Relationship between Cardiorespiratory Fitness and Executive Function in Young Adults: Mediating Effects of Gray Matter Volume
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cardiorespiratory Fitness Testing
2.3. Executive Function Assessment
2.3.1. Flanker Task
2.3.2. 2-Back Task
2.3.3. More-Odd Shifting Task
2.4. MRI Data Acquisition
2.4.1. T1-Weighted Image Data Acquisition
2.4.2. GMV Data Pre-Processing
2.5. Statistical Analysis
3. Results
3.1. Demographics, CRF, and EF of the Two Groups
3.2. Effects of CRF on the GMV of the Two Groups
3.3. Analysis of CRF, GMV, and EF
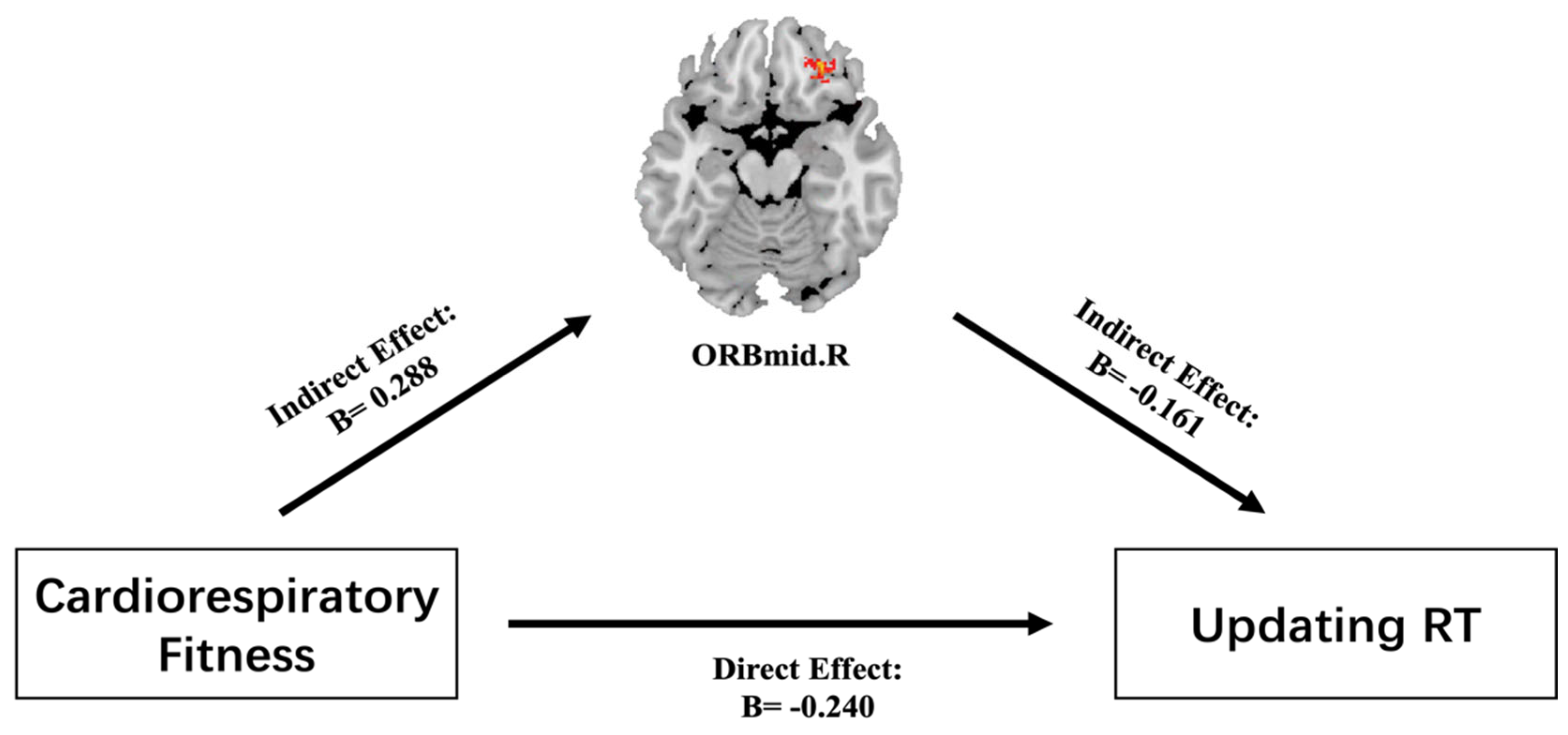
3.4. Mediating Role of GMV between CRF and Updating RT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capouskova, K.; Kringelbach, M.L.; Deco, G. Modes of cognition: Evidence from metastable brain dynamics. Neuroimage 2022, 260, 119489. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.Y.; Hubbard, A.E.; Shaw, L.M.; Trojanowski, J.Q.; Petersen, R.C.; Aisen, P.S.; Weiner, M.W.; Jagust, W.J. Longitudinal change of biomarkers in cognitive decline. Arch. Neurol. 2011, 68, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Gracey, F.; Kelly, G.; Jim, J. A new model to guide identity-focused multidisciplinary rehabilitation for children and young people following acquired brain injury: I-FoRM. Neuropsychol. Rehabil. 2022, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, J.N.; Fares, C.; Bader, R. Relationship between emotional expressions and lifestyle changes among university students during COVID-19 lockdown in Lebanon. J. Infect. Dev. Ctries. 2022, 16, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Floody, P.; Soto-García, D.; Caamaño-Navarrete, F.; Carter-Thuillier, B.; Guzmán-Guzmán, I.P. Negative Physical Self-Concept Is Associated to Low Cardiorespiratory Fitness, Negative Lifestyle and Poor Mental Health in Chilean Schoolchildren. Nutrients 2022, 14, 2771. [Google Scholar] [CrossRef]
- Rodriguez, J.C.; Peterman, J.E.; Fleenor, B.S.; Whaley, M.H.; Kaminsky, L.A.; Harber, M.P. Cardiopulmonary Exercise Responses in Individuals with Metabolic Syndrome: The Ball State Adult Fitness Longitudinal Lifestyle Study. Metab. Syndr. Relat. Disord. 2022. [Google Scholar] [CrossRef]
- McAllister, M.J.; Gonzalez, D.E.; Leonard, M.; Martaindale, M.H.; Bloomer, R.J.; Pence, J.; Martin, S.E. Firefighters with higher cardiorespiratory fitness demonstrate lower markers of cardiovascular disease risk. J. Occup. Environ. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- McDermott, C.E.; Vincent, H.K.; Mathews, A.E.; Cautela, B.G.; Sandoval, M.; Tremblay, A.; Langkamp-Henken, B. Impact of probiotic supplementation on exercise endurance among non-elite athletes: Study protocol for a randomized, placebo-controlled, double-blind, clinical trial. Trials 2022, 23, 603. [Google Scholar] [CrossRef]
- Birnbaumer, P.; Weiner, L.; Handl, T.; Tschakert, G.; Hofmann, P. Effects of Different Durations at Fixed Intensity Exercise on Internal Load and Recovery-A Feasibility Pilot Study on Duration as an Independent Variable for Exercise Prescription. J. Funct. Morphol. Kinesiol. 2022, 7, 54. [Google Scholar] [CrossRef]
- Petkus, A.J.; Jarrahi, B.; Holschneider, D.P.; Gomez, M.E.; Filoteo, J.V.; Schiehser, D.M.; Fisher, B.E.; Van Horn, J.D.; Jakowec, M.W.; McEwen, S.C.; et al. Thalamic volume mediates associations between cardiorespiratory fitness (VO(2)max) and cognition in Parkinson’s disease. Parkinsonism. Relat. Disord. 2021, 86, 19–26. [Google Scholar] [CrossRef]
- Carnevale, D.; Elferink-Gemser, M.; Filgueiras, A.; Huijgen, B.; Andrade, C.; Castellano, J.; SiIva, D.; Vasconcellos, F. Executive Functions, Physical Abilities, and Their Relationship with Tactical Performance in Young Soccer Players. Percept. Mot. Skills 2022, 129, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Keenan, S.; Cooke, M.B.; Chen, W.S.; Wu, S.; Belski, R. The Effects of Intermittent Fasting and Continuous Energy Restriction with Exercise on Cardiometabolic Biomarkers, Dietary Compliance, and Perceived Hunger and Mood: Secondary Outcomes of a Randomised, Controlled Trial. Nutrients 2022, 14, 3071. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E.; Catasta, G. Lifestyle behaviours predicting major cardiovascular diseases mortality in a practically extinct cohort of middle-aged men followed-up for 61 years. Acta Cardiol. 2022. [Google Scholar] [CrossRef]
- Petersen, K.S.; Murphy, J.; Whitbread, J.; Clifton, P.M.; Keogh, J.B. The Effect of a Peanut-Enriched Weight Loss Diet Compared to a Low-Fat Weight Loss Diet on Body Weight, Blood Pressure, and Glycemic Control: A Randomized Controlled Trial. Nutrients 2022, 14, 2986. [Google Scholar] [CrossRef]
- Guio de Prada, V.; Ortega, J.F.; Ramirez-Jimenez, M.; Morales-Palomo, F.; Pallares, J.G.; Mora-Rodriguez, R. Training intensity relative to ventilatory thresholds determines cardiorespiratory fitness improvements in sedentary adults with obesity. Eur. J. Sport Sci. 2019, 19, 549–556. [Google Scholar] [CrossRef]
- Hammami, A.; Randers, M.B.; Kasmi, S.; Razgallah, M.; Tabka, Z.; Chamari, K.; Bouhlel, E. Effects of soccer training on health-related physical fitness measures in male adolescents. J. Sport Health Sci. 2018, 7, 169–175. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Park, J.; Lee, M.; Kim, J.Y.; Kim, K.C.; Kim, S.H.; Im, J.A.; Chu, S.H.; Suh, S.H.; et al. Association between cardiorespiratory fitness and the prevalence of metabolic syndrome among Korean adults: A cross sectional study. BMC Public Health 2014, 14, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Raghuveer, G.; Hartz, J.; Lubans, D.R.; Takken, T.; Wiltz, J.L.; Mietus-Snyder, M.; Perak, A.M.; Baker-Smith, C.; Pietris, N.; Edwards, N.M. Cardiorespiratory Fitness in Youth: An Important Marker of Health: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e101–e118. [Google Scholar] [CrossRef]
- Ruotsalainen, I.; Glerean, E.; Karvanen, J.; Gorbach, T.; Renvall, V.; Syväoja, H.J.; Tammelin, T.H.; Parviainen, T. Physical activity and aerobic fitness in relation to local and interhemispheric functional connectivity in adolescents’ brains. Brain Behav. 2021, 11, e01941. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chad-Friedman, E.; Botdorf, M.; Riggins, T.; Dougherty, L.R. Early childhood cumulative risk is associated with decreased global brain measures, cortical thickness, and cognitive functioning in school-age children. Dev. Psychobiol. 2021, 63, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; Saliba, B.J.; Raine, L.B.; Picchietti, D.L.; Hillman, C.H. Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. J. Pediatr. 2013, 162, 543–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaddock, L.; Pontifex, M.B.; Hillman, C.H.; Kramer, A.F. A review of the relation of aerobic fitness and physical activity to brain structure and function in children. J. Int. Neuropsychol. Soc. 2011, 17, 975–985. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.G.; Zhu, L.N.; Yan, J.; Yin, H.C. Neural Basis of Working Memory Enhancement after Acute Aerobic Exercise: fMRI Study of Preadolescent Children. Front. Psychol. 2016, 7, 1804. [Google Scholar] [CrossRef] [Green Version]
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Malkowski, E.; Alves, H.; Kim, J.S.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; et al. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia 2010, 48, 1394–1406. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.D.; McMorris, C.A.; Longman, R.S.; Leigh, R.; Hill, M.D.; Friedenreich, C.M.; Poulin, M.J. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol. Aging 2010, 31, 2047–2057. [Google Scholar] [CrossRef]
- Boots, E.A.; Schultz, S.A.; Oh, J.M.; Larson, J.; Edwards, D.; Cook, D.; Koscik, R.L.; Dowling, M.N.; Gallagher, C.L.; Carlsson, C.M.; et al. Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging Behav. 2015, 9, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Yaffe, K.; Fiocco, A.J.; Lindquist, K.; Vittinghoff, E.; Simonsick, E.M.; Newman, A.B.; Satterfield, S.; Rosano, C.; Rubin, S.M.; Ayonayon, H.N.; et al. Predictors of maintaining cognitive function in older adults: The Health ABC study. Neurology 2009, 72, 2029–2035. [Google Scholar] [CrossRef] [Green Version]
- Wayne, P.M.; Walsh, J.N.; Taylor-Piliae, R.E.; Wells, R.E.; Papp, K.V.; Donovan, N.J.; Yeh, G.Y. Effect of tai chi on cognitive performance in older adults: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 2014, 62, 25–39. [Google Scholar] [CrossRef]
- Andel, R.; Crowe, M.; Pedersen, N.L.; Fratiglioni, L.; Johansson, B.; Gatz, M. Physical exercise at midlife and risk of dementia three decades later: A population-based study of Swedish twins. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 62–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Themanson, J.R.; Hillman, C.H. Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience 2006, 141, 757–767. [Google Scholar] [CrossRef]
- Zou, L.; Loprinzi, P.D.; Yeung, A.S.; Zeng, N.; Huang, T. The Beneficial Effects of Mind-Body Exercises for People With Mild Cognitive Impairment: A Systematic Review With Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1556–1573. [Google Scholar] [CrossRef]
- Zukowski, L.A.; Tennant, J.E.; Iyigun, G.; Giuliani, C.A.; Plummer, P. Dual-tasking impacts gait, cognitive performance, and gaze behavior during walking in a real-world environment in older adult fallers and non-fallers. Exp. Gerontol. 2021, 150, 111342. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, C.E.; Strang, A.; Anderson, E.; Jurcsisn, J.; Johnson, E.; Das, T.; Kuchan, M.J.; Barbey, A.K. Author Correction: Enhanced physical and cognitive performance in active duty Airmen: Evidence from a randomized multimodal physical fitness and nutritional intervention. Sci. Rep. 2021, 11, 3820, Erratum in Sci. Rep. 2020, 10, 17826. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.; Lambourne, K.; Szabo-Reed, A.N. Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Med. Sci. Sports Exerc. 2016, 48, 1197–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Hermoso, A.; Ramírez-Vélez, R.; Saavedra, J.M. Exercise, health outcomes, and pædiatric obesity: A systematic review of meta-analyses. J. Sci. Med. Sport 2019, 22, 76–84. [Google Scholar] [CrossRef]
- Robinson, L.E.; Stodden, D.F.; Barnett, L.M.; Lopes, V.P.; Logan, S.W.; Rodrigues, L.P.; D’Hondt, E. Motor Competence and its Effect on Positive Developmental Trajectories of Health. Sports Med. 2015, 45, 1273–1284. [Google Scholar] [CrossRef]
- Scott, S.P.; MJ, D.E.S.; Koehler, K.; Petkus, D.L.; Murray-Kolb, L.E. Cardiorespiratory Fitness Is Associated with Better Executive Function in Young Women. Med. Sci. Sports Exerc. 2016, 48, 1994–2002. [Google Scholar] [CrossRef] [Green Version]
- Carol, R.N.; Schreiber Compo, N. The effect of encoding duration on implicit and explicit eyewitness memory. Conscious. Cogn. 2018, 61, 117–128. [Google Scholar] [CrossRef]
- Herting, M.M.; Nagel, B.J. Differences in brain activity during a verbal associative memory encoding task in high- and low-fit adolescents. J. Cogn. Neurosci. 2013, 25, 595–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colcombe, S.J.; Erickson, K.I.; Raz, N.; Webb, A.G.; Cohen, N.J.; McAuley, E.; Kramer, A.F. Aerobic fitness reduces brain tissue loss in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging 2014, 35 (Suppl 2), S20–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, S.M.; Hayes, J.P.; Cadden, M.; Verfaellie, M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front. Aging Neurosci. 2013, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [Green Version]
- Wittfeld, K.; Jochem, C.; Dörr, M.; Schminke, U.; Gläser, S.; Bahls, M.; Markus, M.R.P.; Felix, S.B.; Leitzmann, M.F.; Ewert, R.; et al. Cardiorespiratory Fitness and Gray Matter Volume in the Temporal, Frontal, and Cerebellar Regions in the General Population. Mayo Clin. Proc. 2020, 95, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, A.M.; Voss, M.W.; Prakash, R.S.; Chaddock, L.; Szabo, A.; White, S.M.; Wojcicki, T.R.; Mailey, E.; McAuley, E.; Kramer, A.F.; et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav. Immun. 2012, 26, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Kim, J.S.; Voss, M.W.; Vanpatter, M.; Pontifex, M.B.; Raine, L.B.; Konkel, A.; Hillman, C.H.; et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010, 1358, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Schwarb, H.; Johnson, C.L.; Daugherty, A.M.; Hillman, C.H.; Kramer, A.F.; Cohen, N.J.; Barbey, A.K. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. Neuroimage 2017, 153, 179–188. [Google Scholar] [CrossRef]
- Lukito, S.; Norman, L.; Carlisi, C.; Radua, J.; Hart, H.; Simonoff, E.; Rubia, K. Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol. Med. 2020, 50, 894–919. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Greenstein, D.; Lerch, J.; Clasen, L.; Lenroot, R.; Gogtay, N.; Evans, A.; Rapoport, J.; Giedd, J. Intellectual ability and cortical development in children and adolescents. Nature 2006, 440, 676–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolger, L.E.; Bolger, L.A.; O’Neill, C.; Coughlan, E.; O’Brien, W.; Lacey, S.; Burns, C.; Bardid, F. Global levels of fundamental motor skills in children: A systematic review. J. Sports Sci. 2021, 39, 717–753. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.; Welsman, J.R. Aerobic fitness: What are we measuring? Med. Sport Sci. 2007, 50, 5–25. [Google Scholar] [CrossRef]
- Chaddock-Heyman, L.; Erickson, K.I.; Kienzler, C.; King, M.; Pontifex, M.B.; Raine, L.B.; Hillman, C.H.; Kramer, A.F. The role of aerobic fitness in cortical thickness and mathematics achievement in preadolescent children. PLoS ONE 2015, 10, e0134115. [Google Scholar] [CrossRef]
- Hillman, C.H.; Kramer, A.F.; Belopolsky, A.V.; Smith, D.P. A cross-sectional examination of age and physical activity on performance and event-related brain potentials in a task switching paradigm. Int. J. Psychophysiol. 2006, 59, 30–39. [Google Scholar] [CrossRef]
- Anderheim, L.; Holter, H.; Bergh, C.; Möller, A. Does psychological stress affect the outcome of in vitro fertilization? Hum. Reprod. 2005, 20, 2969–2975. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; Yi, S.; Fitzpatrick, A.; Gupta, V.; Prak, P.R.; Oum, S.; Logerfo, J.P. Appropriate body mass index and waist circumference cutoff for overweight and central obesity among adults in Cambodia. PLoS ONE 2013, 8, e77897. [Google Scholar] [CrossRef] [Green Version]
- Ratcliff, R. Methods for dealing with reaction time outliers. Psychol. Bull. 1993, 114, 510–532. [Google Scholar] [CrossRef]
- Hanna, C.; Hamilton, J.; Arnavut, E.; Blum, K.; Thanos, P.K. Brain Mapping the Effects of Chronic Aerobic Exercise in the Rat Brain Using FDG PET. J. Pers. Med. 2022, 12, 860. [Google Scholar] [CrossRef]
- Hou, J.H.; Ou, Y.N.; Xu, W.; Zhang, P.F.; Tan, L.; Yu, J.T. Association of peripheral immunity with cognition, neuroimaging, and Alzheimer’s pathology. Alzheimers Res. Ther. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Baez, S.; Pinasco, C.; Roca, M.; Ferrari, J.; Couto, B.; García-Cordero, I.; Ibañez, A.; Cruz, F.; Reyes, P.; Matallana, D.; et al. Brain structural correlates of executive and social cognition profiles in behavioral variant frontotemporal dementia and elderly bipolar disorder. Neuropsychologia 2019, 126, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, L.; Cheng, X.; Ge, H.; Hu, G.; Xue, C.; Qi, W.; Xu, W.; Chen, S.; Gao, R.; et al. Functional Integrity of Executive Control Network Contributed to Retained Executive Abilities in Mild Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 710172. [Google Scholar] [CrossRef] [PubMed]


| Variable | Low CRF Group | High CRF Group |
|---|---|---|
| Number | 65 (Female 36) | 65 (Female 26) |
| Age | 19.307 ± 0.634 | 19.12 ± 0.516 |
| BMI/(kg × m−2) | 22.834 ± 3.869 *** | 20.85 ± 2.552 *** |
| VO2max/(L × kg−1 × min−1) | 0.791 ± 0.201 *** | 1.75 ± 0.345 *** |
| Relative VO2max/(mL × min−1 × kg−1) | 12.598 ± 2.166 *** | 28.43 ± 3.064 *** |
| Inhibition | ||
| RT/ms | 19.393 ± 19.531 * | 12.66 ± 12.228 * |
| ACC/% | 0.926 ± 0.120 | 0.943 ± 0.127 |
| Updating | ||
| RT/ms | 1143.294 ± 300.018 * | 1032.98 ± 198.260 * |
| ACC/% | 0.595 ± 0.224 * | 0.681 ± 0.191 * |
| Cognitive flexibility | ||
| RT/ms | 378.713 ± 114.807 *** | 269.954 ± 70.261 *** |
| ACC/% | 0.897 ± 0.079 | 0.909 ± 0.120 |
| Regions | Mini Coordinates | Activation Cluster | Max t-Statistic | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Right ParaHippocampal | 19.5 | −12 | −31.5 | 116 | 4.95 |
| Right Frontal-Mid-Orb | 27 | 42 | −16.5 | 315 | 5.76 |
| Right Putamen | 24 | −4.5 | 9 | 1167 | 5.83 |
| Left Putamen | −22.5 | −1.5 | 9 | 938 | 6.30 |
| Left Caudate | −3 | 6 | −6 | 52 | 5.08 |
| Left Thalamus | −3 | −22.5 | 7.5 | 1003 | 6.42 |
| Right Thalamus | 13.5 | −7.5 | 9 | 126 | 4.72 |
| Region | Low CRF Group GMV/mm3 | High CRF Group GMV/mm3 | Cohen’s d |
|---|---|---|---|
| Right ParaHippocampal | 0.515 ± 0.036 | 0.527 ± 0.049 | −0.266 |
| Right Frontal-Mid-Orb | 0.428 ± 0.043 + | 0.456 ± 0.048 + | −0.603 |
| Right Putamen | 0.524 ± 0.051 | 0.543 ± 0.059 | −0.346 |
| Left Putamen | 0.537 ± 0.046 | 0.550 ± 0.057 | −0.247 |
| Left Caudate | 0.405 ± 0.037 | 0.423 ± 0.058 | −0.381 |
| Left Thalamus | 0.496 ± 0.052 | 0.506 ± 0.062 | −0.172 |
| Right Thalamus | 0.542 ± 0.050 | 0.546 ± 0.060 | −0.066 |
| Regression Equation (n = 130) | Simulation Index | Coefficient Significance | ||||
|---|---|---|---|---|---|---|
| Result Variables | Forecast Variables | R | R2 | F(df) | B | t |
| Updating RT | 0.239 | 0.057 | 7.802 | |||
| Gender | 0.043 | 0.486 | ||||
| Age | 0.122 | 1.409 | ||||
| BMI | 0.043 | 0.479 | ||||
| CRF | −0.240 | −2.793 * | ||||
| GMV | 0.187 | 0.083 | 11.558 | |||
| Gender | 0.082 | 0.946 | ||||
| Age | −0.115 | −1.341 | ||||
| BMI | −0.062 | −2.459 | ||||
| CRF | 0.288 | 3.400 * | ||||
| Updating RT | 0.285 | 0.081 | 5.616 | |||
| Gender | 0.056 | 0.644 | ||||
| Age | 0.105 | 1.212 | ||||
| BMI | 0.018 | 0.202 | ||||
| GMV | −0.161 | −1.814 * | ||||
| CRF | −0.193 | −2.177 * | ||||
| Effect Value | Boot SE Standard Error | Boot LLCI Limit | Boot ULCI Limit | Proportion | |
|---|---|---|---|---|---|
| Total effect | −0.240 | 0.086 | −0.410 | −0.070 | 100% |
| Indirect effect | −0.047 | 0.025 | −0.101 | −0.004 | 19.6% |
| Direct effect | −0.193 | 0.089 | −0.370 | −0.018 | 80.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, L.; Cai, K.; Dong, X.; Xiong, X.; Liu, Z.; Chen, A. Relationship between Cardiorespiratory Fitness and Executive Function in Young Adults: Mediating Effects of Gray Matter Volume. Brain Sci. 2022, 12, 1441. https://doi.org/10.3390/brainsci12111441
Liu Y, Zhu L, Cai K, Dong X, Xiong X, Liu Z, Chen A. Relationship between Cardiorespiratory Fitness and Executive Function in Young Adults: Mediating Effects of Gray Matter Volume. Brain Sciences. 2022; 12(11):1441. https://doi.org/10.3390/brainsci12111441
Chicago/Turabian StyleLiu, Yuexin, Lina Zhu, Kelong Cai, Xiaoxiao Dong, Xuan Xiong, Zhimei Liu, and Aiguo Chen. 2022. "Relationship between Cardiorespiratory Fitness and Executive Function in Young Adults: Mediating Effects of Gray Matter Volume" Brain Sciences 12, no. 11: 1441. https://doi.org/10.3390/brainsci12111441
APA StyleLiu, Y., Zhu, L., Cai, K., Dong, X., Xiong, X., Liu, Z., & Chen, A. (2022). Relationship between Cardiorespiratory Fitness and Executive Function in Young Adults: Mediating Effects of Gray Matter Volume. Brain Sciences, 12(11), 1441. https://doi.org/10.3390/brainsci12111441






