 18, 1529–1539. [Google Scholar]
- Roux, F.-E.; Boulanouar, K.; Lotterie, J.-A.; Mejdoubi, M.; LeSage, J.P.; Berry, I. Language Functional Magnetic Resonance Imaging in Preoperative Assessment of Language Areas: Correlation with Direct Cortical Stimulation. Neurosurgery 2003, 52, 1335–1347. [Google Scholar] [CrossRef]
- Bizzi, A.; Blasi, V.; Falini, A.; Ferroli, P.; Cadioli, M.; Danesi, U.; Aquino, D.; Marras, C.; Caldiroli, D.; Broggi, G. Presurgical Functional MR Imaging of Language and Motor Functions: Validation with Intraoperative Electrocortical Mapping. Radiology 2008, 248, 579–589. [Google Scholar] [CrossRef]
- Meier, M.P.; Ilmberger, J.; Fesl, G.; Ruge, M.I. Validation of functional motor and language MRI with direct cortical stimulation. Acta Neurochir. 2013, 155, 675–683. [Google Scholar] [CrossRef]
- Giussani, C.; Roux, F.-E.; Ojemann, J.; Sganzerla, E.P.; Pirillo, D.; Papagno, C. Is Preoperative Functional Magnetic Resonance Imaging Reliable for Language Areas Mapping in Brain Tumor Surgery? Review of Language Functional Magnetic Resonance Imaging and Direct Cortical Stimulation Correlation Studies. Neurosurgery 2010, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Sollmann, N.; Hauck, T.; Ille, S.; Meyer, B.; Ringel, F. Repeated mapping of cortical language sites by preoperative navigated transcranial magnetic stimulation compared to repeated intraoperative DCS mapping in awake craniotomy. BMC Neurosci. 2014, 15, 20. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Wesseling, P.; Capper, D. WHO 2016 Classification of gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2016. Available online: https://www.r-project.org/ (accessed on 1 October 2022).
- Rösler, J.; Niraula, B.; Strack, V.; Zdunczyk, A.; Schilt, S.; Savolainen, P.; Lioumis, P.; Mäkelä, J.; Vajkoczy, P.; Frey, D.; et al. Language mapping in healthy volunteers and brain tumor patients with a novel navigated TMS system: Evidence of tumor-induced plasticity. Clin. Neurophysiol. 2014, 125, 526–536. [Google Scholar] [CrossRef]
- Freigang, S.; Fresnoza, S.; Mahdy Ali, K.; Zaar, K.; Jehna, M.; Reishofer, G.; Rammel, K.; Studencnik, F.; Ischebeck, A.; von Campe, G. Impact of Priming on Effectiveness of TMS in Detecting Language-eloquent Brain Areas in Tumor Patients. J. Neurol. Surg. A. Cent. Eur. Neurosurg. 2020, 81, 111–129. [Google Scholar] [CrossRef]
- Rosenstock, T.; Picht, T.; Schneider, H.; Koch, A.; Thomale, U.-W. Left perisylvian tumor surgery aided by TMS language mapping in a 6-year-old boy: Case report. Child’s Nerv. Syst. 2019, 35, 175–181. [Google Scholar] [CrossRef]
- Jung, J.; Lavrador, J.-P.; Patel, S.; Giamouriadis, A.; Lam, J.; Bhangoo, R.; Ashkan, K.; Vergani, F. First United Kingdom Experience of Navigated Transcranial Magnetic Stimulation in Preoperative Mapping of Brain Tumors. World Neurosurg. 2019, 122, e1578–e1587. [Google Scholar] [CrossRef]
- Negwer, C.; Sollmann, N.; Ille, S.; Hauck, T.; Maurer, S.; Kirschke, J.S.; Ringel, F.; Meyer, B.; Krieg, S.M. Language pathway tracking: Comparing nTMS-based DTI fiber tracking with a cubic ROIs-based protocol. J. Neurosurg. 2017, 126, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Picht, T.; Mäkelä, J.P.; Meyer, B.; Ringel, F.; Krieg, S.M. Navigated transcranial magnetic stimulation for preoperative language mapping in a patient with a left frontoopercular glioblastoma: Case report. J. Neurosurg. 2013, 118, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Sobottka, S.B.; Bredow, J.; Beuthien-baumann, B.; Reiss, G.; Schackert, G.; Steinmeier, R. Comparison of Functional Brain PET Images and Intraoperative Brain-Mapping Data Using Image-Guided Surgery. Comput. Aided Surg. 2002, 7, 317–325. [Google Scholar] [CrossRef]
- Thiel, A.; Herholz, K.; Von Stockhausen, H.M.; Van Leyen-Pilgram, K.; Pietrzyk, U.; Kessler, J.; Wienhard, K.; Klug, N.; Heiss, W.D. Localization of language-related cortex with 15O-labeled water PET in patients with gliomas. Neuroimage 1998, 7, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.T.; Sturz, L.; Schreckenberger, M.; Spetzger, U.; Meyer, G.F.; Setani, K.S.; Sabri, O.; Buell, U. Preoperative mapping of cortical language areas in adult brain tumour patients using PET and individual non-normalised SPM analyses. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 951–960. [Google Scholar] [CrossRef]
- Zukotynski, K.A.; Fahey, F.H.; Vajapeyam, S.; Ng, S.S.; Kocak, M.; Gururangan, S.; Kun, L.E.; Poussaint, T.Y. Exploratory evaluation of MR permeability with 18F-FDG PET mapping in pediatric brain tumors: A report from the pediatric brain tumor consortium. J. Nucl. Med. 2013, 54, 1237–1243. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Jiang, Z.; Nguchu, B.A.; Zhou, Y.; Wang, Y.; Wang, H.; Li, Y.; Zhu, Y.; Wu, F.; et al. Decoding and mapping task states of the human brain via deep learning. Hum. Brain Mapp. 2020, 41, 1505–1519. [Google Scholar] [CrossRef]
- Wiesen, D.; Sperber, C.; Yourganov, G.; Rorden, C.; Karnath, H.O. Using machine learning-based lesion behavior mapping to identify anatomical networks of cognitive dysfunction: Spatial neglect and attention. NeuroImage 2019, 201, 116000. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, N.; Yan, J.; Cheng, J.; Lu, J.; Wu, J. Resting-state functional connectivity predicts individual language impairment of patients with left hemispheric gliomas involving language network. NeuroImage Clin. 2019, 24, 102023. [Google Scholar] [CrossRef]
- Kasabov, N.K.; Doborjeh, M.G.; Doborjeh, Z.G. Mapping, learning, visualization, classification, and understanding of fMRI Data in the NeuCube evolving spatiotemporal data machine of spiking neural networks. IEEE Trans. Neural Networks Learn. Syst. 2017, 28, 887–899. [Google Scholar] [CrossRef]
- Gazit, T.; Andelman, F.; Glikmann-Johnston, Y.; Gonen, T.; Solski, A.; Shapira-Lichter, I.; Ovadia, M.; Kipervasser, S.; Neufeld, M.Y.; Fried, I.; et al. Probabilistic machine learning for the evaluation of presurgical language dominance. J. Neurosurg. 2016, 125, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. White Matter Tracts and Diffuse Lower-Grade Gliomas: The Pivotal Role of Myelin Plasticity in the Tumor Pathogenesis, Infiltration Patterns, Functional Consequences and Therapeutic Management. Front. Oncol. 2022, 12, 855587. [Google Scholar] [CrossRef] [PubMed]
- Aabedi, A.A.; Young, J.S.; Chang, E.F.; Berger, M.S.; Hervey-Jumper, S.L. Involvement of White Matter Language Tracts in Glioma: Clinical Implications, Operative Management, and Functional Recovery After Injury. Front. Neurosci. 2022, 16, 932478. [Google Scholar] [CrossRef]
- Luzzi, S.; Lucifero, A.G.; Martinelli, A.; Del Maestro, M.; Savioli, G.; Simoncelli, A.; Lafe, E.; Preda, L.; Galzio, R. Supratentorial high-grade gliomas: Maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurg. Focus 2021, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Essayed, W.I.; Zhang, F.; Unadkat, P.; Cosgrove, G.R.; Golby, A.J.; O’Donnell, L.J. White matter tractography for neurosurgical planning: A topography-based review of the current state of the art. NeuroImage. Clin. 2017, 15, 659–672. [Google Scholar] [CrossRef]
- Sun, G.C.; Wang, F.; Chen, X.L.; Yu, X.G.; Ma, X.D.; Zhou, D.B.; Zhu, R.Y.; Xu, B. nan Impact of Virtual and Augmented Reality Based on Intraoperative Magnetic Resonance Imaging and Functional Neuronavigation in Glioma Surgery Involving Eloquent Areas. World Neurosurg. 2016, 96, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Abhinav, K.; Yeh, F.C.; Mansouri, A.; Zadeh, G.; Fernandez-Miranda, J.C. High-definition fiber tractography for the evaluation of perilesional white matter tracts in high-grade glioma surgery. Neuro. Oncol. 2015, 17, 1199–1209. [Google Scholar] [CrossRef]
- Deman, P.; Bhattacharjee, M.; Tadel, F.; Job, A.S.; Rivière, D.; Cointepas, Y.; Kahane, P.; David, O. IntrAnat Electrodes: A Free Database and Visualization Software for Intracranial Electroencephalographic Data Processed for Case and Group Studies. Front. Neuroinform. 2018, 12, 40. [Google Scholar] [CrossRef]
- Stephani, C.; Koubeissi, M. Differences of Intracranial Electrical Stimulation Thresholds in the Human Brain. Brain Stimul. 2015, 8, 724–729. [Google Scholar] [CrossRef]
- Trebaul, L.; Deman, P.; Tuyisenge, V.; Jedynak, M.; Hugues, E.; Rudrauf, D.; Bhattacharjee, M.; Tadel, F.; Chanteloup-Foret, B.; Saubat, C.; et al. Probabilistic functional tractography of the human cortex revisited. Neuroimage 2018, 181, 414–429. [Google Scholar] [CrossRef]
- Han, S.J.; Teton, Z.; Gupta, K.; Kawamoto, A.; Raslan, A.M. Novel Use of Stimulating Fence-Post Technique for Functional Mapping of Subcortical White Matter During Tumor Resection: A Technical Case Series. Oper. Neurosurg. 2020, 19, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Fountas, K.N.; Smith, J.R. Subdural electrode-associated complications: A 20-year experience. Stereotact. Funct. Neurosurg. 2007, 85, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.M.; Reif, P.S.; Spyrantis, A.; Cattani, A.; Freiman, T.M.; Seifert, V.; Wagner, M.; You, S.J.; Schubert-Bast, S.; Bauer, S.; et al. Invasive EEG-electrodes in presurgical evaluation of epilepsies: Systematic analysis of implantation-, video-EEG-monitoring- and explantation-related complications, and review of literature. Epilepsy Behav. 2019, 91, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Mathon, B.; Clemenceau, S.; Hasboun, D.; Habert, M.O.; Belaid, H.; Nguyen-Michel, V.H.; Lambrecq, V.; Navarro, V.; Dupont, S.; Baulac, M.; et al. Safety profile of intracranial electrode implantation for video-EEG recordings in drug-resistant focal epilepsy. J. Neurol. 2015, 262, 2699–2712. [Google Scholar] [CrossRef]
- Serletis, D.; Bulacio, J.; Bingaman, W.; Najm, I.; González-Martínez, J. The stereotactic approach for mapping epileptic networks: A prospective study of 200 patients. J. Neurosurg. 2014, 121, 1239–1246. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, J.; Bulacio, J.; Alexopoulos, A.; Jehi, L.; Bingaman, W.; Najm, I. Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: Early experience from a North American epilepsy center. Epilepsia 2013, 54, 323–330. [Google Scholar] [CrossRef]
- González-Martínez, J.; Bulacio, J.; Thompson, S.; Gale, J.; Smithason, S.; Najm, I.; Bingaman, W. Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery 2016, 78, 169–179. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, J.; Mullin, J.; Bulacio, J.; Gupta, A.; Enatsu, R.; Najm, I.; Bingaman, W.; Wyllie, E.; Lachhwani, D. Stereoelectroencephalography in children and adolescents with difficult-to-localize refractory focal epilepsy. Neurosurgery 2014, 75, 258–268. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, J.; Mullin, J.; Vadera, S.; Bulacio, J.; Hughes, G.; Jones, S.; Enatsu, R.; Najm, I. Stereotactic placement of depth electrodes in medically intractable epilepsy: Technical note. J. Neurosurg. 2014, 120, 639–644. [Google Scholar] [CrossRef]
- Blauwblomme, T.; David, O.; Minotti, L.; Job, A.S.; Chassagnon, S.; Hoffman, D.; Chabardes, S.; Kahane, P. Prognostic value of insular lobe involvement in temporal lobe epilepsy: A stereoelectroencephalographic study. Epilepsia 2013, 54, 1658–1667. [Google Scholar] [CrossRef]
- Vadera, S.; Mullin, J.; Bulacio, J.; Najm, I.; Bingaman, W.; Gonzalez-Martinez, J. Stereoelectroencephalography following subdural grid placement for difficult to localize epilepsy. Neurosurgery 2013, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]

| Mean or Median * | SD or IQR * | p (Shapiro-Wilk Test) | ||
|---|---|---|---|---|
| Age (years) | 58 | 9.38 | 0.22 | |
| Number of stimulation sessions | 1 * | 1–2 * | <0.001 | |
| Cumulative duration of stimulation (h) | 2 * | 1.5–2.5 * | 0.009 | |
| Cumulative implantation time (h) | 72 * | 48–96 * | <0.001 | |
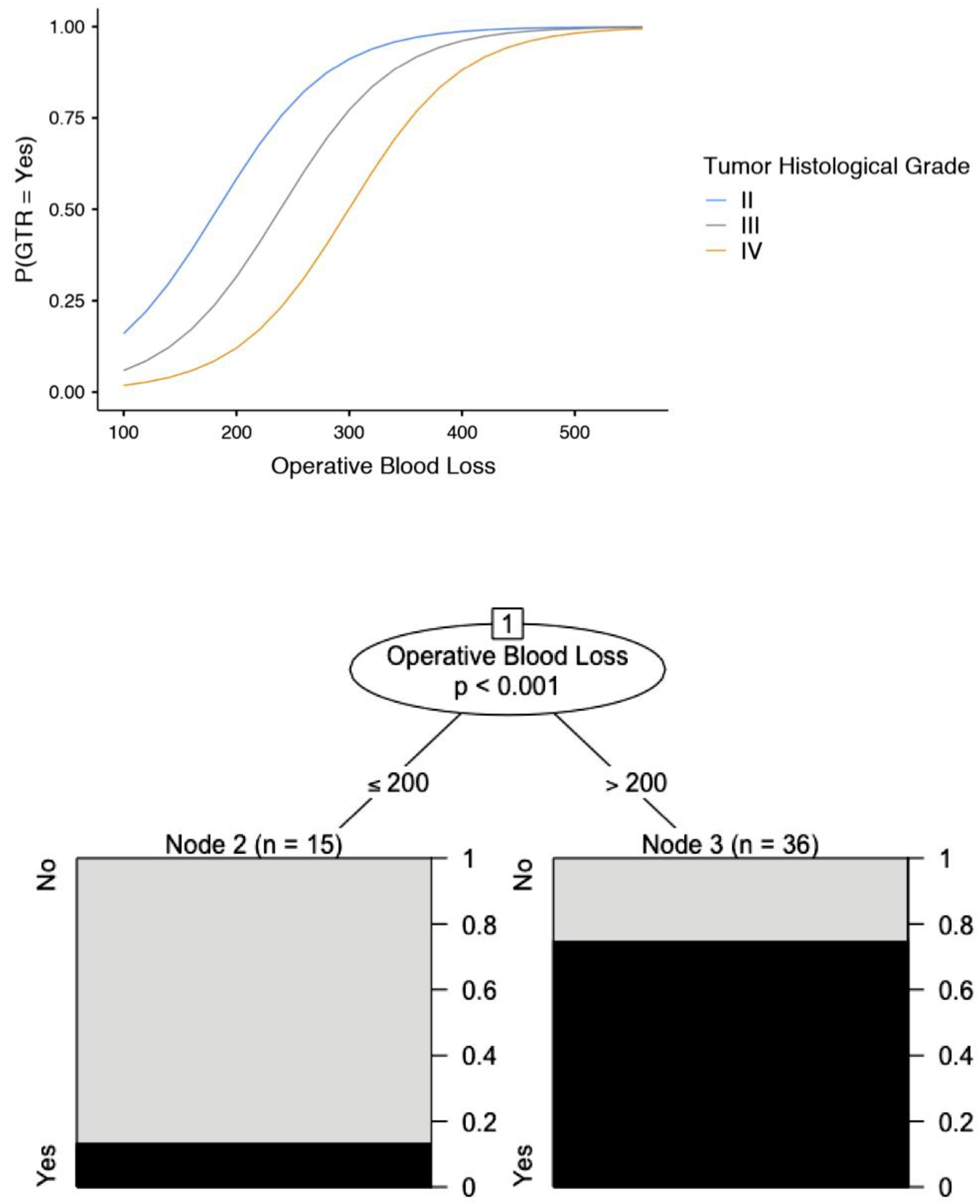
| Intraoperative blood loss (mL) | 286 | 96 | 0.157 | |
| Extent of resection (%) | 95 | 85–98 | <0.001 | |
| Counts | % | p (Chi-square) | ||
| Gender | Female | 21 | 41.2 | 0.262 |
| Male | 30 | 58.8 | ||
| Availability of 1HMRS | No | 13 | 25.5 | <0.001 |
| Yes | 38 | 74.5 | ||
| Availability of fMRI | No | 7 | 13.7 | <0.001 |
| Yes | 44 | 86.3 | ||
| Availability of DTI/FA | No | 19 | 37.3 | 0.092 |
| Yes | 32 | 62.7 | ||
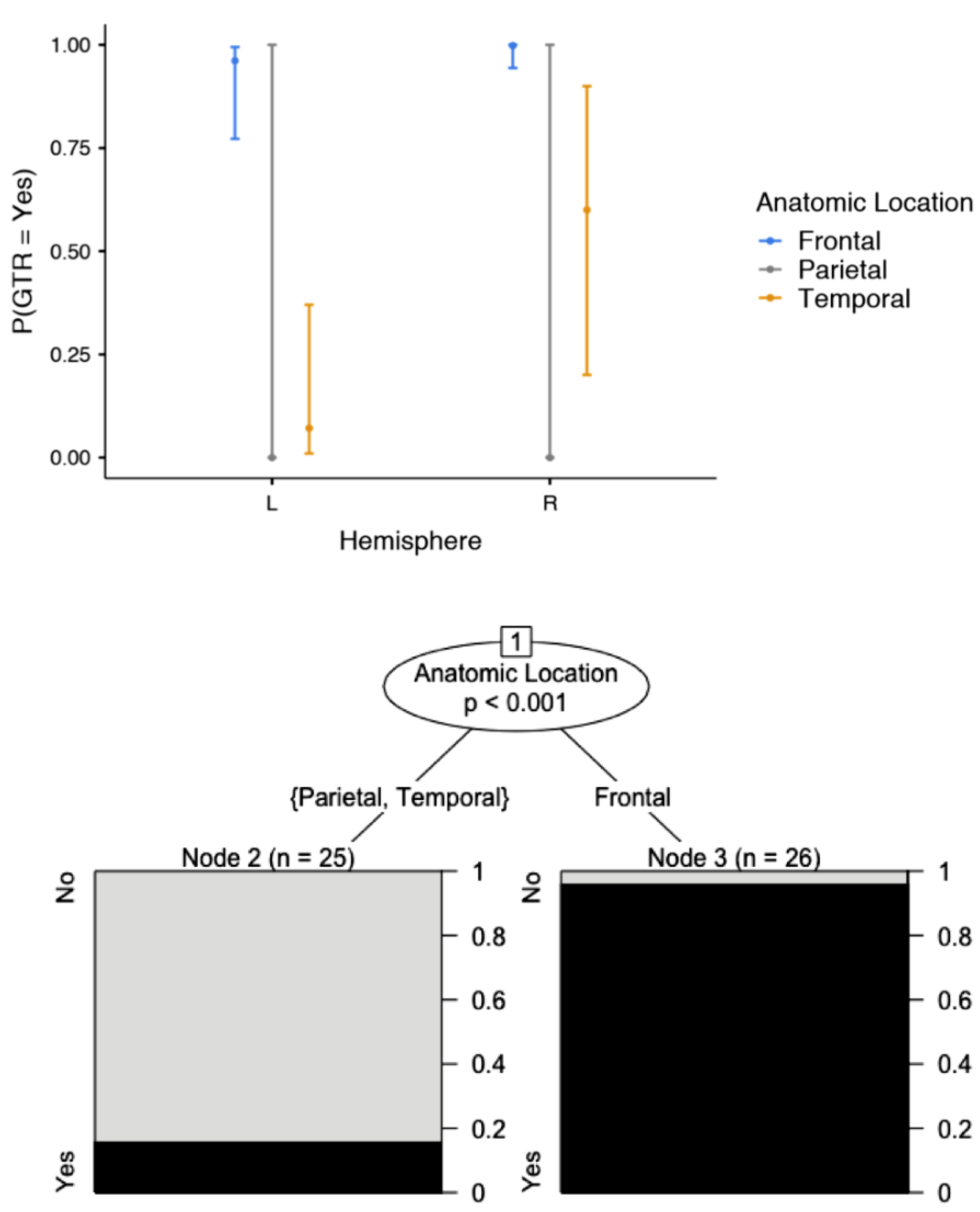
| Location (hemisphere) | Left | 41 | 80.4 | <0.001 |
| Right | 10 | 19.6 | ||
| Location (lobe) | Frontal | 26 | 51 | 0.002 |
| Parietal | 6 | 11.8 | ||
| Temporal | 19 | 37.2 | ||
| Stimulation associated complications | No | 41 | 80.4 | <0.001 |
| CSF leak | 3 | 5.9 | ||
| Seizures | 7 | 13.7 | ||
| Perioperative complications | No | 34 | 67.7 | 0.024 |
| Transient Dysphasia | 11 | 21.6 | ||
| Transient Hemiparesis | 3 | 5.9 | ||
| SDH | 2 | 3.9 | ||
| Seizures | 1 | 2.1 | ||
| WHO tumour histological grade | II | 9 | 17.6 | <0.001 |
| III | 15 | 29.4 | ||
| IV | 27 | 52.9 | ||
| GTR | No | 22 | 43.1 | 0.327 |
| Yes | 29 | 56.9 |
| Risk Factor | Reference | Comparator | Univariate Analysis | |
|---|---|---|---|---|
| Crude OR (95% CI) | p | |||
| Age (years) | Per year | (-) | 0.962 (0.899–1.02) | 0.219 |
| Gender | Female | Male | 0.71 (0389–1.25) | 0.234 |
| Availability of 1HMRS | No | Yes | 0.879 (0.448–1.66) | 0.694 |
| Availability of fMRI | No | Yes | 1.993 (0.872–5.47) | 0.103 |
| Availability of DTI/FA | No | Yes | 1.15 (0.644–2.042) | 0.639 |
| Location (hemisphere) | Left | Right | 0.497 (0.218–1.17) | 0.067 |
| Location (lobe) | Frontal | Parietal | 0 (0-Infinity) | 0.993 |
| Temporal | 0.011 (0.005–0.073) | <0.001 | ||
| Number of stimulation sessions | Per stimulation | (-) | 1.21 (0.656–2.46) | 0.562 |
| Cumulative duration of stimulation | Per hour of stimulation | (-) | 1.40 (0.822–2.53) | 0.236 |
| Stimulation associated complications | No | Yes | 0.497 (0.218–1.17) | 0.067 |
| Cumulative implantation time | Per hour of stimulation | (-) | 1.00 (.0981–1.03) | 0.780 |
| Intraoperative blood loss | Per mL of blood lost | (-) | 1.02 (1.00–1.028) | 0.001 |
| WHO tumor grade | II | III | 0.429 (0.05–2.567) | 0.377 |
| IV | 0.265 (0.035–1.337) | 0.136 | ||
| Risk Factor | Reference | Comparator | Multivariate Analysis | |
|---|---|---|---|---|
| Adjusted OR (95% CI) | p | |||
| Location (hemisphere) | Left | Right | 19.5 (1.30–293) | 0.032 |
| Location (Lobe) | Frontal | Parietal | 0 (0-Infinity) | 0.997 |
| Temporal | 0.00308 (0.0–0.553) | <0.001 | ||
| Risk Factor | Reference | Comparator | Univariate Analysis | |
|---|---|---|---|---|
| Crude OR (95% CI) | p | |||
| Age (years) | Per year | (-) | 1.026 (0.963–1.098) | 0.433 |
| Gender | Female | Male | 1.461 (0.799–2.818) | 0.232 |
| Availability of 1HMRS | No | Yes | 0.859 (0.448–1.697) | 0.650 |
| Availability of fMRI | No | Yes | 0.561 (0.234–1.27) | 0.165 |
| Availability of DTI/FA | No | Yes | 0.609 (0.329–1.107) | 0.106 |
| Location (hemisphere) | Left | Right | 2.023 (0.994–4.331) | 0.055 |
| Location (Lobe) | Frontal | Parietal | 3.333 (0.504–22.71) | 0.2 |
| Temporal | 2.424 (0.675–9.179) | 0.178 | ||
| Number of stimulation sessions | Per stimulation | (-) | 0.568 (0.201–1.172) | 0.187 |
| Cumulative duration of stimulation | Per hour of stimulation | (-) | 0.859 (0.476–1.482) | 0.593 |
| Stimulation associated Complications | No | Yes | 1.555 (0.759–3.202) | 0.220 |
| Cumulative Implantation Time | Per hour of stimulation | (-) | 0.980 (0.951–1.005) | 0.133 |
| Intraoperative blood loss | Per mL of blood lost | (-) | 0.998 (0.991–1.004) | 0.497 |
| WHO tumour grade | II | III | 0.625 (0.11–3.404) | 0.587 |
| IV | 0.526 (1.108–2.60) | 0.418 | ||
| GTR | No | Yes | 0.618 (0.333–1.115) | 0.114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fountas, K.N.; Brotis, A.; Paschalis, T.; Kapsalaki, E. The Role of Extra-Operative Cortical Stimulation and Mapping in the Surgical Management of Intracranial Gliomas. Brain Sci. 2022, 12, 1434. https://doi.org/10.3390/brainsci12111434
Fountas KN, Brotis A, Paschalis T, Kapsalaki E. The Role of Extra-Operative Cortical Stimulation and Mapping in the Surgical Management of Intracranial Gliomas. Brain Sciences. 2022; 12(11):1434. https://doi.org/10.3390/brainsci12111434
Chicago/Turabian StyleFountas, Kostas N., Alexandros Brotis, Thanasis Paschalis, and Eftychia Kapsalaki. 2022. "The Role of Extra-Operative Cortical Stimulation and Mapping in the Surgical Management of Intracranial Gliomas" Brain Sciences 12, no. 11: 1434. https://doi.org/10.3390/brainsci12111434
APA StyleFountas, K. N., Brotis, A., Paschalis, T., & Kapsalaki, E. (2022). The Role of Extra-Operative Cortical Stimulation and Mapping in the Surgical Management of Intracranial Gliomas. Brain Sciences, 12(11), 1434. https://doi.org/10.3390/brainsci12111434









